case report
Dedifferentiated Liposarcoma with Heterologous and Homologous Elements-A Report of Three Cases
University Hospital, Pathology, Lewis-Katz Medical School, Philadelphia, PA, United States.
*Corresponding Author: Daniela Proca,University Hospital, Pathology, Lewis-Katz Medical School, Philadelphia, PA, United States
Citation: Samir A, Ahmed L, Israh A, Jian J, F, Daniela P. (2023). Dedifferentiated Liposarcoma with Heterologous and Homologous Elements- A Report of Three Cases, International Journal of Medical Case Reports and Reviews, BRS Publishers. 2(5); DOI: 10.59657/2837-8172.brs.23.028
Copyright: © 2023 Daniela Proca, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 14, 2023 | Accepted: August 02, 2023 | Published: August 22, 2023
Abstract
Background: Osteosarcoma (OS) heterologous component in dedifferentiated liposarcoma (DDL) is rare, but should be considered and MDM2/ CDK-4 testing employed. Myxoid liposarcoma-like (ML) homologous differentiation with simultaneous MDM2 and DDDIT3 amplification is also unusual, and needs to be recognized as it has implications for diagnosis.
Case series: We report three special cases of DDL, two in the retroperitoneum and one in the abdomen: a DDL with OS differentiation, confirmed by MDM2 and CDK4 positive testing, as well as two DDL with homologous ML differentiation confirmed by simultaneous positive MDM2 and DDIT3 testing. In case of a DDL with heterologous osseous differentiation, it is important to sample extensively and identify WDL areas, in order to exclude the diagnosis of extraosseous OS. Without the rare foci of WDL, the extensive high grade spindled DDL may have been misinterpreted as extraosseous OS. MDM2 and CDK4 were useful for the final diagnosis. FISH testing for MDM2 and DDIT3 amplification is also necessary in DDL with ML foci. WDL and DDL can contain ML component, a diagnostic challenge when confronted with a small biopsy. Such tumors have been shown to contain co-amplifications of MDM2 and DDIT3, further adding to a diagnostic dilemma.
Conclusion: The presence of pleomorphic cells or lipoblasts in WDL or DDL, as seen in our two cases, should prompt considering MDM2 FISH testing in conjunction with DDIT3, rather than DDIT3 alone. Furthermore, the first case illustrates the utility of MDM2 and CDK4 testing in ruling out other high-grade sarcomas when a DDL diagnosis is uncertain due to absent WDL or presence of heterologous component, particularly high-grade OS.
Keywords: liposarcoma; myxoid; dedifferentiated; osteosarcoma; DDIT3; MDM2; immunohistochemistry
Introduction
As introducedby Dahlin and Beabout [1], the tem dedifferentiation refers to a low-grade tumor that evolves to a less differentiated one. Evans described dedifferentiated liposarcoma composed of well-differentiated liposarcoma juxtaposed to foci of high-grade no lipogenic sarcoma [2]. Over 65% of the dedifferentiated liposarcomas (DDL) arise in the retroperitoneum, being the most common soft tissue sarcoma in that location. Other locations include the spermatic cord, extremities, head and neck, and trunk. Histologically, dedifferentiated liposarcoma (DDL) often shows areas of high-grade, poorly differentiated sarcoma; in about 10% of cases, the dedifferentiated component shows divergent differentiation, such as smooth or skeletal muscle, vascular, osseous, chondroid and neural origins [3, 4]. The presence of heterologous differentiation in dedifferentiated liposarcomas (DDL) may not affect the clinical outcome, but the prognostic significance is still subject of debate among pathologists [5-8]. Gronchi et al purported that myogenic differentiation in dedifferentiated liposarcoma (DDL) is associated with a worse outcome, in concordance with other reports [7]. Several studies have reported that most retroperitoneal sarcomas diagnosed as poorly differentiated sarcomas are in fact dedifferentiated liposarcomas [8-10]. We report three dedifferentiated liposarcomas (DDL) with special features; one occupies the retroperitoneum and shows heterologous osteoid differentiation (OS) with MDM2 amplification; the second tumor occupies the abdominal cavity and shows homologous myxoid differentiation (ML–like) with simultaneous MDM2 and DDDIT3 amplification; the third tumor is a de-differentiated liposarcoma (DDL) that occupies the retroperitoneum and it also shows myxoid differentiation (ML–like) with simultaneous MDM2 and DDDIT3 amplification.
