Research Article
Construction and Analysis of Instant Effect Formulation Involving Box-Behnken
- Tushar Sonare
- Aman Kumar
- Kratika Khadsondni
- Krutika Mandloi
- Akash Yadav *
- Dinesh Kumar Jain
IPS Academy College of Pharmacy, Knowledge Village, Rajendra Nagar, A.B. Road, Indore-452012, India.
*Corresponding Author: Akash Yadav, IPS Academy College of Pharmacy, Knowledge Village, Rajendra Nagar, A.B. Road, Indore-452012, India.
Citation: Sonare T., Kumar A., Khadsondni K., Mandloi K., Yadav A., et al. (2025). Construction and Analysis of Instant Effect Formulation Involving Box-Behnken, Journal of Clinical Research and Clinical Trials, BioRes Scientia Publishers. 4(3):1-15. DOI: 10.59657/2837-7184.brs.25.050
Copyright: © 2025 Akash Yadav, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 10, 2025 | Accepted: March 12, 2025 | Published: March 20, 2025
Abstract
This research investigated the creation of fast-dissolving tablets containing glipizide, utilizing a Box-Behnken design and direct compression method. The study aimed to incorporate natural super disintegrants such as Plantago ovata seed mucilage and the natural binder Guar gum, in addition to the synthetic super disintegrant cross-povidone. Detailed pre-formulation studies were carried out to verify the compatibility between glipizide and all the excipients used in the formulation. The resulting fast-dissolving tablets were thoroughly assessed for weight variation, hardness, friability, disintegration time, in vitro drug release profile, and stability. The Box-Behnken design proved to be an effective approach for optimizing the amounts of both natural and synthetic super disintegrants, with the goal of achieving quick disintegration and an optimal drug release profile. The expected result is a promising formulation of fast-dissolving glipizide tablets that could greatly enhance patient compliance, particularly for diabetic patients who have trouble swallowing. This formulation has the potential to improve therapeutic effectiveness and patient ease in managing diabetes.
Keywords: diabetes mellitus; box-behnken design; plantago ovata seed mucilage; glipizide; fast dissolving tablet
Introduction
Diabetes mellitus is a long-term metabolic condition characterized by persistently high blood sugar levels, which can gradually harm essential organs such as the liver, blood vessels, eyes, and kidneys, along with other bodily systems. If not managed properly, diabetes can result in serious health issues over time. There are mainly two types of diabetes: Type 1 and Type 2. Type2 diabetes is the most form and mainly affects, though it is increasingly diagnosed in younger individuals due to changes in. This condition arises when the body becomes resistant to insulin or when the pancreas does not generate sufficient insulin to maintain normal blood sugar levels. Over the last thirty years, the incidence of type 2 diabetes has notably risen worldwide, across all income brackets, primarily due to escalating obesity rates, poor dietary habits, and lack of physical activity.
On the other hand, Type 1 diabetes, also referred to as juvenile diabetes or insulin-dependent diabetes, is an autoimmune disorder in which the pancreas produces little to no insulin. This type typically emerges during childhood or adolescence but can also develop in adults. People with diabetes, regardless of the type, rely on consistent and affordable healthcare access, particularly for vital medications like insulin, to manage their condition and avert life-threatening complications. Diabetes represents a significant global health issue, resulting in around 1.5 million fatalities each year. The quantity of diagnosed diabetes cases continues to rise steadily across the globe, creating considerable challenges for healthcare systems. Tackling this escalating epidemic necessitates immediate global action. It is crucial to implement comprehensive strategies aimed at reducing both diabetes and obesity rates by 2025. This includes public health initiatives, better healthcare accessibility, and policies encouraging healthier lifestyles. Effectively combating diabetes requires a unified international approach to alleviate further health and economic strains [1-4].
Glipizide is a key medication used to manage Type 2 diabetes mellitus. It is classified as a sulfonylurea and has a molecular weight of 445.536 g/mol. The primary action of this drug is to increase insulin production as well as improve insulin sensitivity. As an oral drug, glipizide lowers blood sugar levels by stimulating the pancreas to produce more insulin. It is essential for controlling high blood sugar levels in Type 2 diabetes and enhancing the body's insulin response. Glipizide is available in several forms, such as sustained-release tablets and oral solutions. It is listed in major pharmacopoeias, including the Indian Pharmacopoeia (IP), British Pharmacopoeia (BP), and United States Pharmacopoeia (USP). Generally taken orally, its biological half-life is between 2 to 6 hours. Glipizide is mainly prescribed for managing non-insulin-dependent diabetes mellitus (Type 2 diabetes) due to its ability to boost insulin production and effectiveness. This medication is vital in diabetes care, providing a consistent approach to lowering blood sugar levels in those with Type 2 diabetes [5-8].
Figure 1: Glipizide.
Pharmaceutical companies focused on medical goals are typically called medicine. Since drugs cannot be taken in their pure form, they must be properly formulated for effective absorption in the body. The oral route has gained prominence as the primary method of drug administration due to its user-friendliness, adaptable design, and high rates of patient compliance. However, medications taken orally can undergo considerable metabolism and interaction in the gastrointestinal tract and liver, which often limits their presence in systemic circulation. Rapid-dissolving tablets, approved by the U.S. FDA in 1998, dissolve or break apart in the mouth without requiring water. These tablets quickly disintegrate when they come into contact with saliva, which improves drug absorption, especially for compounds that do not dissolve well in water. Although formulating soluble compounds presents certain challenges, enhancing solubility remains crucial for improving bioavailability.
The dispersion process has surfaced as an effective approach to tackle this issue. These fast-dissolving tablets provide a viable medication option for patients who struggle with oral drug intake, including children, individuals undergoing medical therapy, or those with mental health issues. Created using a soft molded matrix characterized by low porosity or compressive strength, these tablets quickly spread in saliva, removing the need for water and offering a user-friendly delivery method for those with difficulties swallowing. They significantly improve the patient experience, especially for individuals who have trouble swallowing, ultimately leading to better treatment outcomes while remaining easy to administer [9-12].
Figure 2: Fast dissolving tablet.
