Research Article
Conversion of B.0 Lineage of Human Corona Virus (Covid-19) Into Notoriously Infecting Less Pathogenic and Immune Escape Omicron B.1.1.529.2.75.2 or BA.2.75.2 Variant
- Asit Kumar Chakraborty MSc., PhD *
Department of Biotechnology and Biochemistry, Oriental Institute of Science and Technology, West Bengal, India.
*Corresponding Author: Asit Kumar Chakraborty, Department of Biotechnology and Biochemistry, Oriental Institute of Science and Technology, West Bengal, India.
Citation: Asit K Chakraborty. (2023). Conversion of B.0 Lineage of Human Corona Virus (Covid-19) Into Notoriously Infecting Less Pathogenic and Immune Escape Omicron B.1.1.529.2.75.2 or BA.2.75.2 Variant. Journal of BioMed Research and Reports, BRS Publishers. 2(1); DOI: 10.59657/2837-4681.brs.23.008
Copyright: © 2023 Asit Kumar Chakraborty, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 10, 2022 | Accepted: January 17, 2023 | Published: January 23, 2023
Abstract
Human corona viruses appeared in December, 2019 at China and then within a span of 2 years such viruses (COVID-19) have gone deletions and mutations generating more infectious and death promoting variants like B.1.1.7 (Alpha) and B.1.617.2 (Delta) which were claimed half million deaths worldwide. The D614G and N501Y point mutations in spike protein appeared important for higher transmission and P4715L mutation in RdRP enzyme of ORF1ab polyprotein was also significant. However, since end of November 2021, an Omicron variant with 29 mutations on RBD domain of spike protein appeared in Africa which known as B.1.1.529 lineage which successively generated BA.1 and BA.2 variants. Omicron virus was highly infectious with immune escape properties but caused mild diseases. BA.2 omicron virus changed into BA.2.75.2 with more immune-escape and evasion properties. All omicron viruses had important 31ERS deletion on N-protein and 3675SGF deletion on nsp6 domain of ORF1ab which likely borrowed from B.1.1.7 by recombination. However, 69HV immune-escape deletion in B.1.1.7 and 157FR deletion in B.1.617.2 were not found in BA.2 variants. Its journey from BA.2.3, BA.2.9, BA.2.12, BA.2.48 etc and finally BA.2.75 variant was happened within a span of 10 months and BA.2.75.2 was highly spreading in India and USA recently. Although BA.2.75.2 variant has unique T607I and D1119N mutations in spike protein, other common N440K, G446S and L452R mutations were necessary for higher immune-escape and transmission including D614G and N501Y mutations. A G44R mutation in ORF3a protein also appeared specific for BA.2.75.2 and a 26 bases deletion in the 3’-UTR (5’-gag gcc acg cgg agt acg atc gag tg-3’) found in omicron viruses may be responsible for weak viral load and pathogenicity as such deletion was not found in deadly B.1.1.7 and B.1.617.2 variants. The genetic changes in BA.2.75 sub-variants as well as other emerging omicron variants like BA.4.6, BA.5.2.1, BE.1.1, BQ.1 and BF.7 also have been discussed.