Case Reports
Case 1: Dedifferentiated Liposarcoma with Heterologous Osteosarcoma component
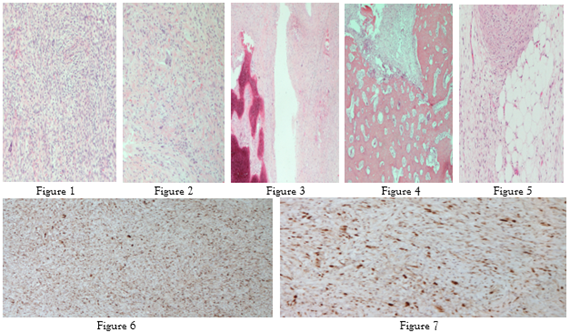
A 68-year-old female with complaints of weight loss and abdominal pain for several months underwent a CT with contrast that demonstrated a left retroperitoneal mass with heterogeneous enhancement, calcifications, and questionable foci of fat. The patient underwent en-bloc tumor resection, left nephrectomy, splenectomy, and distal pancreatectomy. The tumor measured grossly 20.5 cm in greatest dimension; it was adherent focally to the pancreas and was encasing the spleen and left kidney, without infiltrating into any of these organs; a separate tumor nodule was identified adjacent to the celiac artery. Margins were focally positive for tumor. Histologically, the tumor was composed mostly of high grade, pleomorphic and mitotically active spindled cells; rare foci of well differentiated liposarcoma (WDL), lipoma-like and sclerosing subtypes, were identified after re-grossing; focal areas of osteosarcoma (OS) were also noted- overall, the findings were consistent with de-differentiated liposarcoma (DDL) with heterologous OS elements (Figures 1, 2, 3, 4, 5, 6, 7). The DDL was positive with vimentin, and negative for desmin, S100, SMA, CD34, Myoglobin, and C-kit. The DDL and WDL areas were positive with MDM-2, CDK-4, INI-1. Focal, weak, nuclear reactivity with STAT-6 was also noted, consistent with reported focal STAT-6 positivity in 11% of DDL due to close proximity of STAT6 to MDM-2 on chromosome 12q. This tumor was diagnosed as DDL with focal OS heterologous elements, FNCLCC grade 3/3. This case illustrates the importance of thorough sampling of large soft tissue tumors in order to reach an accurate diagnosis. The predominance of high-grade spindled sarcoma would have suggested undifferentiated pleomorphic sarcoma, while areas of malignant osteoid raised the possibility of extraosseous OS, both unusual entities in the retroperitoneum. In the end, foci of WDL and MDM2 and CDK4 positivity tied the diagnosis together. Dedifferentiation occurs in up to 10% of WDL, but heterologous OS differentiation is very rare.
Figures: 1&2: DDL, spindled areas with pleomorphic cells (HE, 40&100x); 3&4, DDL with OS foci (HE, 100x); 5, WDL (HE, 100x); 6, DDL CDK4+ (40x); DDL MDM2 + (100x).