Box-Behnken Experimental Design
Response Surface Methodology (RSM) is centered on exploring the connection between control variables and response variables, which facilitates the optimization of processes. A vital element of RSM is the strategic design of experiments, essential for reducing costs and demanding careful consideration of factors like mixture and level. Methods such as full factorial designs, Box-Behnken Design (BBD), and central composite designs serve as fundamental instruments in optimization research. The Box-Behnken Design, accessible through Design Expert software (version 7.0, Stat-Ease Inc.), is especially useful, as it accommodates the investigation of three levels and provides reliable findings across 15 experimental trials, featuring five repeated runs to enhance consistency. This methodology employs a second-order polynomial model to delineate the relationships among variables. Utilizing these optimized experimental designs speeds up the progress of pharmaceutical research by making data collection and analysis more efficient, thereby boosting research effectiveness and productivity [13-15].
Table 1: The table showing the independent variable and the levels that are selected.
| S. No. | Independent Variable | levels | |
| -1(lower level) | +1(higher level) | ||
| 1. | Cross-Povidone | 1 | 4 |
| 2. | Plantago Ovata Seed Mucilage | 2 | 10 |
| 3. | Guar Gum | 1 | 2 |
Table 2: Dependent Variables.
| Response | Variables | Unit |
| 1. | Dissolution | (%) |
| 2. | Disintegration Time | Minute |
| 3. | Hardness | kg/cm2 |
Table 3: Formulation Runs as per Box Behnken design.
| Runs | Cross-Povidone | Isabgol Husk | Guar Gum |
| 1. | 2.5 | 10 | 1 |
| 2. | 2.5 | 2 | 2 |
| 3. | 1 | 10 | 1.5 |
| 4. | 2.5 | 6 | 1.5 |
| 5. | 1 | 6 | 1 |
| 6. | 2.5 | 6 | 1.5 |
| 7. | 1 | 6 | 2 |
| 8. | 1 | 2 | 1.5 |
| 9. | 2.5 | 2 | 1 |
| 10. | 2.5 | 10 | 2 |
| 11. | 2.5 | 6 | 1.5 |
| 12. | 4 | 6 | 2 |
| 13. | 4 | 10 | 1.5 |
| 14. | 4 | 2 | 1.5 |
| 15. | 4 | 6 | 1 |
Materials and Methods
Glipizide was provided as a sample by USV Private Limited, located in the 2nd Stage of Peenya Industrial Estate, Bangalore, Karnataka. Microcrystalline cellulose was sourced from Maple Biotech Pvt. Ltd., Pune, Maharashtra, while saccharin was obtained from Ranbaxy, New Delhi. Talc and magnesium stearate were supplied by S.D. Fine Chem Ltd., Mumbai, Maharashtra. All other solvents and reagents for the formulation were procured from the market. These ingredients were carefully selected from reputable suppliers to ensure the final product's quality and effectiveness, maintaining high standards within the pharmaceutical industry. The attention to sourcing and quality control emphasizes the commitment to producing reliable and effective medications for end-users.
Table 4: Physical and chemical parameters of Glipizide.
| S. No. | Parameter | Predicted value |
| Molecular formula | C21H27N5O4S | |
| Molecular structure |
| |
| IUPAC name | N-(4-[N-(cyclohexyl carbamoyl) sulfonyl] phenethyl)-5-methylpyrazine-2-carboxamide | |
| Molecular weight | 445.536 g/mol | |
| BCS class | Class II | |
| pH and pKa | 5.9 and 6.0 | |
| Log P | 1.91 or 1.83 | |
| Crystallinity | Crystalline solid | |
| Melting point | 208-209 0C (406 to 408 °F) | |
| Solubility | Soluble in organic solvents like methanol, acetone |
Extraction
Plantago ovata seed mucilage, commonly referred to as Ispaghula, start by soaking the seeds in distilled water for 48 hours. This soaking period allows the seeds to take in water, causing them to swell and release their natural mucilage. After soaking, bring the seeds to a boil for several minutes. This step facilitates the extraction of mucilage from the seeds. Once the boiling is complete, the mucilage must be separated from the solid seed material. This can be achieved by pressing the mixture through muslin fabric, ensuring efficient extraction of the mucilage. To assist in the separation and concentration of the mucilage, add an equal volume of acetone to the filtrate. This addition helps precipitate the mucilage from the liquid, making it easier to collect. The extracted mucilage is then dried using a bowl dryer set to 40°C to eliminate any residual moisture and produce a stable end product. After the mucilage has been thoroughly dried, it is ground into a fine powder. This powdered mucilage is sifted through a #80 sieve to achieve a consistent texture. The final output is a fine powder that can be utilized for various medicinal and therapeutic applications, such as a dietary fiber supplement or a natural laxative [16-18].
Figure 3: Plantago ovata seeds and husk.
Table 5: Evaluation parameters of extracted material.
| S. No. | Evaluation | Result |
| 1. | Colour | Light buff |
| 2. | Odour | Faint |
| 3. | Taste | Neutral Taste |
| 4. | Appearance | Lightweight |
| 5. | Nature | Hydrophilic |
| 6. | Melting point | - |
| 7. | pH | 6.5-8.5 |
| 8. | Swelling Index | 10ml/g |
Angle of Repose: The maximum angle at which a build-up of loose powder remains stable without collapsing is known as the angle of repose. It acts as a gauge of the cohesiveness and flow properties of the powder. Better flow is indicated by lower angles, and worse flow is indicated by greater angles. A mound of powder is progressively built up on a level surface until it starts to slump in order to be measured.

Bulk Density: The mass of a powder divided by its whole volume, which includes the empty spaces between particles, is known as its bulk density. It is commonly given in quantities such as g/cm³ or kg/L and indicates how well the powder packs together. Using tools like a graduated cylinder, a known volume of the powder-including its vacant spaces-must be weighed in order to make a determination.

Tapped Density: The maximum bulk density attained when a powder is tapped or vibrated to remove interparticle gaps is known as "tapped density." The powder is tapped repeatedly in a container until no more volume loss takes place, indicating the powder's packing capabilities under compaction.

Hausner's Ratio: Hausner's ratio is the ratio of tapped density to bulk density, calculated using the formula:

It offers information about the packing efficiency and flow behavior of the powder. Good flowability is indicated by a ratio near 1, and poor flowability is indicated by values greater than 1.
