Research Article
Concurrent finding of Breast cancer and axillary Lymph nodes in Receptor Positive Patients
- Vinod Kumar Singhal *
- Mohammad Merajuddin
- Raafat Samuel Fares
- Adil Mohammed Suleman
- Nufra senopher Mohamed Sarfraz
- Hatem Moussa Faris Dawood Al Aswad
- Vidher V V Singhal
- Umm Heba Asif
Department of General Surgery, Prime Hospital, Dubai, United Arab Erimates.
*Corresponding Author: Vinod Kumar Singhal,Department of General Surgery, Prime Hospital, Dubai, United Arab Erimates
Citation: Vinod K. Singhal, M. Merajuddin, Raafat S. Fares, Adil M. Suleman, Nufra S. M Sarfraz et al. (2023). Concurrent finding of Breast cancer and axillary Lymph nodes in Receptor Positive Patients. Journal of Surgical Case Reports and Reviews. BioRes Scientia Publishers. 2(2):1-6. DOI: 10.59657/2993-1126.brs.23.017
Copyright: © 2023 Vinod Kumar Singhal, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 13, 2023 | Accepted: October 28, 2023 | Published: October 30, 2023
Abstract
Background and Objectives: The most frequent site of disease involvement in breast cancer that has progressed from the initial lesion is the axillary lymph nodes (ALN). There are still no reliable ways to anticipate ALN status prior to surgery, despite sentinel node biopsy being a reliable means of managing ALN.
Materials and Methods: This was a retrospective study of female patients with invasive breast cancer at Tertiary care hospital between January 2020 and January 2023. The clinical data and tumor characteristics for all invasive breast cancers (425) had been collected in breast cancer database. This study was approved by the institutional review board and ethics committee. Breast cancer patients who had undergone surgery were identified. The exclusion criteria were: 1) ductal carcinoma in situ; 2) neoadjuvant therapy; 3) bilateral invasive breast cancer; and 4) patients without breast-conserving surgery with ALND or sentinel lymph node sampling or modified radical mastectomy. SVM was used for analysis.
Results: Of these, 242 patients had positive ALNM. The mean tumor size was 24.4 mm (SD=1.5); 38% of the patients had T1 tumors (<20 mm), followed by 52% with T2 tumors and 10% with T3 tumors. The histology grade was predominantly grade II (57%), with 28% grade III and 15% grade I tumors. Lymph vascular invasion was found in 200 patients (47%). The ER/PR/HER2 profile showed 270 patients (63.5%) had luminal A type tumors, followed by 16.5% with triple-negative tumors, 13.1% with luminal B type tumors, and 6.9% with HER2-positive tumors. A higher AUC value indicated a better diagnostic performance. The AUC value of this study was 0.754 by SVM.
Conclusion: It is possible to forecast ALN status using a model that is just dependent on clinically common pathologic data derived from the source tumor. This could support the surgeon's decision-making for ALN management and breast cancer counseling.
Keywords: axillary lymph node; metastasis; breast carcinoma; support vector machine
Introduction
The most frequent site of disease involvement in breast cancer that has progressed from the initial lesion is the axillary lymph nodes (ALN). ALN status is a significant prognostic factor in invasive breast cancer in women [1,2]. Complete ALN dissection (ALND) was a common surgical technique in the early 20th century for tumor staging and treatment. However, ALND was also associated with numerous distressing clinical issues; well-documented morbidities include lymphedema, paranesthesia’s, and significant nerve and vascular damage. These issues prompted medical professionals to look for alternative methods for determining ALN status [3,4]. The primary location of ALN metastasis (ALNM) from breast cancer is the sentinel lymph node. Sentinel lymph node biopsy (SLNB) was advantageous to patients in terms of surgical morbidities and precise tumor staging. In the assessment of axillary status in early invasive breast cancer, SLNB is now a valid and accepted technique. To accurately forecast ALN status prior to surgery, there are currently no reliable approaches [5]. Since ALND is the major cause of morbidity in breast cancer surgery, there may be a technique to inform the surgeon in certain patient subgroups using prediction models for lymph node involvement.
Materials And Methods
This was a retrospective study of female patients with invasive breast cancer at Tertiary care hospital between January 2020 and January 2023. The clinical data and tumor characteristics for all invasive breast cancers had been collected in breast cancer database. This study was approved by the institutional review board and ethics committee. Breast cancer patients who had undergone surgery were identified. The exclusion criteria were: 1) ductal carcinoma in situ; 2) neoadjuvant therapy; 3) bilateral invasive breast cancer; and 4) patients without breast-conserving surgery with ALND or sentinel lymph node sampling or modified radical mastectomy.