Keywords: SARS-CoV-2; Large RNA viruses; BA.2.75.2; omicron viruses; alpha and delta variants; rna recombination; respiratory infections; immune escape mutants
Introduction
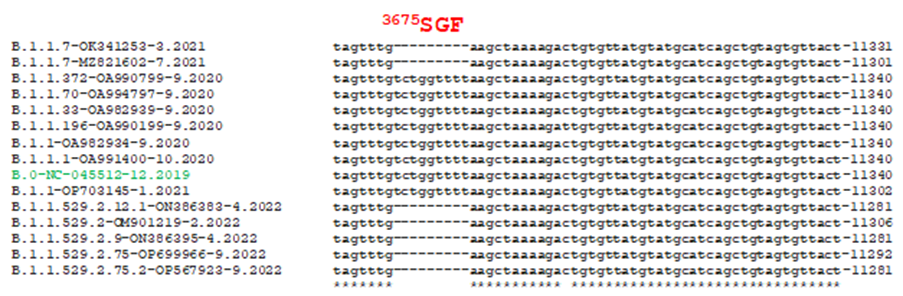
Human corona virus appeared in 2019 in the Wuhan province of China although related viruses like CoV-229E, CoV-HKU1, CoV-NL63 and MERS-CoV were known since 2003 [1]. SARS-CoV-2 has caused huge infections worldwide within 2 years and 6.4 million deaths were reported [2]. It caused many point mutations and deletions creating dominant forms like alpha, beta, delta and very recently omicron [3]. COVID-19 is a large positive-sense RNA virus with a compact 29,980 nucleotides-long genome. It had structural proteins (S, M, N, E) at the 3’- end and 5’ two (ORF1ab, ORF1a) very large poly-proteins (2/3 of the genome; in same reading frames) which were degraded into sixteen (nsp1-nsp16) non-structural proteins [4] including RNA topoisomerase (nsp2) [5], two proteases (nsp3 and nsp5) [6,7], RNA-dependent RNA polymerase (nsp12) [8], RNA helicase (nsp13) [9], uridine specific endoribonuclease (nsp15) [10] and methyl transferases (nsp16) [11] (figure-1). ORF1ab protein was reported as 7096-7092 AA in different variants due to 141KSF and or 3675SGF deletions where Wuhan corona virus was 7096 AA.
Spike protein of B.0 viruses is 1273 AA and stays as trimeric class 1 transmembrane glycoprotein. It’s RBD domain (335-515 aa) acts as receptor binding domain to bind ACE-2 receptor of host lung cells for virus entry [12]. Spike protein 1-13 AA acts as signal peptide and other two domains, S1 (14-685 AAs) and S2 (686 to 1273 AAs) are also important. S-protein also contains fusion contact peptide (788-806 AA) as well as two hepta-peptide (HPPHCPC) repeats at 1163 and 1213 positions as well as furine cleavage point [13]. RNA sequencing clearly established many variants were generated within 2 years due to point mutations and deletions. In USA Wuhan-D614G mutant first peak between March-August, 2020, Alpha (B.1.1.7) 2nd peak with 69HV deletion immune-escape mutant between January-June, 2021 followed by 3rd peak of Delta (B.1.617.2, AY.X) with 157FR deletion mutant between June to December, 2021. Since last week of December, 2022 4th peak of Omicron BA.1 variant (B.1.1.519) spread was evident followed by BA.2 variant spread since April, 2022. From June-July, 2022, omicron BA.4/BA.5 variants were dominating worldwide.
Figure 1A
Figure 1B
Figure 1C
Figure 1D
Spike protein in COVID-19 Alpha is 1270 AA due to deletions of 69HV and 145Y positions but Delta variant has 157FR deletion only (S=1271 AA). Spike protein of Omicron BA.1 variant has 69HV, 143VYY and 212L deletions as well as 215EPE three amino acid insertion but no 24LPP deletion (1270 AA) [14]. Spike protein of Omicron BA.4 and BA.5 corona viruses are 1268 AA due to deletions of 24LPP and 69HV but no 212L deletion or 215EPE insertion. Spike protein of omicron BA.2 has 1270 AA due to 24LPP deletion but no 69HV and 143VYY deletions or 215EPE insertion. 69HVdeletion found in B.1.1.7 also acquired in BA.1/4/5 but BA.2. Among the other structural proteins N-protein (419 AAs) binds to leader RNA of replicating corona virus and also regulates host-pathogen interactions. Three AA deletions (31ERS) were found in N-protein (416 AAs) in all omicron corona viruses (BA.1/2/4/5 and BE.1/BK.1/ XE.1/XBB.1/BQ.1) and was very useful for diagnostics [14-16]. Three amino acid deletions (3675SGF) were found in ORF1ab protein (nsp6 protein domain) of Alpha and Omicron BA.2/BA.4/BA.5 (ORF1ab=7093) viruses but at the same region 3674LSG deletion as well as extra 2083S deletion were found in omicron BA.1 corona virus (ORF1ab=7092 AA) but no such deletions in Delta variant (ORF1ab=7096 AA). Whereas, extra three amino acids (141KSF) deletions were found in omicron BA.4 variant only (ORF1ab=7090 AAs) and such change was utilized to identify BA.4 omicron variant. Further, D614G mutations were detected in all variants and such mutation increased 80% higher transmission. N501Y mutation was appeared first in alpha variant but also located in omicron variants BA.1/BA.4/BA.5 but not in BA.2 and such mutation increased transmission by more than 20% with more immune escape properties [17-20]. We will discuss the generation of BA.2.75.2 from B.0 Wuhan virus illustrating important mutations and deletions.