Carr's Index: Carr's index, or compressibility index, quantifies the powder's compressibility and is calculated as follows:

It shows the extent of volume reduction brought about by compression or tapping. Superior flowability is indicated by lower Carr's index values, whereas reduced flow or enhanced cohesion are implied by higher values.
Partition Coefficient: Glipizide, a second-generation sulfonylurea utilized for the treatment of type 2 diabetes, is a lipophilic substance with limited solubility in water. Its partition coefficient (Log P) is between 2.4 and 2.6, suggesting that it dissolves better in lipid environments compared to aqueous solutions. characteristic facilitates its passage through lipid membranes, enhancing oral absorption. However, an excessive degree of lipophilicity could impede its dissolution and bioavailability. In laboratory settings, the partition coefficient of Glipizide is measured by equilibrating it in n-Octanol and water, subsequently calculating the ratio of concentrations in both phases.

Glipizide Identification Studies: Glipizide 10mg was accurately weighed and dissolved in 10ml methanol and subsequent dilution was made to get required concentrations (1000µg/ml). The wave length of maximum absorbance (λmax) of this clear solution was determined from 200-400nm methanol used as blank.
Preparation of Stock Solution: Glipizide 10 mg was dissolved in methanol 10 ml (1000 µg/ml) Stock solution I. From this solution diluted with methanol up to 100ml (100 µg/ml) Stock solution II was prepared.
Preparation of Sample Solution: Aliquots of 0.5, 1, 1.5, 2, and 2.5 ml were taken from Stock Solution II and diluted to a total volume of 10 ml, leading to concentrations of 5, 10, 15, 20, and 25 µg/ml, respectively. The absorbance for each diluted solution was recorded at 268 nm using a UV-visible spectrophotometer (Model 1800), comparing each measurement to the corresponding blank solution. Standard curves were created by plotting the known concentrations along the X-axis against the associated absorbance values on the Y-axis. These curves served to define the relationship between concentration and absorbance, facilitating the precise determination of unknown concentrations in later analyses.
Melting point of Glipizide: The melting point of Glipizide was established using an electric melting point device. A capillary tube, sealed at one end, was filled with the drug sample and positioned in the appropriate slot of the apparatus. The device was turned on, and the tube was monitored closely through the built-in magnifying glass for any indication of melting. The temperature at which the sample-initiated melting was carefully recorded from the thermometer. This initial temperature was noted as the melting point of the sample. To guarantee accuracy and reliability, the procedure was repeated three times, and the average of the three measurements was taken as the definitive melting point value. This technique yielded dependable and precise results for characterizing Glipizide [19-23].
Box-Behnken Experimental Design: The Box-Behnken Experimental Design, carried out using Design Expert® software (version 7.0, Stat-Ease Inc.), was utilized to create glipizide tablets aimed at quick medication release. The research explored the effects of three variables—Sodium starch glycolate, Guar Gum, and Plantago ovata seed mucilage each evaluated at three levels, from low to high. This detailed methodology enabled the discovery of the best combination of ingredients for producing fast-dissolving tablets with maximum effectiveness. Adhering to the structure of a three-factor and three-level design, a total of 15 experiments were performed, which included 3 central points to examine experimental variability and design accuracy. The main responses or dependent variables measured were average tablet hardness, in vitro dissolution time, and in vitro disintegration time, as detailed in Table 1. Selected Independent variables and Dependent variables: The selected independent variables were displayed in Table 6.
Table 6: Independent variables with levels.
| Factor | Name | Unit | Minimum | Maximum |
| A. | Cross-povidone | mg | 1 | 4 |
| B. | Plantago ovata seed mucilage | mg | 2 | 10 |
| C. | Guar gum | mg | 1 | 2 |
The selected Dependent variables were displayed in Table 7.
Table 7: Dependent variables.
| Response | Variables | Unit |
| 1. | Dissolution | (%) |
| 2. | Disintegration Time | Minute |
| 3. | Hardness | kg/cm2 |
Table 8: Composition of experimental batches of tablets.
| Std | Runs | Independent Variables | Dependent Variables | ||||
| Cross-povidone (mg) | Plantago ovata seed mucilage (mg) | Guar Gum (mg) | Dissolution (%) | Disintegration (Second) | Hardness (Kg/cm2) | ||
| 13 | 1 | 2.5 | 6 | 1.5 | 93.81 | 19.16 | 2.6 |
| 12 | 2 | 2.5 | 10 | 2 | 94.82 | 17.11 | 2.9 |
| 5 | 3 | 1 | 6 | 1 | 90.17 | 20.46 | 2.1 |
| 2 | 4 | 4 | 2 | 1.5 | 96.34 | 14.11 | 2.4 |
| 4 | 5 | 4 | 10 | 1.5 | 98.43 | 13.12 | 2.3 |
| 6 | 6 | 4 | 6 | 1 | 95.17 | 14.32 | 2.1 |
| 10 | 7 | 2.5 | 10 | 1 | 95.43 | 15.48 | 2.2 |
| 1 | 8 | 1 | 2 | 1.5 | 91.39 | 21.12 | 2.4 |
| 15 | 9 | 2.5 | 6 | 1.5 | 94.82 | 17.42 | 2.3 |
| 14 | 10 | 2.5 | 6 | 1.5 | 93.12 | 16.52 | 2.6 |
| 8 | 11 | 4 | 6 | 2 | 97.47 | 14.34 | 2.8 |
| 3 | 12 | 1 | 10 | 1.5 | 95.17 | 18.46 | 2.4 |
| 7 | 13 | 1 | 6 | 2 | 93.27 | 15.48 | 2.8 |
| 11 | 14 | 2.5 | 2 | 2 | 91.65 | 21.23 | 2.9 |
| 9 | 15 | 2.5 | 2 | 1 | 92.87 | 22.47 | 2.3 |
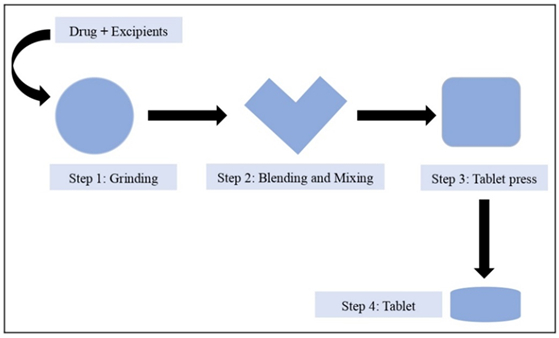
Formulation of Glipizide by using Direct Compression Method
- Premix Glipizide, talc, polyvinylpyrrolidone k30, sodium saccharin, and magnesium stearate until combined.