Methodology
Age at diagnosis, tumor size, lymph node positivity, histological grade, estrogenic receptor (ER) status, progesterone receptor (PR) status, and human lymph vascular invasion (LVI) and the state of the epidermal growth factor receptor 2 (HER2). To find patients who may have been at risk for ALN metastases prior to surgery, all data was retrospectively evaluated. total of 425 invasive breast cancer patients were analyzed using tumor biological parameters that included age, tumor size, grade, estrogenic receptor, progesterone receptor, lymph vascular invasion, and HER2, to test their ability to predict ALN involvement.
Statistical Analysis
A statistical learning theory called the support vector machine (SVM) was created to divide data points into two categories. SVMs are effective statistical techniques for classifying data. The SVM algorithm was used in the study to categorize the axillary status of breast cancer. The receiver operating efficiency served as the performance indicator. Analysis of characteristic curves (ROC) was utilized to gauge the effectiveness of the suggested system. P-value <0>
Results
Table 1: Demographic and Tumor features of Breast cancer patients Axillary Lymph node metastasis
| Variables | Negative | Positive | Total |
| Age, mean (SD) | 49.12 (11.06) | 50.43 (11.38) | 50 (11.30) |
| Clinical factors | |||
| Tumor size,mean (SD) | 2.08 (1.164) | 2.91 (1.659) | 24.44 (1.461) |
| <2cm> | 40 (47.98) | 121 (44.27) | 200 (45) |
| 2–5 cm | 60 (47.98) | 120 (35.75) | 180 (40) |
| 5 cm | 16 (15.04) | 29 (10.98) | 45 (15) |
| Grade | |||
| 1 | 143 (19.27) | 72 (12.35) | 215 (16.23) |
| 2 | 15 (55.93) | 120 (54.89) | 135 (55.47) |
| 3 | 18 (24.80) | 57 (32.76) | 75 (28.30) |
| Pathological factors | |||
| Estrogen receptor | |||
| Negative | 56 (34.50) | 77 (30.36) | 133 (32.68) |
| Positive | 186 (65.50) | 106 (69.64) | 292 (67.32) |
| Progesterone receptor | |||
| Negative | 91 (39.22) | 183 (31.39) | 274 (35.77) |
| Positive | 51 (60.78) | 100 (68.61) | 151 (64.23) |
| HER2 | |||
| Negative | 61 (83.02) | 104 (76.16) | 165 (80.00) |
| Positive | 126 (16.98) | 139 (23.84) | 265 (20.00) |
| ER/PR/Her2 profile | |||
| Triple negative | |||
| No | 98 (80.59) | 114 (88.16) | 212 |
| Yes | 130 (19.41) | 83 (11.84) | 213 |
| HER2-positive | |||
| No | 112 | 222 | 334 |
| Yes | 50 | 41 | 91 |
| Luminal A | |||
| No | 170 | 208 | 378 |
| Yes | 42 | 5 | 47 |
| Luminal B | |||
| No | 66 | 49 | 115 |
| Yes | 212 | 98 | 310 |
| Lymph vascular invasion (LVI) | |||
| No | 155 (60) | 70 (40) | 225 (53) |
| Yes | 110 (55) | 90 (45) | 200 (47) |
A total of 425 patients with a mean age of 50 years (SD=11.3) were included in this study. Of these, 242 patients had positive ALNM. The mean tumor size was 24.4 mm (SD=1.5); 38% of the patients had T1 tumors (<20 mm), followed by 52% with T2 tumors and 10% with T3 tumors. The histology grade was predominantly grade II (57%), with 28% grade III and 15% grade I tumors. Lymph vascular invasion was found in 200 patients (47%). The ER/PR/HER2 profile showed 270 patients (63.5%) had luminal A type tumors, followed by 16.5% with triple-negative tumors, 13.1% with luminal B type tumors, and 6.9% with HER2-positive tumors.
Table 2: Metastasis analysis of Axillary Lymph node based on SVM
| SVM | Axillary Lymph node Metastasis | p-value | |
| Positive | Negative | ||
| Positive | 142 | 73 | 0.01* |
| Negative | 100 | 110 | |
| 242 | 183 | ||
Table 2 presents the observed results of SVM classification. Discordant results were noted between SVM and pathology reports in cases; 100 out of 242 patients were positive for ALNM but were classified as negative, and 73 out of 183 negative ALNM patients were classified as positive for ALNM by SVM and these results were statistically significant (p<0>
Table 3: Correlation between estrogenic receptor and progesterone receptor status of metastatic lymph node with estrogenic receptor and progesterone receptor status of primary tumor.
| Tumor mass | ALN | p-value | ||
| ER+ | PR+ | HER- | ||
| ER+ and PR + | 1.2 | 1.67 | 1.54 | 0.01* |
| ER- and PR- | 1.1 | 1.67 | 1.12 | 0.01* |
| HER-2 + | 1.2 | 1.67 | 1.54 | 0.01* |
| HER 2- | 0.11 | 0.21 | 0.84 | 0.01* |
As per table 3 it was seen a positive correlation between all the receptors for breast carcinoma with metastatic axillary lymph node (ALN) and it was significant (p>0.05).
