Research Article
Comparison of Diagnostic Accuracy of Different Sites for Transcutaneous Bilirubin Measurement in Neonates
1Pediatrics department, Faculty of Medicine, Menoufia University, Egypt.
2Medical Biochemistry & Molecular Biology, Faculty of Medicine, Menoufia University, Egypt.
3Menouf General Hospital, Menoufia, Egypt.
*Corresponding Author: Nagwan Y. Saleh, Pediatrics department, Faculty of Medicine, Menoufia University, Egypt.
Citation: Saleh NY, Ibrahim M.M., Nada A.R.M., El-Banna A. A. (2024). Comparison of Diagnostic Accuracy of Different Sites for Transcutaneous Bilirubin Measurement in Neonates. Journal of Clinical Paediatrics and Child Health Care, BioRes Scientia Publishers. 1(1):1-9. DOI: 10.59657/2997-6111.24.005
Copyright: © 2024 Nagwan Y. Saleh, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 23, 2024 | Accepted: March 08, 2024 | Published: March 25, 2024
Abstract
Background: Jaundice is one of the most prevalent diseases needing medical care in newborns. Hyperbilirubinemia is a benign illness, but chronic exposure to high serum bilirubin levels can cause kernicterus in infants.
Objectives: To determine and compare the diagnostic accuracy of transcutaneous bilirubin (TcB) measured at various body sites in neonates (full term and preterm).
Patients and Methods: This was a prospective observational trial performed on 131 neonates, who were admitted into the neonatal intensive care unit (NICU) of Menoufia University Hospital and Al Helal Insurance Hospital. This study conducted during the period time from March 2022 till January 2023.
Results: No significant variation regarding mean different of TSB - TcB Forehead (P>0.05) was reported. While, there were significant difference regarding TSB - TCB Sternum and TSB - TcB Interscapular with mean change (0.98±2.39, 1.28±2.62), (P<0.001) respectively. Also, there was a significant positive correlation between TSB with TcB forehead, TcB sternum and TcB interscapular (P<0.001), also between TcB forehead with TcB sternum and TcB interscapular (P<0.001), and between TcB sternum with TcB interscapular (P<0.001).
Conclusions: TcB using forehead TcBF, sternum TcBS, and interscapular TcBI sites is valid as a tool for screening preterm neonates for neonatal jaundice, as it showed a strong and significant correlation and predictive accuracy between TSB and levels of TcBS, TcBF, and TcBI in preterm newborns. The forehead site had the lowest false negative rate and the highest sensitivity.
Keywords: bilirubin; interscapular; jaundice; sternum
Introduction
Preterm newborns in the Neonatal Intensive Care Unit (NICU) frequently have hyperbilirubinemia. Within the first week of life, about 60% of full-term infants and 80% of preterm infants develop jaundice [1]. Up to 10% of newborns are thought to have severe neonatal hyperbilirubinemia. Usually, hyperbilirubinemia is a benign illness, but chronic exposure to high serum bilirubin levels can cause kernicterus in infants, irreversibly damaging the hippocampus, globus pallidus, subthalamic nuclei, and oculomotor nucleus, among other elements of the central nervous system [2]. Although jaundice is easily diagnosed, it needs immediate attention. It causes multiple complications if not treated properly [3]. Preterm newborns have higher risk for bilirubin encephalopathy and kernicterus than full-term neonates, due to their heightened neonatal red cell, gastrointestinal, and hepatic immaturity [4]. All susceptible newborns with jaundice should have their serum bilirubin levels evaluated in order to avoid these issues and to begin appropriate management, such as phototherapy or blood transfusion, as soon as possible [5]. Total serum bilirubin (TSB) estimation is the gold standard for diagnosing hyperbilirubinemia; however, it is invasive, necessitates blood collection, is inconvenient due to vein puncture technical issues, discomfort, pain, delay in results, and causes anxiety for parents. So, it's crucial to lessen the number of blood draws and the volume of blood the infant loses as a result of blood draws [6].
The initial purpose of transcutaneous bilirubinometry was to potentially replace invasive blood sampling. When used as an adjunct to TSB monitoring, the use of a TcB metre in preterm newborns can be a trustworthy noninvasive screening technique for hyperbilirubinemia and may help to lessen unpleasant stimuli and iatrogenic blood loss [7]. The sternum area, the forehead, and the interscapular area are typically the areas for TcB measurements. It has been suggested that the interscapular area is a better location for TcB measurement than the sternum area. However, it is still unclear which measuring site is the most accurate [5]. Studies have shown that TcB measurement is reliable. However, the precision of TcB measurement is debatable and varies depending on a number of variables, such as the preterm birth atatus, race, and the site of the TcB measurement. [8]. our objective was to determine and compare the diagnostic accuracy of TcB measured at various body sites in neonates (full term and pre term).