- Add the Isabgol husk and guar gum directly to the powder mixture before mixing to ensure it is well mixed and evenly distributed. Cross-povidone is added and mixing continues until a homogeneous dispersion is obtained.
- Add Microcrystalline Cellulose, remaining as required in total weight of tablet.
- Involves integrating the data directly into the tablet using the appropriate tablet tool.
- Quality control tests such as hardness, brittleness, disintegration and testing of tablets to ensure they meet the set standards. as well as appropriate documentation and storage to ensure durability and safety [24-26].
Figure 4: Formulation of Fast Dissolving Tablets by using Direct Compression Method.
Post-compression Studies
Organoleptic Characters: Each batch of tablets is evaluated for sensory properties, including appearance, color, odor and shape, through direct observation and analysis.
Thickness and Diameter: Measure tablet thickness and diameter length using vernier caliper. Five tablets were selected from each batch and their measurements averaged to give a representative value expressed in millimeters.
Hardness: Test the hardness of the tablets using the Monsanto hardness tester. Three pieces were selected from each map and the average hardness value was recorded. These values are expressed in kilograms per square centimeter (kg/cm2).
Friability: Friability of tablets was assessed using Roche friability tester as per the guidelines prescribed in Indian Pharmacopoeia (IP) standards. Initially, the weight of 20 pieces was recorded as the starting weight (Wi). Set the grinder to run at 25 revolutions per minute (rpm) for 4 minutes. Thereafter the tablets were reprocessed (Wf). Then, the friability percentage (%F) of each batch of tablets was calculated using the given formula:

It is worth noting that friability should not exceed 1.0% as per the standards set by the United States Pharmacopoeia (USP), Indian Pharmacopoeia (IP) and British Pharmacopoeia (BP).
Weight Variation: Twenty tablets were selected from each batch before weighing, individual weight was measured and averaged. The weight was measured as per the specifications mentioned in the Indian Pharmacopoeia (I.P.). According to the I.P standard the average difference in weight should not exceed 5% and two tablets and no tablet should exceed two times the percentage.

In-Vitro Disintegration Time: Tablet disintegration time was evaluated using a USP disintegration tester in water maintained at 37 ± 2 °C. As standard partial disintegration, six tablets from each batch were placed in one liter of distilled water. The time required to complete disintegration of all six tablets was recorded. Ideally, the duration of all tablets should not exceed 3 minutes. If more than one tablet failed to meet this criterion, the study was repeated using 12 tablets. It is expected that no more than 2 out of 18 tablets will differ from the unseparated standards.
In-Vitro Dissolution Studies: The release rate of Glipizide tablets was evaluated using a USP Type II dissolution apparatus in paddle configuration. Place one tablet in 6 dissolution vials, each containing 900 ml of separation medium, pre-equilibrated at 37 ± 0.5 °C and mixed at 50 rpm. At the appointed time, remove 2 ml aliquots of the dissolution medium and replace them with fresh dissolution medium. The sample was then filtered, diluted, and analyzed spectrophotometrically at 268 nm to determine the concentration of the drug relative to the corresponding concentration. This process is done on all batches. The average percentage of Glipizide released at different time points was determined from the sample image and plotted against time.
Calculation of % drug release involves the following steps:
Determined concentration of drug released by using formula:

Y is absorbance, m is slope, C is intercept, X is concentration (µg/ml)

Stability Studies: In this study, all formulations were evaluated for one-month stability in accordance with the rapid study criteria established by the International Committee for Harmonization (ICH). The sample was carefully wrapped in aluminum foil and stored in airtight glass. The tablets were stored at three different temperatures. Over a period of 10, 20 and 30 days, the tablets are removed from storage and analyzed for physical properties, separation properties and drug content [27-33].
Result and Discussion
Organoleptic properties: Organoleptic properties of the drug sample were found to be as given in table below.
Table 9: Organoleptic properties of Glipizide.
| S. No. | Organoleptic Characteristics | Result |
| 1. | Color | White to off-white crystalline compound |
| 2. | Nature | Crystalline powder |
| 3. | Odour | Odorless |
| 4. | Taste | Slightly bitter |
Melting Point of Glipizide: The final reading was obtained by averaging the results of three independent experiments, resulting in a temperature of 209°C.
Partition Coefficient: The partition coefficient of Glipizide is found to be 1.91.
pH Determination of Glipizide: The pH range glipizide is adjusted to 6.8 to 7.0 during preparation to ensure that it is stable and effective in treating diabetes.
Solubility Study of Glipizide: A solubility assessment was conducted to examine how Glipizide dissolves in methanol. During this experiment, different quantities of Glipizide were introduced into flasks that contained a consistent volume of methanol. The mixtures were continuously stirred for 24 hours to guarantee that the drug fully dissolved. After the stirring process, the solutions were filtered to eliminate any undissolved Glipizide particles, resulting in a clear solution. The concentration of the dissolved Glipizide was determined using a UV-Vis spectrophotometer, comparing absorbance values with a calibration curve that correlated absorbance to known concentrations. By considering the amount of Glipizide added, the volume of methanol used, and the absorbance data, the solubility was calculated in milligrams per milliliter (mg/mL). This method could also be modified to investigate how temperature and various solvents impact Glipizide's solubility, offering useful insights for formulation development.