Table 4: Diagnostic criteria of ALN metastasis
| Criteria | Percentage | 95% CI |
| Sensitivity | 75.80 | 0.73-0.80 |
| Specificity | 74.26 | 0.70-0.75 |
| Positive predictive value | 69.88 | 0.68-0.72 |
| Negative predictive value | 80.36 | 0.78-0.82 |
| Accuracy | 75.44 | 0.72-0.76 |
As per table 4 the sensitivity and specificity were 76% and 74%, respectively. The positive predictive value was 69% and negative predictive value was 80%. The accuracy rate was 75%.
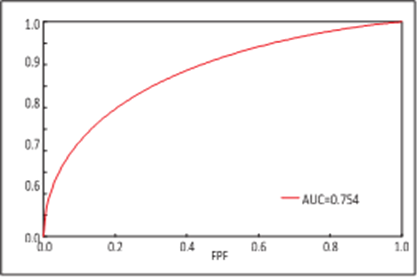
Figure 1: Diagnostic criteria based on ROC curve
As per figure 1 ROC curve were used to analyze the diagnostic performance by clinical pathology features. The cut off value to balance sensitivity and specificity was 0.000325. If the output of SVM was smaller than 0.000325, then it was defined as negative for ALNM, and values greater than or equal to 0.000325 were classified as positive axillary nodal status. A higher AUC value indicated a better diagnostic performance. The AUC value of this study was 0.754 by SVM.
Discussion
Complete ALN is a crucial step in the staging of cancer and local disease management. However, ALN has certain negative side effects, such as numbness, oedema, and significant nerve and vascular damage [6,7]. Today, axillary nodal status is evaluated in large part by SLNB. The efficiency and low incidence of postoperative complications of SLNB are its main advantages. Small tumor patients may benefit from SLNB. In fact, SLNB is frequently done on individuals who have clinically negative axillary nodes. In determining the extent of axillary nodal involvement, primary pathologic characteristics and clinical symptoms are helpful [8,9,10]. Clarifying the connection between ALNM and pathologic traits was our main goal. The SVM has been discovered to be a beneficial diagnostic tool when working with binominal data based on our prior research [10]. In this work, we developed a potent statistical technique using pathologic characteristics, such as tumor size, LVI and histological grade, as well as ER, PR, and HER2 status. The ROC curve was used to examine the SVM output in our investigation, and the diagnostic performance (AUC value=0.754) was satisfactory. The link between ALNM and pathologic traits is still up for dispute. Tumor size, LVI, and histological grade were significant predictors of ALNM. LVI is characterized by the presence of cancer cells that have invaded lymphatic or blood vessels. Aggressive tumor behavior and the capacity for metastasis are associated with positive LVI [11]. Numerous investigations have consistently demonstrated that LVI is a reliable indicator of ALNM. In cases of severe axillary nodal involvement, the odds ratio (LVI presence vs. negative) is considerable. The incidence of ALNM is minimal in small invasive breast cancers with negative LVI. The incidence of non-sentinel lymph node metastases and single tumor cells in the sentinel lymph node is similarly increased by the LVI [11,12]. The presence of LVI as the most important predictor is well accepted. Our study also confirms LVI is the strongest single predictor in ALNM. Additionally significant ALNM indicators include tumor size and histology grade. If a patient has a big tumor and a high histology grade, the likelihood of ALNM is considerable. Our data show that patients with big tumors (tumors that are more than 5 cm in size) and high histological grades are more likely to have ALNM, which is in line with findings from earlier research [13]. SLNB may be advantageous for patients with minor invasive breast cancer [14]. According to the results of our study, patients who have tumors that are less than 2 cm in size and have low- to medium-grade histology are appropriate candidates for SLNB. The condition of the local lymph nodes is essential for tumor staging and surgical planning. Although earlier research identified a few crucial variables in predicting ALNM, the predictive power was insufficient. The use of SLNB in early breast cancer is now widespread thanks to advancements in surgical technique, and it is spreading to patients who are clinically ALN-negative. Less surgical complications from SLNB than from complete ALND, as well as effectiveness and accuracy after a long-term follow-up, are advantages [15,16]. It is obvious how important SLNB is. As per Singh S et al the ER‑PR status of tumor mass and lymph node was compared. Of 60 cases, 35% of the cases were positive for both ER and PR in both lymph node and tumor mass. On the other hand, 60% of the cases showed a similar status of receptor negativity in both the tumor mass and lymph nodes. Hence, the concordance level between the tumor mass and the lymph node was 98.33% for ER and 96.66% for PR. When statistical analysis was done using the Chi‑square test, it was found that the P value was equal to 0.0001 which was significant statistically. The results are similar to our study [17]. In this investigation, it was discovered that the clinical pathologic characteristics were helpful in predicting the prognosis of cancer. We thus provide an additional method, not a replacement for SLNB, for assessing axillary status prior to surgery.