Case 2: Dedifferentiated Liposarcoma with Myxoid Homologous component
A 78-year-old male with history of prostate cancer and renal cell carcinoma with complaints of constipation and abdominal discomfort had an abdominal/ pelvic MRI performed to show a well-defined intra-peritoneal heterogeneously enhancing mass of low attenuation, with layering internal hyper densities and interspersed locules of fat. A wide en-block resection of the mass with left and transverse colectomy, as well as additional mesenteric margin resection, was performed. The tumor measured grossly 30.1 cm in greatest dimension; it infiltrated through the left colonic wall and was present at the margins. Histologically, the tumor was composed of areas of well-differentiated liposarcoma, dedifferen-tiated liposarcoma, as well as areas of myxoid liposarcoma. FISH analysis in one block that had both myxoid and dedifferentiated tumor showed MDM2 and DDIT3 amplification; immunohistochemical stains for MDM2 and CDK4 were performed on three different blocks that showed different histopathologic features and they were positive in areas of WDL and DDL (Figures 1, 2, 3, 4, 5). Of course, this being a heterogenous lipocytic tumor with different histologic grades, the final diagnosis had to be a unifying one to match the best overall grade and prognostic predictive value. This was diagnosed as DDL with focal ML morphology, FNCLCC grade 3/3.
Figures: 1, 2: DDL (HE, 100x); 3, 4, ML (HE, 100x); 5, DDL, high pleomorphism (HE, 100x); 6, ML (HE, 40x); ML (HE, 200x); WDL (HE, 100x); MDM2 (100x); CDK4 (200x).
Case 3: Dedifferentiated Liposarcoma with Myxoid Homologous component
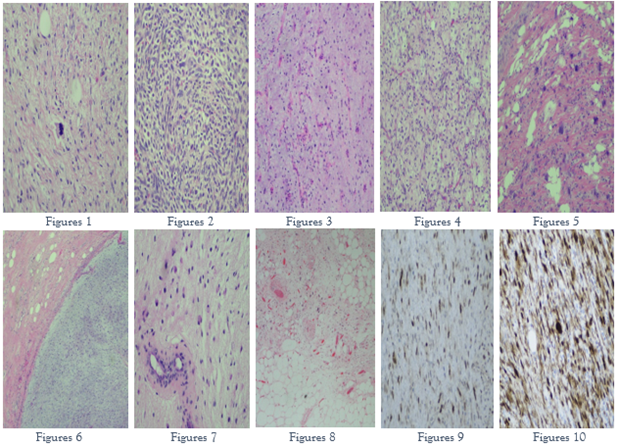
A 70 yo female with new onset of constipation, abdominal pain and dysuria had a CT scan of the chest, abdomen, and pelvis. This demonstrated a large right retroperitoneal mass with contralateral displacement of the right kidney and abutment of its associated artery/vein. A wide en block resection of the retroperitoneal mass with associated radical right nephrectomy, as well as additional soft tissue margin resection, was performed. Grossly, the tumor measured 40 cm in greatest dimension and was adherent to the renal capsule and right adrenal gland, without infiltrating into their parenchyma. Histologically, the tumor was composed of areas of well-differentiated liposarcoma, dedifferentiated liposarcoma, as well as areas of myxoid liposarcoma. FISH analysis in one block that had both myxoid and dedifferentiated tumor showed MDM2 and DDIT3 amplification (Figures 6, 7, 8, 9, 10). The final diagnosis was DDL with focal ML morphology, FNCLCC grade 2/3 - no significant necrosis was present, as opposed to case 2 that showed 50% tumor necrosis. The additional soft tissue margin was negative for tumor.