Table 10: Solubility of glipizide in solvents.
| S. No. | Name of solvent | Solubility |
| 1. | Methanol | Freely soluble |
| 2. | Ethanol | Practically soluble |
| 3. | Acetone | Slightly soluble |
| 4. | Methylene chloride | Slightly soluble |
| 5. | Water | Practically insoluble |
Table 11: Flow properties of powder blend.
| S. No | Angle of Repose (θ) | Bulk Density (g/cm2) | Tapped Density (g/cm2) | Hausner’s Ratio | Carr’s Ratio (%) |
| 1. | 33.23 | 0.52 | 0.64 | 1.23 | 18.40 |
| 2. | 32.57 | 0.48 | 0.53 | 1.12 | 11.32 |
| 3. | 31.71 | 0.52 | 0.60 | 1.15 | 13.33 |
| 4. | 34.47 | 0.49 | 0.57 | 1.16 | 14.03 |
| 5. | 33.21 | 0.57 | 0.64 | 1.12 | 10.93 |
| 6. | 32.68 | 0.53 | 0.62 | 1.16 | 14.51 |
| 7. | 30.33 | 0.48 | 0.55 | 1.14 | 12.72 |
| 8. | 32.51 | 0.51 | 0.59 | 1.19 | 14.87 |
| 9. | 33.16 | 0.49 | 0.62 | 1.15 | 16.90 |
| 10. | 34.45 | 0.48 | 0.57 | 1.15 | 14.02 |
| 11. | 33.22 | 0.53 | 0.65 | 1.23 | 18.40 |
| 12. | 31.72 | 0.52 | 0.59 | 1.16 | 13.32 |
| 13. | 33.22 | 0.56 | 0.64 | 1.11 | 10.93 |
| 14. | 33.23 | 0.52 | 0.65 | 1.22 | 18.42 |
| 15. | 32.52 | 0.51 | 0.58 | 1.19 | 14.86 |
Post-compression Studies
Organoleptic Characters
Table 12: Organoleptic properties of Glipizide Tablets.
| S. No. | Organoleptic Characteristics | Result |
| 1. | Color | White to off-white |
| 2. | Shape | Circular |
| 3. | Odour | Odorless |
| 4. | Taste | Palatably sweet |
Identification of Glipizide by UV Spectrophotometry
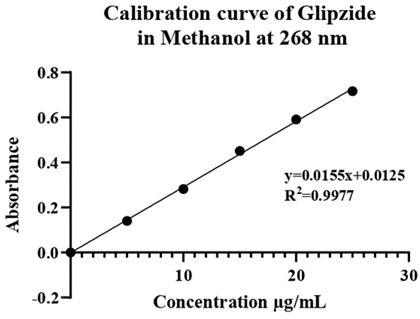
Derivation of drug spectrum: The methanol stock solution of Glipizide, with a concentration of 10µg/ml, exhibited a peak absorption wavelength (λmax) at 268 nm. The absorbance observed at this wavelength was measured to be 0.1404.
Preparation of calibration curve of Glipizide in water: The calibration curve of glipizide dissolved in methanol was plotted using a UV spectrophotometer and measured at a wavelength of 268 nm. This curve was created from 5 dilutions of stock solution, each at 5 µg/ml. The resulting curve is shown in Table 13.
Table 13: Calibration curve data of Glipizide in methanol.
| S. No. | Concentration (μg/ml) | Absorbance at 268 |
| 1 | 5 | 0.1404 |
| 2 | 10 | 0.2824 |
| 3 | 15 | 0.4513 |
| 4 | 20 | 0.5914 |
| 5 | 25 | 0.7170 |
Figure 5: Calibration curve of Glipizide.
Table 14: Post-compression parameters of tablets.
| Formulation code | Thickness (mm) | Hardness (Kg/cm2) | Friability (%) | Disintegration Time (Sec) |
| FDT 1 | 1.96±0.2 | 1.9 | 0.40 | 20.16 |
| FDT 2 | 2.02±0.2 | 1.8 | 0.42 | 21.48 |
| FDT 3 | 1.99±0.2 | 2.1 | 0.48 | 19.46 |
| FDT 4 | 2.01±0.2 | 1.9 | 0.43 | 20.12 |
| FDT 5 | 2.0±0.2 | 2.3 | 0.44 | 22.12 |
| FDT 6 | 1.98±0.2 | 1.8 | 0.47 | 20.12 |
| FDT 7 | 1.99±0.2 | 1.9 | 0.56 | 19.48 |
| FDT 8 | 2.02±0.2 | 2.0 | 0.58 | 20.12 |
| FDT 9 | 1.98±0.2 | 1.9 | 0.44 | 20.11 |
| FDT 10 | 1.99±0.2 | 1.8 | 0.43 | 18.42 |
| FDT 11 | 2.01±0.2 | 1.9 | 0.47 | 19.49 |
| FDT 12 | 2.00±0.2 | 2.1 | 0.45 | 19.40 |
| FDT 13 | 1.97±0.2 | 2.0 | 0.49 | 20.13 |
| FDT 14 | 1.99±0.2 | 1.8 | 0.48 | 22.16 |
| FDT 15 | 2.01±0.2 | 1.9 | 0.49 | 24.11 |
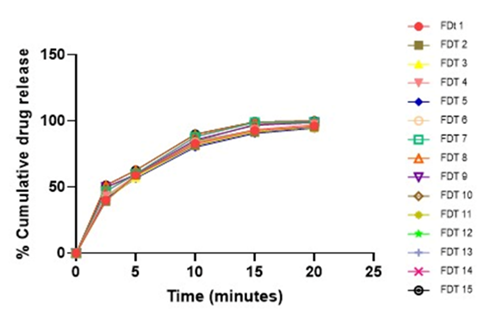
In-vitro Dissolution Studies
Table 15: Cumulative drug release of Glipizide Tablets formulations (F1-F15).
| Formulation code | Time (Minutes) | ||||
| 2.5 | 5 | 10 | 15 | 20 | |
| FDT 1 | 40.11 | 58.95 | 82.93 | 92.81 | 96.21 |
| FDT 2 | 39.37 | 59.19 | 81.26 | 91.26 | 95.37 |
| FDT 3 | 39.93 | 57.43 | 81.74 | 91.43 | 95.11 |
| FDT 4 | 43.54 | 58.68 | 83.37 | 93.51 | 97.47 |
| FDT 5 | 41.13 | 56.70 | 80.24 | 90.67 | 94.58 |
| FDT 6 | 42.39 | 58.41 | 83.98 | 93.23 | 94.69 |
| FDT 7 | 46.75 | 59.96 | 88.02 | 98.89 | 99.00 |
| FDT 8 | 51.35 | 62.70 | 89.74 | 99.28 | 99.89 |
| FDT 9 | 49.96 | 59.67 | 85.78 | 96.68 | 98.89 |
| FDT 10 | 49.76 | 58.78 | 84.91 | 97.41 | 98.79 |
| FDT 11 | 40.93 | 57.43 | 81.74 | 92.43 | 96.11 |
| FDT 12 | 42.37 | 58.41 | 83.91 | 93.25 | 94.61 |
| FDT 13 | 42.46 | 58.41 | 84.98 | 93.23 | 95.69 |
| FDT 14 | 46.73 | 59.96 | 88.01 | 98.89 | 98.99 |
| FDT 15 | 51.34 | 62.70 | 89.71 | 99.28 | 99.88 |
Figure 6: Cumulative drug release of Glipizide Tablets (F1-F15).