Spike protein in COVID-19 Alpha is 1270 AA due to deletions of 69HV and 145Y positions but Delta variant has 157FR deletion only (S=1271 AA). Spike protein of Omicron BA.1 variant has 69HV, 143VYY and 212L deletions as well as 215EPE three amino acid insertion but no 24LPP deletion (1270 AA) [14]. Spike protein of Omicron BA.4 and BA.5 corona viruses are 1268 AA due to deletions of 24LPP and 69HV but no 212L deletion or 215EPE insertion. Spike protein of omicron BA.2 has 1270 AA due to 24LPP deletion but no 69HV and 143VYY deletions or 215EPE insertion. 69HVdeletion found in B.1.1.7 also acquired in BA.1/4/5 but BA.2. Among the other structural proteins N-protein (419 AAs) binds to leader RNA of replicating corona virus and also regulates host-pathogen interactions. Three AA deletions (31ERS) were found in N-protein (416 AAs) in all omicron corona viruses (BA.1/2/4/5 and BE.1/BK.1/ XE.1/XBB.1/BQ.1) and was very useful for diagnostics [14-16]. Three amino acid deletions (3675SGF) were found in ORF1ab protein (nsp6 protein domain) of Alpha and Omicron BA.2/BA.4/BA.5 (ORF1ab=7093) viruses but at the same region 3674LSG deletion as well as extra 2083S deletion were found in omicron BA.1 corona virus (ORF1ab=7092 AA) but no such deletions in Delta variant (ORF1ab=7096 AA). Whereas, extra three amino acids (141KSF) deletions were found in omicron BA.4 variant only (ORF1ab=7090 AAs) and such change was utilized to identify BA.4 omicron variant. Further, D614G mutations were detected in all variants and such mutation increased 80% higher transmission. N501Y mutation was appeared first in alpha variant but also located in omicron variants BA.1/BA.4/BA.5 but not in BA.2 and such mutation increased transmission by more than 20% with more immune escape properties [17-20]. We will discuss the generation of BA.2.75.2 from B.0 Wuhan virus illustrating important mutations and deletions.
Methods
We searched PubMed to get idea on published papers on BA.2.75 variants and also searched SARS-CoV-2 NCBI database using BLAST-N and BLAST-X search methods to get related sequences. Multi-alignment of protein was done by MultAlin software (Corpet, F., 1988; Katoh & Standley., 2013) and multi-alignment of DNA by CLUSTAL-Omega software, EMBL-EBI (Sievers, et al., 2011; Wallace, 2005); Yang, et al., 2014). Hairpin structure of ~ 120-200nt sequence was done by OligoAnalyzer 3.1 software (Integrated DNA Technologies). The protein 3-D structure was determined by SWISS-Model software with normal vs. mutant peptides (Gao, et al., 2022; Waterhouse, et al., 2018; Bienert, et al., 2017).