Discussion
Dedifferentiated liposarcomas (DDL) may exhibit heterologous differentiation in approximately 10% of cases to include rhabdomyosarcomata’s, leiomyo-sarcomata’s, osteosarcoma Tous, angiosarcoma Tous and chondrosarcomata’s elements. Dedifferentiated areas can be a major diagnostic challenge and extensive sampling of the tumors is necessary in order to uncover different foci of differentiation [11,12,13]. We report three interesting dedifferentiated liposarcomas, two in the retroperitoneum and one in the abdomen: a dedifferentiated liposarcoma with heterologous osteosarcoma Tou’s differentiation, confirmed by MDM-2 and CDK-4 positive testing, as well as two dedifferentiated liposarcoma with homologous myxoid differentiation confirmed by positive MDM2 and DDIT3 testing [14-20]. The osteosarcoma Tous component seen in Case 1, could also be interpreted as extraosseous osteosarcoma. Low-grade extraosseous osteosarcoma may show amplified MDM2 [14-16]. Therefore, MDM2 amplification in the osteosarcoma foci is expected, but MDM2 and CDK4 positivity were present in our case in multiple different blocks, to include well differentiated liposarcoma and spindled de-differentiated areas. In addition, foci of OS varied from low to intermediate histologic grade. The combination of several features was useful for the final diagnosis: 1. tumor localization- retroperitoneum, adherent to the pancreas, left kidney, and spleen 2. histopathology of this large tumor, with very rare foci of well differentiated liposarcoma, as well as predominant high grade-spindled dedifferentiated sarcoma, and occasional low to intermediate grade OS, and 3. finally, the MDM2 and CDK4 positivity in WDL, DDL, and heterologous foci.
Cases 2 and 3 raise the possibility of collision tumors, or synchronous well differentiated liposarcoma/ dedifferentiated liposarcoma and a myxoid liposarcoma. Collision tumors are neoplasms of the same organ or anatomical location, which are composed of at least two different tumors without mixed or transitional zone between the tumor components; in our case we had MDM2 and DDDIT3 positive foci in the same block, a mixture of WDL, ML and DDL.
Simultaneous MDM2 and DDDIT3 amplification in the same tumor has been previously reported by Mantilla et al [22], however it appears to be a rare phenomenon and needs to be recognized because a pure myxoid liposarcoma (ML) with no round cell features may be a low-intermediate grade sarcoma, while the two DDL with homologous myxoid differentiation were a grade 3/3 FNCLCC in case 2 (differentiation 3, mitoses 1, necrosis 2) and grade 2/3 FNCLCC in case 3 (differentiation 3, mitoses 1, necrosis 1). Scapa et al (23) showed in a recent study that all 46 cases of myxoid liposarcoma (6 out of which had round cell morphology) showed strong, diffuse immunoreactivity with DDIT3, while other examined lipomatous and myxoid neoplasms were negative for DDIT3 expression; limited, very weak immunoreactivity was very rarely detected in dedifferentiated liposarcoma and pleomorphic lipo-sarcoma and did not correlate with DDIT3 ampli-fication or myxoid liposarcoma like morphology [21-23]. Cases 2 and 3 had FISH amplification in one block for MDM2 and DDIT3, with corresponding histology for DDL and ML which makes the argument of dedifferentiation and subsequent homologous and heterologous differentiation as two possible ways of progression for DDL. Well-differentiated and dedifferentiated liposarcomas can contain prominent myxoid stroma, which can present as a diagnostic challenge when confronted with a small biopsy sample. Such tumors have been shown to contain co-amplifications of MDM2 and DDIT3 [22-25], further adding to a potential diagnostic dilemma. Helpful morphologic clues to exclude the presence of a ML would be absence of the characteristic uniform histomorphology with presence of pleomorphic cells/lipoblasts in the tumor. Presence of the latter would be more supportive of a WDL/DDL. Hence, if any pleomorphic or lipoblast tumor cells are seen in WDL/ DDL, then MDM2 FISH should be considered first or in conjunction with DDIT3, rather than DDIT3 alone. MDM2-amplified WDL and DDL not uncommonly display myxoid liposarcoma-like features including myxoid stroma and characteristic “chicken-wire” like vascular patterns. In a series of 48 cases (Mantilla et al, 22), for the first time the amplification of DDIT3 was identified in one-third of all cases of DDL evaluated, and was significantly associated with myxoid liposarcoma-like morphologic features and homologous lipoclastic differentiation.