Response data for all 15 batches by using Box-Behnken Experimental Design (FI–F15).
Response 1: Dissolution (%)
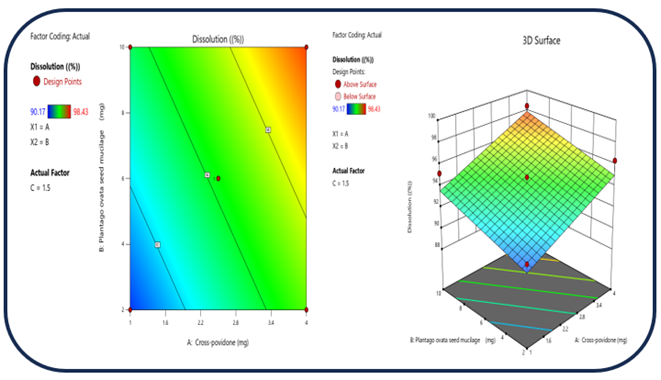
Figure 7: The 2D contour plot and the three-dimensional 3D map shows how the total amounts of super disintegrates (X1) and (X2) affects the dissolution.
ANOVA for Linear model
Response 1: Dissolution
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
| Model | 56.30 | 3 | 18.77 | 13.27 | 0.0006 | Significant |
| A- Cross-povidone | 37.89 | 1 | 37.89 | 26.78 | 0.0003 | |
| B-Plantago ovata seed mucilage | 16.82 | 1 | 16.82 | 11.89 | 0.0054 | |
| C-Guar gum | 1.59 | 1 | 1.59 | 1.13 | 0.3113 | |
| Residual | 15.56 | 11 | 1.41 | |||
| Lack of Fit | 14.10 | 9 | 1.57 | 2.14 | 0.3585 | Not Significant |
| Pure Error | 1.46 | 2 | 0.7310 | |||
| Cor Total | 71.86 | 14 |
Response 2: Disintegrating time (Second)
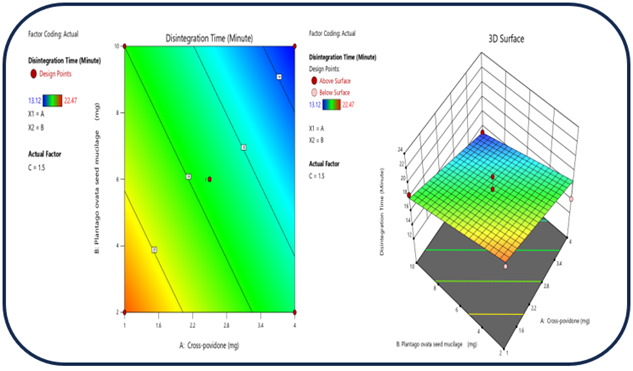
Figure 8: The 2D contour plot and the three-dimensional 3D map shows how the total amounts of super disintegrates (X1) and (X2) affect the disintegration time.
ANOVA for Linear model
Response 2: Disintegration Time
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
| Model | 78.01 | 3 | 26.00 | 6.22 | 0.0100 | Significant |
| A- Cross-povidone | 48.17 | 1 | 48.17 | 11.52 | 0.0060 | |
| B-Plantago ovata seed mucilage | 27.23 | 1 | 27.23 | 6.51 | 0.0269 | |
| C-Guar gum | 2.61 | 1 | 2.61 | 0.6242 | 0.4462 | |
| Residual | 46.01 | 11 | 4.18 | |||
| Lack of Fit | 42.41 | 9 | 4.71 | 2.62 | 0.3071 | Not Significant |
| Pure Error | 3.60 | 2 | 1.80 | |||
| Cor Total | 124.02 | 14 |
Response 3: Hardness (Kg/cm2)
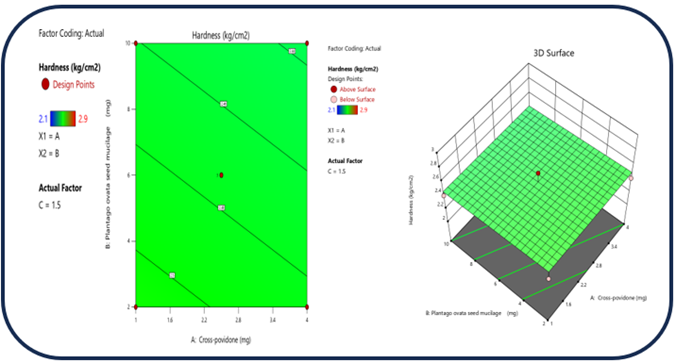
Figure 9: The 2D contour plot and the three-dimensional 3D map shows how the total amounts of super disintegrates (X1) and (X2) affects the Hardness.