Results
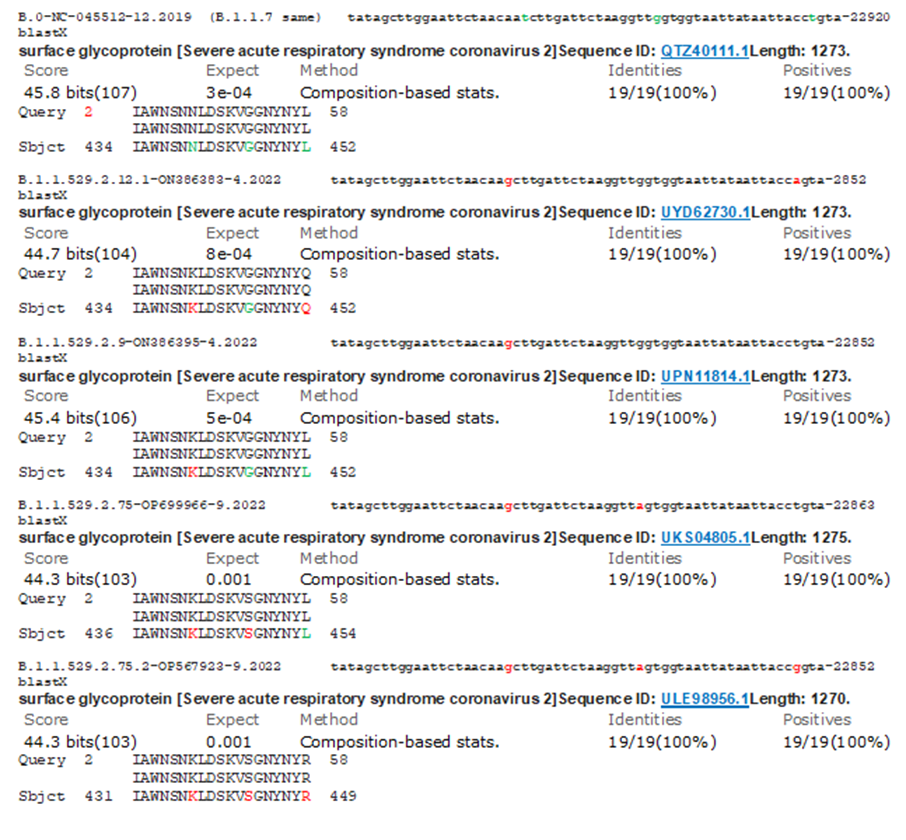
We performed multi-alignment of different corona virus genomes since its isolation in December, 2019 to December, 2022 to find the genetic changes with time and to give an idea how BA.2.75.2 omicron corona virus was formed. Severe Delta corona viruses including Alpha and Beta variants generated from Wuhan virus caused havoc deaths between March, 2021-September, 2022 worldwide. However, spike modified omicron viruses since December 2022, had higher transmission and immune-escape but no death usually occurred unless co-morbidity. Figure 1A showed the part of the multi-alignment where we detected 3675SGF deletion in all BA.2 variant including BA.2.75.2 and such deletion was first found in B.1.1.7 lineage which highly spread worldwide between March, 2021 to August, 2021. On the other hand, B.1.1.7 had 69HV deletion on spike which was not located in BA.2 omicron variants (figure-1B). Figure 1C demonstrated a 24LPP new deletion on the spike NH2 terminus and such deletion was not found in B.1.1.7 early lineage or afterwards lineages (B.1.1.172; B.1.1.372). Such higher lineages neither had 3675SGF deletion indicating SGF three amino acids deletion in nsp6 protein might have role in higher transmission due to virus stability. We further showed the portion of Multi-alignment describing 31ERS N-protein deletion in BA.2 variant and such deletion was prominent in BA.1/4/5 omicron variants (figure-1D). A 3’-UTR deletion located in omicron BA.2.75.2 as well as omicron BA.4 and omicron BA.5 variants but not in Alpha, Delta and omicron BA.1 variant (figure-2).
Figure 2A
Figure 2: 3’-UTR deletion located in BA.2.75.2 as well as other BA.2 variant but not in B.1.1.7 and B.1.1.372 (2A) as well as in highly transmissible Delta, Omicron BA.1, BA.4, BA.5, BF.7 and BA.5.2.1 corona viruses (2B).
Figure 3(A): Multi-alignment to show the BA.2.75 and BA.2.75.2 distinct mutations in the spike.
Figure 3(B): BlastX search to get mutant AAs. All three spike mutations (N440K, G446S, L452R) was found in BA.2.75.2 and such changes might be responsible for high transmission and immune-escape which were not located in B.0 as well as high transmissible B.1.1.7. variant.