Conclusion
It is possible to forecast ALN status using a model that is just dependent on clinically common pathologic data derived from the source tumor. This could support the surgeon's decision-making for ALN management and breast cancer counselling.
Conflict of Interest
None declared
References
- American Joint Committee on Cancer. (2010). AJCC Cancer Staging Manual. 7thed. Springer, New York, NY.
Publisher | Google Scholor - Kwang In K, Keechul J, Se Hyun P, Hang Joon K. (2012). Support vector machines for texture classification. Pattern Analysis and Machine Intelligence. IEEE Transactions on, 24:1542-1550.
Publisher | Google Scholor - Qing S, Wenjie H, Wenfang X. (2012). Robust support vector machine with bullet hole image classification. Systems, Man, and Cybernetics. Part C: Applications and Reviews. IEEE Transactions on, 32:440-48.
Publisher | Google Scholor - Silberman AW, McVay C, Cohen JS. (2014). Comparative morbidity of axillary lymph node dissection and the sentinel lymph node technique: implications for patients with breast cancer, Ann Surg. 240:1-6.
Publisher | Google Scholor - Arana S, Vasquez-Del-Aguila J, Espinosa M. Lymphatic mapping could not be impaired in the presence of breast carcinoma and coexisting small lymphocytic lymphoma, Am J Case Rep. 14:322-325.
Publisher | Google Scholor - Bhosale SJ, Kshirsagar AY, Sulhyan SR, Jagtap SV. N. (2013). Matrix-producing metaplastic breast carcinoma - a rare malignancy. Am J Case Rep,14:213-215.
Publisher | Google Scholor - Testori A, Meroni S, Moscovici OC. (2018). Surgical sentinel lymph node biopsy in early breast cancer. Could it be avoided by performing a preoperative staging procedure? A pilot studies. Med Sci Monit,18(9):543-549.
Publisher | Google Scholor - Chen ST, Hsiao YH, Huang YL. (2019). Comparative analysis of logistic regression, support vector machine and artificial neural network for the differential diagnosis of benign and malignant solid breast tumors by the use of three-dimensional power Doppler imaging. Korean J Radiol, 10:464-471.
Publisher | Google Scholor - Huang YL, Chen DR, Jiang YR. (2018). Computer-aided diagnosis using morphological features for classifying breast lesions on ultrasound. Ultrasound Obstet Gynecol, 32:565-572.
Publisher | Google Scholor - Nos C, Harding-MacKean C, Freneaux P. (2013). prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg, 90:1354-1360.
Publisher | Google Scholor - Hwang RF, Krishnamurthy S, Hunt KK. (2013). Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol, 10:248-254.
Publisher | Google Scholor - Mittendorf EA, Sahin AA, Tucker SL. (2018). Lymphovascular invasion and lobular histology are associated with increased incidence of isolated tumor cells in sentinel lymph nodes from early-stage breast cancer patients. Ann Surg Oncol, 15:3369-3377.
Publisher | Google Scholor - Rivadeneira DE, Simmons RM, Christos PJ. (2014). Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg, 191:1-6.
Publisher | Google Scholor - Nixon AJ, Schnitt SJ, Gelman R. (2016). Relationship of tumor grade to other pathologic features and to treatment outcome of patients with early-stage breast carcinoma treated with breast-conserving therapy. Cancer, 78:1426-1431.
Publisher | Google Scholor - Harden SP, Neal AJ, Al-Nasiri N. (2011). Predicting axillary lymph node metastases in patients with T1 infiltrating ductal carcinoma of the breast. Breast, 10:155-159.
Publisher | Google Scholor - Coombs N, Chen W, Taylor R, Boyages J. (2017). A decision tool for predicting sentinel node accuracy from breast tumor size and grade. Breast J, 13:593-598.
Publisher | Google Scholor - Singh S, Shukla S, Singh A, Acharya S, Kadu RP, Bhake A. (2020). Comparison of estrogen and progesterone receptor status in tumor mass and axillary lymph node metastasis in patients with carcinoma breast. Int J App Basic Med Res, 10:117-121.
Publisher | Google Scholor