ANOVA for Linear model
Response 3: Hardness
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
| Model | 0.9175 | 3 | 0.3058 | 22.16 | <0> | Significant |
| A- Cross-povidone | 0.0012 | 1 | 0.0012 | 0.0906 | 0.7691 | |
| B-Plantago ovata seed mucilage | 0.0050 | 1 | 0.0050 | 0.3622 | 0.5595 | |
| C-Guar gum | 0.9112 | 1 | 0.9112 | 66.02 | <0> | |
| Residual | 0.1518 | 11 | 0.0138 | |||
| Lack of Fit | 0.0918 | 9 | 0.0102 | 0.3401 | 0.8959 | Not Significant |
| Pure Error | 0.0600 | 2 | 0.0300 | |||
| Cor Total | 1.07 | 14 |
Table 16: pre compressional data of optimized batch (FDT 16).
| S. No. | Pre-compressional evaluation parameter | Results |
| 1. | Bulk density | 0.48 g/cm2 |
| 2. | Tapped density | 0.62 g/cm2 |
| 3. | Hausner’s ratio | 1.29 |
| 4. | Carss index | 22.58% |
| 5. | Angle of repose | ~30° to 35° |
Table 17: post compressional data of optimized batch (FDT 16).
| S. No. | Post-compression evaluation parameter | Results |
| 1. | Thickness | 2.98±0.2mm |
| 2. | Hardness | 2.357 kg/cm2 |
| 3. | Disintegration time | 13.8 seconds |
| 4. | Friability | 0.49 % |
| 5. | % Drug release | 97.981 % |
Table 18: In vitro drug release for optimized batch (MBT 16).
| Formulation batch | 2.5 min | 5 min | 10 min | 15 min | 20 min |
| FDT 1 | 42.11 | 59.95 | 76.93 | 89.81 | 97.21 |
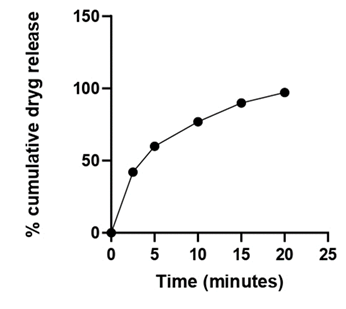
Figure 10: Percent drug release of batch ODT 16 (Optimized Batch).
The obtained Percent drug release of batch ODT 16 (optimized batch) has 97.21% of drug release in 20 minutes, and the optimized batch was evaluated for all the pre and post compressional parameters required for quality control of dosage form and the obtained results was found between satisfied range.
Stability Studies
The stability studies of fast-dissolving tablets of Glipizide at a 100 mg dose indicated that the best formulation remained stable even after storing at 40±20°C / 75±5% RH for 3 months. The tablets were visually examined for any physical changes, evaluated for drug content, and in vitro drug release at monthly intervals. The results showed that the formulation maintained its drug release profile within the specified limits, demonstrating stability over the study period.
Conclusion
This research effectively employed the Box-Behnken design alongside the direct compression technique to create fast-dissolving glipizide tablets. The study examined both natural super disintegrants, such as Plantago ovata and guar gum, and a synthetic option, crosspovidone, to find the best mix for quick disintegration and optimal drug release. Extensive pre-formulation tests confirmed that the components were compatible, ensuring their stability and effectiveness. The resulting formulation holds significant promise for improving patient adherence, especially for diabetic patients who struggle with swallowing standard tablets. By focusing on ease of use, this method aligns with principles of patient-centered care, providing a practical approach to enhancing treatment compliance. Nonetheless, additional research is suggested to assess the viability of large-scale production and to conduct in-vivo studies that confirm the formulation’s clinical effectiveness. This development represents a noteworthy step forward in pharmaceutical technology, addressing the unique needs of individuals with diabetes.
Declarations
Acknowledgement
I want to genuinely thank IPS Academy College of Pharmacy for offering the crucial resources, facilities, and assistance necessary for executing this study successfully. I am truly grateful for the continuous encouragement and guidance received during this journey. Furthermore, I extend my heartfelt appreciation to all my colleagues and peers for their meaningful discussions, helpful suggestions, and steadfast moral support, which were vital in bringing this work to fruition. Their collaboration and encouragement were key in navigating challenges and achieving the success of this project.
Competing Interest
The authors have declared that no competing interest exists.
References
- Husain, A., Joshi, S., Choudhary, A.N., Ajmal, M., Khan, M., et al. (2024). Computational Design and Toxicity Prediction of Oxazole Derivatives Targeting Pparγ as Potential Therapeutics for Diabetes Mellitus in Comparison to Rosiglitazone and Pioglitazone, Journal of The Chilean Chemical Society, 69(1):6056-6064.
Publisher | Google Scholor - Parasuraman, S., Thinagaran, J.N. (2023). Evaluation of the Antidiabetic Activity of Hesperidin on Streptozotocin-Induced Diabetes Mellitus in Swiss Albino Mice, Free Radicals and Antioxidants, 13(1):46-49.
Publisher | Google Scholor - Tiwary, N., Sharma, N., Singh, S., Behl, T., Zahoor, I. (2024). Understanding The Pharmacological and Nanotechnological Facets of Dipeptidyl Peptidase-4 Inhibitors in Type II Diabetes Mellitus: A Paradigm in Therapeutics, Bionanoscience, 14(1):211-229.
Publisher | Google Scholor - Ozkan, C.K., Esim, O., Savaser, A. And Ozkan, Y. (2021). An Overview of Excipients Classification and Their Use in Pharmaceuticals, Current Pharmaceutical Analysis, 17(3):360-374.
Publisher | Google Scholor - Lv, W., Wang, X., Xu, Q. And Lu, W. (2020). Mechanisms and Characteristics of Sulfonylureas and Glinides, Current Topics in Medicinal Chemistry, 20(1):37-56.
Publisher | Google Scholor - Li, Z., Mei, S., Dong, Y., She, F., Li, Y., et al. (2020). Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery-A Critical Review, Pharmaceutics, 12(6):522.
Publisher | Google Scholor - El-Dakroury, W.A., Zewail, M.B., Amin, M.M. (2023). Design, Optimization, and In-Vivo Performance of Glipizide-Loaded O-Carboxymethyl Chitosan Nanoparticles in Insulin-Resistant/Type 2 Diabetic Rat Model, Journal of Drug Delivery Science and Technology, 79:104040.
Publisher | Google Scholor - Ubhe, T.S., Gedam, P. (2020). A Brief Overview on Tablets and Its Types, Journal of Advancement in Pharmacology, 1(1):21-31.
Publisher | Google Scholor - Biswajit, B.A., Mankad, A., Dutta, A. (2022). Methylphenidate Fast Dissolving Films: Development, Optimization Using Simplex Centroid Design and In Vitro Characterization’, Turkish Journal of Pharmaceutical Sciences, 19(3):251.