However, figure-4 (A, B and C) demonstrated unique BA.2.75.2 variant specific mutations T607I (4A), D1199N (4B) in spike protein and G44R (4C) in ORF3a protein. No other variant has such mutation till now (Alpha, Delta, BA.1, BA.2, BA.4, BA.5 and BF.7). All those above mutations in the spike were responsible for greater immune escape but role of ORF3a G44R mutation was not clear. However, ORF3a may be involved in RNA binding and Arginine (R) may increase RNA binding efficiency.
Figure 4A: Demonstration of another BA.2.75.2 specific genetic mutation (T607I) in spike (4a). Such mutation was not found in Alpha, Delta and omicron BA.1, BA.2, BA.4, BA.5, BA.5.2, BA.5.2.1, BF.7 as well as BA.2.75. (4b). BlastX search to find AA change was shown in figure-4C.
Figure 4B: Multi-alignment and BlastX search to demonstrate D1199N genetic change in spike protein of BA.2.75.2 variant.
Figure 4C: Multi-alignment and BlastX search to demonstrate G44R genetic change in ORF3a protein of BA.2.75.2 variant.
The figure-5 demonstrated the time course of generation of BA.2.75 which in our assay found July, 2022 but other report showed May, 2022. It also showed how 24LPP deletion in spike was important for BA.2.75 sub-lineages but not 69HV, neither 143VYY, nor 157FR.
Figure 5: Multi-alignment of spike proteins of BA.2.75 variants and time course of BA.2.75 generation with 24LPP deletion but no 69HV deletion. The Date of Generation of BA.2.75 was estimated here as July, 2022 based on NCBI SARS-CoV-2 Database. Parts of the alignment were shown.
The figure-6 showed the important passage of BA.2.75.2 from BA.2 which was initiated from B.0 Wuhan corona virus after so many mutations since December, 2019.
Figure 6: Flow chart of generation of different highly infectious corona virus variant of concern with time.
Discussion
Corona virus transmission was huge and cell to cell transmission was reported whereas saliva may be a route of such transmission [19,20]. Alpha and Delta corona viruses caused million deaths whereas Omicron spread also havoc but it caused mild disease and no oxygen requirement or hospitalization was necessary unless co-morbidity. Many mutations and deletions were reported in most genes of SARS-CoV-2. However, D614G spike mutation was very necessary for deadly disease. Analysis found that ORF1ab protein had less mutations compared to spike protein. The Nsp2 RNA topoisomerase I120F mutants were significant in Australia [16]. COVID-19 Nsp15 protein H235Y mutation was a marker for Delta clade C and K260R mutation was a marker for Delta clade E. Among the many mutations reported, N74N, D79D, L214L, L217L and N278N were synonymous and one (D220Y) was non-synonymous found in more than 10,000 isolates worldwide [21]. Ziegler et al (2021) demonstrated that interferon production of nasal epithelial cells was highly impaired in severe corona virus infection with weak antibody production. Huang et al (2022) showed that SARS-CoV-2 viral entry factors such as ACE2 and TMPRSS members were highly enriched in epithelial cells of oral cavity and saliva may be a potential route of virus transmission. Multiple variants were generated by RNA recombination and thus single human might carry multiple species due to multiple infections at different time [22]. We found that D614G and N501Y mutation were carried into omicron viruses including BA.2.75, BA.2.75.1 and BA.2.75.2 variants. HIV RNA virus is only 9.8kb which infects CD4+ lymphocytes through gp120 spike protein causing immune-deficiency (AIDS) so that other pathogens can grow in host. It contains reverse transcriptase enzyme that coverts RNA into double-stranded DNA which then integrates into host chromosome [24.25]. Such process was not reported for COVID-19. The RdRp enzyme of COVID-19 had P4715L mutation and such enzyme was a target for many drugs like remdesivir and favipiravir. However, so far, few vaccines were approved against COVID-19 and very effective to generate antibodies against corona viruses [26,27]. Serum generated in COVID-19 infected host is very good drug to treat other SARS-CoV-2 infected person but in case of omicron virus infections old serum seems not effective [28]. Thus, BA.2.75 variant’s therapy was not successful yet using human serum of previously infected person [29,30]. The BA.2.75 variant hCoV-19/Japan/TY41-716/2022 (TY41-716) had nine amino acid changes (K147E, W152R, F157L, I210V, G257S, D339H, G446S, N460K, and Q493 [reversion]) in its S protein as compared with a BA.2 isolate (hCoV-19/Japan/UT-NCD1288-2N/2022) and completely resistant to Imdevimab [31]. The BA.2.75.2 and BA.4.6 variants both showed complete escape from Cilgavimab and a combination of Cilgavimab and Tixagevimab but was sensitive to Bebtelovimab [32,33]. However, accumulation of so many distinct mutations and deletions in the spike and ORF1ab proteins from earlier deadly B.1.1.7 and B.1.617.2 variants signals that BA.2.75.2 variant may gain more dominant marker to stay as important omicron corona viruses [34,35].