Patients And Methods
This prospective observational trial was performed on 131 neonates, who were admitted into the NICU of Menoufia University Hospital and Al Helal Insurance Hospital from March 2022 till January 2023. All cases were categorized into three groups: 1st group: samples for the measurements of transcutaneous bilirubin (TcB) were obtained from forehead, 2nd group: samples for the measurements of TcB were obtained from sternum, 3rd group: samples for the measurements of TcB were obtained from inter-scapular region. Ethics approval and consent to participate: This research protocol was approved prior to conduction by the research ethics committee, Faculty of Medicine, Menoufia University. The parents provided a written informed consent and approval number was 2/2022 PEDI 28. Inclusion criteria: All newborns with severe clinical jaundice who require TSB measurement. Exclusion criteria: Newborns who have poor perfusion (capillary refill time > 3 s) and those who already started receiving phototherapy. Neonates with a condition that could interfere with TcB measurements, such as congenital malformation, hydrops fetalis, infection, diffuse cutaneous conditions, or purpura.
All cases underwent the following: Medical history: Gestational age: Last menstrual period (LMP), if known, prenatal ultrasonography, or Ballard technique were used to establish the gestational age of the newborns. Perinatal history such as (mode of delivery, maternal illness, gestational age, birth weight, Apgar score, sex, history of convulsions or cyanosis). Family history of neonatal jaundice. Postnatal history. Age at which jaundice firstly appear. Clinical examination: Both general and systemic examinations were performed with specific attention on anthropometric measures, vital signs, neurological examination and presence of Cephal-hematoma). Laboratory investigations: Complete blood count (CBC) and Reticulocyte count 2ml of blood on EDTA tube by Advia 2120 apparatus which use laser light scatter technology for determination of blood count and platelet count. Liver function tests: the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using AU480 Beckman apparatus was measured. C-reactive protein (CRP): level of CRP serum was assessed using Mispa-i2 by slide latex agglutination test (Rapitex CRP kit). Coombs’s test. Serum bilirubin: Using aseptic procedure, 0.5 ml of blood was withdrawn from a peripheral vein and submitted to the laboratory without delay. The diazo technique was used to determine the total serum bilirubin level.
Statistical analysis: Statistical Package for the Social Sciences (SPSS) V23 was used for data analysis (Armonk, NY: IBM Corp.) There were two sections of statistics: Descriptive statistics: the presentation of quantitative data as median and range, Analytical statistics: Chi-square test (2), Student t test (t, Mann Whitney test (U), paired t test. P values of ≤0.05 deemed of significant level.
Results
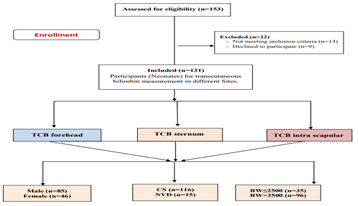
A flowchart of the study cases is shown in Figure 1. Of the 153 participants (Neonates) for TcB measurement in different Sites at Menoufia University Hospital and Al Helal Insurance Hospital. 22 patients were excluded from the research (9 cases declined consent and 13 cases did not meet the inclusion criteria, 131 cases participated in the research and were classified into two groups with male (n=85) and female (n=46), (Figure 1).
Figure 1: Flowchart of patients for transcutaneous bilirubin measurement in different Sites.
Table 1: Demographic characteristics of study participants (N=131).
| Variables | The studied patients (n=131) | |
| Mean ±SD | Range | |
| Gestational age/weeks | 37.44±0.98 | 35-39 |
| Birth weight/Kg | 2.95±2.07 | 1.7-26 |
| Sex | No. | % |
| Male | 85 | 64.9 |
| Female | 46 | 35.1 |
| Mode of delivery | ||
| NVD | 15 | 11.5 |
| CS | 116 | 88.55 |
| Age of jaundice 1st appear/days | ||
| 1day | 11 | 8.4 |
| 2days | 44 | 33.6 |
| 3 days | 50 | 38.2 |
| 4 days | 16 | 12.2 |
| 5 days | 5 | 3.8 |
| 7 days | 2 | 1.5 |
| 8 days | 2 | 1.5 |
| 9 days | 1 | 0.8 |
CS: Cesarean section, NVD: Normal vaginal delivery
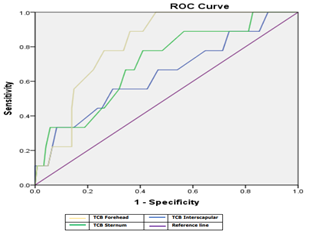
Figure 2: ROC curve of different bilirubin measurements among all cases groups.