Publisher | Google Scholor - Gupta, A., Patil, N., Yadav, A., Jain, D.K. (2022). Breaking the Mold: Understanding the Mechanism of Fast Dissolving Tablets, European Chemical Bulletin, 3447-3457.
Publisher | Google Scholor - Sharma, A.N., Upadhyay, P.K., Bajpai, M., Easwari, T.S., Bhadauria, P., et al. (2023). Formulation and Evaluation of Fast Dissolving Tablets of Nimesulide and Paracetamol, Materials Today: Proceedings, 294-298.
Publisher | Google Scholor - Roy, H. (2018). Box-Behnken Design for Optimization of Formulation Variables for Fast Dissolving Tablet of Urapidil, Asian Journal of Pharmaceutics, 12(3):946-954.
Publisher | Google Scholor - Roy, H., Nandi, S., Pavani, U., Lakshmi, U., Reddy, T.S., et al. (2020). Optimization and Quality by Design Approach for Piroxicam Fast Dissolving Tablet Formulations Using Box-Behnken Design, Current Drug Therapy, 15(2):152-165.
Publisher | Google Scholor - Singh, A., Chauhan, C.S. (2024). Formulation and Optimization of Valganciclovir-Loaded Nanosponges, International Journal of Drug Delivery Technology, 14(1):1-8.
Publisher | Google Scholor - Sharma, S., Chauhan, S. (2021). A Review: An Overview of Natural Super Disintegrants, Research Journal of Topical and Cosmetic Sciences, 12(1):13-24.
Publisher | Google Scholor - Dungarwal, U.N., Atish, S.M. (2021). Comprehensive Overview of Natural Super Disintegrants, International Research Journal of Pharmaceutical and Biosciences, 5-7.
Publisher | Google Scholor - Tyagia, L. (2024). A Comprehensive Review on Super Disintegrants: Accelerating Dissolution for Enhanced Drug Performance, International Journal of Medical Science, 34-49.
Publisher | Google Scholor - Sallam, A.A., Omari, D.M. (2024). Preformulation Considerations in Pharmaceutical Formulation Process, Dosage Forms, Formulation Developments and Regulations, 395-441.
Publisher | Google Scholor - Butola, M., Bhatt, V., Nainwal, N., Jakhmola, V., Dobhal, K., et al. (2023). Preparation and Evaluation of Polymer-Fused Metformin Hydrochloride Sustained Release Tablet, Indian Journal of Pharmaceutical Education and Research, 57(3):711-717.
Publisher | Google Scholor - Kakad, S.B., Rachh, P.R. (2022). Effect of Hydrophilic Polymer and Binder on Drug Release of Metformin Hcl Sustained Release Tablet, International Journal of Health Sciences, 3343-6635.
Publisher | Google Scholor - Tomar, A., Wadhwani, S., Bhadauria, R.S. (2019). A Preformulation Study of Pure Metformin Hcl, International Journal of Pharmaceutical Erudition, 8(4):1-9.
Publisher | Google Scholor - Tyagia, L. (2024). A Comprehensive Review on Superdisintegrants: Accelerating Dissolution for Enhanced Drug Performance, International Journal of Medical Science, 34-49.
Publisher | Google Scholor - Othman, A.M., Alburyhi, M.M., Al-Hadad, G.H. (2024). Formulation and Evaluation of Captopril Mouth Dissolving Tablets, European Journal of Pharmaceutical and Medical Research, 11(1):18-28.
Publisher | Google Scholor - Swati, S., Suma, R.L., Prasanna, S.N., Kusuma, A., Sri, S.N. (2020). Formulation and Evaluation of Glipizide Fast Dissolving Tablets, Journal of Pharmaceutical Sciences and Research, 12(5):609-618.
Publisher | Google Scholor - Aklima, A., Baral, P.K., Amin, M.T., Emon, T.I., Hossain, M.S. (2020). Formulation and Quality Optimization of Effervescent Tablet of Glipizide: An Approach to Comfort Anti-Diabetic Medication, Modern Health Science, 3(2):14-28.
Publisher | Google Scholor - Joshi, R., Garud, N., Akram, W. (2020). Fast Dissolving Tablets: A Review, International Journal of Pharmaceutical Science Research, 11(4):1562-1570.
Publisher | Google Scholor - Kusuma, A.N., Kumar, R.S. (2024). Optimization of Fast-Dissolving Tablets of Carvedilol Using 23 Factorial Designs, International Journal of Applied Pharmaceutics, 1:98-107.
Publisher | Google Scholor - Gupta, A., Yadav, A., Jain, D.K. (2024). Fast Dissolving Tablets for The Treatment of Acute and Chronic Diseases, International Journal of Pharmaceutical Drug Design, 1(2):1-8.
Publisher | Google Scholor - Turanli, Y., Birer, M., Birer, Y.T., Uyar, R., Dikmen, B.Y. et al. (2024). Oral Fast-Dissolving Risperidone-Loaded Electrospun Nanofiber Drug Delivery Systems for Antipsychotic Therapy, Journal of Drug Delivery Science and Technology, 92:1-9.
Publisher | Google Scholor - Alburyhi, M.M., Noman, M.A., Saif, A.A., Salim, Y.A., Abdullah, J.H. (2024). Formulation and Evaluation of Domperidone Orodispersible Tablets, World Journal of Pharmacy and Pharmaceutical Sciences, 13(3):49-68.
Publisher | Google Scholor - Chatterjee, A., Gupta, R. (2023). Formulation and Evaluation of Fast Dissolving Tablets of Piroxicam Using Various Concentration of Different Super Disintegrating Agents, International Journal of Health Advancement and Clinical Research, 1(4):90-97.
Publisher | Google Scholor - Akdag, Y., Gulsun, T., Izat, N., Cetin, M., Oner, L., et al. (2020). Evaluation of Preparation Methods for Orally Disintegrating Tablets, Medicine, 9(1):265-269.
Publisher | Google Scholor - Akdag, Y., Gulsun, T., Izat, N., Cetin, M., Oner, L., et al. (2020). Characterization and Comparison of Deferasirox Fast Disintegrating Tablets Prepared by Direct Compression and Lyophilization Methods, Journal of Drug Delivery Science and Technology, 57:101760-101772.
Publisher | Google Scholor