Acknowledgement
I thank NCBI for free SARS-CoV-2 Database and free BLAST program to serve humanity worldwide. The free MultAlign software and CLUSTAL-omega software were also acknowledged.
Competing interest
The author declares no conflict of interest.
References
- Peiris M, Poon L.L.M. (2021). Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) (Coronaviridae). Encyclopedia of Virology, 814-824.
Publisher | Google Scholor - Lu G, Wang Q, Gao G.F. (2015). Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 23:468-478.
Publisher | Google Scholor - Ge X.Y, Li J.L, Yang X.L, Chmura A.A, Zhu G, et al. (2013). Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature, 503:535-538.
Publisher | Google Scholor - Chakraborty A.K & Chanda A. (2021). New Biotechnological Exploration on COVID-19 Proteins: Functions, Mutational Profiles and Molecular Targets for Drug Design. Sun Text Rev Virol. 2(1):115.
Publisher | Google Scholor - Chakraborty, A.K. (2020). Coronavirus Nsp2 Protein Homologies to the bacterial DNA Topoisomerase I and IV Suggest Nsp2 Protein is an unique RNA Topoisomerase with novel target for drug and vaccine development. Virol Mycol. 9:185.
Publisher | Google Scholor - Noske G.D, Nakamura A.M, Gawriljuk V.O, Fernandes R.S, et al. (2021). A Crystallographic Snapshot of SARS-CoV-2 Main Protease Maturation Process. J Mol Biol. 433:167118.
Publisher | Google Scholor - Rut W, L, Z, Zmudzinski M, Patchett S, Nayak D, et al. (2020). Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design. Sci Adv.
Publisher | Google Scholor - Pathania S, Rawal R.K, Singh P.K. (2022). RdRp (RNA-dependent RNA polymerase): A key target providing anti-virals for the management of various viral diseases. J Mol Struct. 1250: 131756.
Publisher | Google Scholor - Chakraborty A.K. (2020). Multi-Alignment comparison of Coronavirus non-structural proteins Nsp13-16 with ribosomal proteins and other DNA/RNA modifying enzymes suggested their roles in the regulation of host protein synthesis. International J Clini Med Informatics, 3:7-19.
Publisher | Google Scholor - Pillon M.C, Frazier M.N, Dillard L.B, et al. (2021). Cryo-EM structures of the SARS-CoV-2 endoribonuclease Nsp15 reveal insight into nuclease specificity and dynamics. Nat Commun. 12:636.
Publisher | Google Scholor - Chakraborty, A.K. (2020). Clinical, Diagnostic and Therapeutic implications of Coronavirus ORFab Polyprotein associated Nsp16 Protein-A bioinformatics approach. Acta Scientific Medical Sciences, 4(5):97-103.
Publisher | Google Scholor - Korber B, Fischer W.M, Gnanakar S, Yoon H, Theiler J, et al. (2020). Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell, 182:812-827.
Publisher | Google Scholor - Lu R, Zhao X, Li J, Niu P, Yang B, et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395:565-574.
Publisher | Google Scholor - Chakraborty AK. (2022). Hyper-variable Spike protein of Omicron corona virus and its differences with Alpha and Delta variants: Prospects of RT-PCR and new vaccine. J Emerg Dis Virol. 7(1):1-13.
Publisher | Google Scholor - Li Q, Nie J, Wu J, Zhang L, Ding R, et al. (2021). SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell, 184:2362-2371.