Conclusion
In summary, we present a series of three DDL cases: one case with heterologous osseous differentiation and very rare WDL foci, which were important to identify in order to exclude the diagnosis of extraosseous OS. This case illustrates the crucial importance of extensive sampling, in order to uncover all possible histologic patterns within a tumor; sampling one section per cm tumor, as is generally recommended, in this case was insufficient to uncover all histologic patterns. The other two cases are DDL with homologous myxoid differentiation, and they raised the question: are they DDL or ML? The answer was only possible through FISH testing for MDM2 and DDIT3 amplification. DDIT3 amplification appears not to affect the prognosis, including recurrence free survival or disease specific survival, nor the rate of distant metastasis [22-25]. The role of DDIT3 in the pathogenesis of DDL as a possible driver/non-driver mutation or regulating adipocytic differentiation requires larger studies to further characterize.
References
- Dahlin, DC, Beabout, JW. (1971). Dedifferentiation of low-grade chondrosarcomas. Cancer, 28:461-466.
Publisher | Google Scholor - Evans, HL. (1979). Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol, 3:507-523.
Publisher | Google Scholor - A.G. Nascimento. (2001). Dedifferentiated liposarcoma. Semin Diagn Pathol, 18:263-266.
Publisher | Google Scholor - Evans, HL, Khurana, KK, Kemp, BL, Ayala, AG. (1994). Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma. Am J Surg Pathol, 18:1150-1157.
Publisher | Google Scholor - Aurello, P, Virgilio, E, Sirimarco, D, Novi, L, D’Angelo, F, Ramacciato, G. (2014). Dedifferentiated liposarcoma of Cipriani, NA, Kurzawa, P, Ahmad, RA. Prognostic value of myoge nic differentiation in undifferentiated pleomorphic sarcomas of soft tissue. Hum Pathol, 45:1504-1508.
Publisher | Google Scholor - Khin Thway. (2019). Well-differentiated liposarcoma and dedifferentiated liposarcoma: An updated review, Seminars in Diagnostic Pathology, 36(2):112-121.
Publisher | Google Scholor - Gronchi, A, Collini, P, Miceli. (2015). R Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am J Surg Pathol, 39:383-393.
Publisher | Google Scholor - Kossivi Dantey, Karen Schoedel, Oleksandr Yergiyev, David Bartlett, Uma N.M. Rao. (2017). Correlation of histological grade of dedifferentiation with clinical outcome in 55 patients with dedifferentiated liposarcomas, Human Pathology, 66:86-92.
Publisher | Google Scholor - Thway K, Jones RL, Noujaim J, Zaidi S, Miah AB, Fisher C. (2016). Dedifferentiated liposarcoma: updates on morphology, genetics, and therapeutic strategies. Adv Anat Pathol, 23:30-40.
Publisher | Google Scholor - Rekhi B, Baheti AD, Patkar S. (2020). Dedifferentiated liposarcoma with heterologous spindle cell rhabdomyoblastic de-differentiation: An unusual pattern expanding the morphological spectrum. Indian J Pathol Microbiol, 63:630-633.
Publisher | Google Scholor - Van Haverbeke C, Van Dorpe J, Lecoutere E, Flucke U, Ferdinande L, Creytens D. (2017). Dedifferentiated Liposarcoma of the Retroperitoneum with Heterologous Osteosarcomatous Differentiation and a Striking Aneurysmal Bone Cyst-Like Morphology. Int J Surg Pathol, 25(4):374-378.
Publisher | Google Scholor - Taishi Fujii, Takuma Arai, Masahiro Sakon, Shinji Sawano, Yoshitaka Momose, Keiko Ishii, Shiro Miwa. (2013). Retroperitoneal dedifferentiated liposarcoma with osteosarcomatous components: a case report. Int J Clin Exp Pathol, 6(7):1427-1431.