Publisher | Google Scholor - Chakraborty, A.K. (2021). Abundant transmission of Corona Virus Nsp2 RNA Topoisomerse I120F mutants with concurrence D614G Spike protein mutation in Australia. J Antiviral Antiretroviral. 13:1.
Publisher | Google Scholor - Huang N, Pérez P, Kato T, Mikami Y, Okuda K. (2021). SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 27(5):892-903.
Publisher | Google Scholor - Ziegler C.G.K, Miao V.N, Owings A.H, et al. (2021). Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell, 184(18):4713-4733.
Publisher | Google Scholor - Zing C, Evans J.P, King T, et al. (2022). SARS-CoV-2 spreads through cell-to-cell transmission. Proc Natl Acad Sci. USA. 119(1):e2111400119.
Publisher | Google Scholor - Teng I.T, Nazzari A.F, Choe M, et al. (2022). Molecular probes of spike ectodomain and its subdomains for SARS-CoV-2 variants, Alpha through Omicron. PLoS One, 17(5):e0268767.
Publisher | Google Scholor - Wilson I.M, Frazier M.N, Li J.L, et al. (2022). Biochemical Characterization of Emerging SARS-CoV-2 Nsp15 Endoribonuclease Variants. bioRxiv.
Publisher | Google Scholor - Wang H, Cui X, Cai X, An T. (2022). Recombination in positive strand RNA viruses. Front Microbiol.
Publisher | Google Scholor - Felt S.A, Sun Y, Jozwik A, et al. (2021) Detection of respiratory syncytial virus defective genomes in nasal secretions is associated with distinct clinical outcomes. Nat Microbiol. 6:672-681.
Publisher | Google Scholor - Singh A.K, Das K. (2022). Insights into HIV-1 Reverse Transcriptase (RT) Inhibition and Drug Resistance from Thirty Years of Structural Studies. Viruses. 14(5):1027.
Publisher | Google Scholor - Dybul M, Attoye T, Baptiste S, et al, (2021). The case for an HIV cure and how to get there. Lancet HIV, 8(1):51-58.
Publisher | Google Scholor - Wang Z, Schmidt F, Weisblum Y, et al. (2021). mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 592(7855): 616-622.
Publisher | Google Scholor - Zhu F.C, Guan X.H, Li Y.H, Huang J.Y, Jiang T, et al. (2020). Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet, 396:479-488.
Publisher | Google Scholor - Wang Q, Guo Y, Iketani S, Nair M.S, Li Z, et al. (2022). Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature, 608(7923):603-608.
Publisher | Google Scholor - Qu P, Evans J.P, Faraone J, et al. (2022). Distinct Neutralizing Antibody Escape of SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7 and BA.2.75.2.
Publisher | Google Scholor - Gruell H, Vanshylla K, Tober-Lau P, et al. (2022). Neutralisation sensitivity of the SARS-CoV-2 omicron BA.2.75 sublineage. Lancet Infect Dis. 22(10):1422-1423.
Publisher | Google Scholor - Takashita E, Yamayoshi S, Fukushi S, et al. (2022). Efficacy of Antiviral Agents against the Omicron Subvariant BA.2.75. N Engl J Med. 387:1236-1238.
Publisher | Google Scholor - Sheward D.J, Kim C, Fischbach J, et al. (2022). Omicron sub-lineage BA.2.75.2 exibits extensive escape from neutralising antibodies. Lancet Infect Dis. 22(11):1538-1540.
Publisher | Google Scholor - Qu P, Evans J.P, Faraone J, et al. (2022). Distinct Neutralizing Antibody Escape of SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7 and BA.2.75.2.
Publisher | Google Scholor - Saito A, Tomura T, Zahradnik J, et al. (2022). Virological characteristics of the SARS-CoV-2 Omicron BA.2.75 variant. Cell Host & Microbe. 30(11):1550-1555.
Publisher | Google Scholor - Scarpa F, Sanna D, Azzena I, et al. (2022). On the SARS-CoV-2 BA.2.75 variant: A genetic and structural point of view. J Med Virol.
Publisher | Google Scholor