Publisher | Google Scholor - Zajicek, A.K., Bridge, J.A., Akers, J.W. et al. (2017). Dedifferentiated liposarcoma of the lower extremity with low-grade dedifferentiation and low-grade osteosarcomatous component. Skeletal Radiol, 46:265-271.
Publisher | Google Scholor - Von Baer, A, Ehrhardt, A, Baumhoer, D. (2014). Immunohistochemical and FISH analysis of MDM2 and CDK4 in a dedifferentiated extraskeletal osteosarcoma arising in the vastus lateralis muscle: differential diagnosis and diagnostic algorithm. Pathol Res Pract. 2014; 210:698-703.
Publisher | Google Scholor - Yoshida, A, Ushiku, T, Motoi. (2010). T Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol, 23:1279-1288.
Publisher | Google Scholor - Fujii T, Arai T, Sakon M, et al. (2013). Retroperitoneal dedifferentiated liposarcoma with osteosarcomatous components: a case report. Int J Clin Exp Pathol, 6(7):1427-1431.
Publisher | Google Scholor - Toms AP, White LM, Kandel R, Bell RS. Low-grade liposarcoma with osteosarcomatous dedifferentiation: radiologic and histological features. Skeletal Radiol. 2003; 32:286-289.
Publisher | Google Scholor - Yu L, Jung S, Hojnowski L, Damron T. (2005). Dedifferentiated liposarcoma of soft tissue with high-grade osteosarcomatous dedifferentiation. Radiographics, 25:1082-1086.
Publisher | Google Scholor - Yoshida A, Ushiku T, Motoi T, Shibata T, Fukayama M, Tsuda H. (2010). Well-differentiated liposarcoma with low-grade osteosarcomatous component an underrecognized variant. Am J Surg Pathol, 34:1361-1366.
Publisher | Google Scholor - Aurello P, Edoardo V, Sirimarco D, Novi L, D’Angelo F, Ramacciato G. (2013). Dedifferentiated liposarcoma of the retroperitoneum with osteosarcomatous component. Int J Surg Pathol. 21(3):314-315.
Publisher | Google Scholor - Sioletic S, Dal Cin P, Fletcher CD, Hornick JL. (2013). Well differentiated and dedifferentiated liposarcomas with prominent myxoid stroma: analysis of 56 cases. Histopathology, 62:287-293.
Publisher | Google Scholor - Jose G. Mantilla, Robert W. Ricciotti, Eleanor Y. Chen, Yajuan J. Liu, Benjamin L. Hoch. (2019). Amplification of DNA damage-inducible transcript 3 (DDIT3) is associated with myxoid liposarcoma-like morphology and homologous lipoblastic differentiation in dedifferentiated liposarcoma. Modern Pathology, 32:585-599.
Publisher | Google Scholor - Scapa, Jason V. MD; Cloutier, Jeffrey M. MD, PhD; Raghavan, Shyam S. MD; Peters-Schulze, Grace BS; Varma, Sushama BS, MS; Charville, Gregory W. (2021). MD, PhD DDIT3 Immunohistochemistry Is a Useful Tool for the Diagnosis of Myxoid Liposarcoma, The American Journal of Surgical Pathology, 45(2):230.
Publisher | Google Scholor - Murshed KA, Abo Samra H, Ammar A. (2021). Well-Differentiated Liposarcoma of the Hypopharynx Exhibiting Myxoid Liposarcoma-like Morphology with MDM2 and DDIT3 Co-Amplification. Head Neck Pathol, 4. doi: 10.1007/s12105-021-01341-5. Epub ahead of print. PMID: 34089125.
Publisher | Google Scholor - Peck T, Gervasio KA, Zhang PJL, Shields CL, Lally SE, Eagle RC Jr, Milman T. (2020). Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma with Myxoid Stroma in a Hereditary Retinoblastoma Survivor. Ocul Oncol Pathol, 6(2):79-86.
Publisher | Google Scholor