Our study showed that, the mean gestational age was 37.44±0.98 and birth weight was 2.95±2.07. Moreover, the most common sex was male (64.9%) than female (35.1%). The most common mode of delivery was CS (88.55%) than NVD (11.5%). Regarding the age of jaundice 1st appear, the most common was 3 days (38.2%) then 2 days (33.6%) followed by 4 days (12.2%), 1day (8.4%), 2 days (3.8%), (7, 8) days (1.5%) and 9 days (10.8%), (Table 2).
Table 2: Laboratory investigation among the studied cases (N=131).
| Variables | The studied Cases (n=131) | |
| Mean ±SD | Range | |
| HB | 15.05±2.40 | 8.7-21.3 |
| HCT | 44.52±8.19 | 0-60.1 |
| PLT | 317.88±82.40 | 124-492 |
| TLC | 11.13±6.99 | 4.5-76 |
| MCV | 99.16±5.16 | 90-110.3 |
| MCH | 35.20±2.05 | 30.8-38.9 |
| Lymph | 40.38±17.39 | 3.2-67 |
| Neutrophil | 46.75±11.94 | 6.6-69.8 |
| SGOT | 24.96±3.70 | 16-32.9 |
| SGPT | 21.67±2.99 | 17-28 |
| CRP/value | 3.31±1.92 | 0.7-12.7 |
| TSB | 16.75±3.10 | 6.1-27.7 |
| DSB | 1.06±0.37 | 0.3-2 |
| TCB Forehead | 17.02±2.95 | 6.5-28 |
| TCB STERNUM | 15.77±2.94 | 5.3-26.2 |
| TCB interscapular | 15.47±2.95 | 5-25.7 |
| APGAR score | 7.87±0.56 | 2.7-9 |
| Retics | 1.45±3.57 | 0.2-29.5 |
| CRP | No. | % |
| Negative | 117 | 89.3 |
| Positive | 14 | 10.7 |
| Coombs’s test | ||
| Negative | 118 | 90.1 |
| Positive | 13 | 9.9 |
Hb: Hemoglobin HCT: Hematocrit PLT: platelet MCV: Mean corpuscular volume MCH: mean corpuscular hemoglobin CRP: C-reactive protein, SGOT: serum glutamic-oxaloacetic transaminase SGPT: Serum Glutamic Pyruvic Transaminase CRP: C-reactive protein TSB: Total serum bilirubin DSB: Direct serum bilirubin TCB: Transcutaneous bilirubinometer
Moreover, the mean HB was 15.05±2.40, HCT was 44.52±8.19, PLT was 317.88±82.40, TLC was 11.13±6.99, MCV was 99.16±5.16, MCH was 35.20±2.05, Lymph was 40.38±17.39, Neutrophil was 46.75±11.94 and APGAR score was 7.87±0.56. (10.7%) patients were positive CRP and 90.1% were negative Coombs’s test. Also, the mean SGOT was 24.96±3.70, SGPT was 21.67±2.99, CRP/value was 3.31±1.92, TSB was 16.75±3.10, DSB was 1.06±0.37, TCB forehead was 17.02±2.95, TCB sternum was 15.77±2.94 and TCB interscapular was 15.47±2.95, (Table 3).
Table 3: ABO and Rh among the studied cases (N=131).
| Variables | The studied Cases (n=131) | |
| ABO | ||
| Blood group of mothers | No. | % |
| A | 37 | % |
| B | 26 | 28.2 |
| AB | 37 | 19.8 |
| O | 31 | 28.2 |
| Blood group of babies | ||
| A | 55 | 23.7 |
| B | 53 | 42 |
| AB | 6 | 40.5 |
| O | 17 | 4.6 |
| Rh | 6 | 13 |
| Mothers | ||
| Negative | 6 | 4.6 |
| Positive | 125 | 95.4 |
| Babies | ||
| Negative | 3 | 2.3 |
| Positive | 128 | 97.7 |
Rh: Rhesus factor ABO: Blood group system
Also, the most blood group of mothers was A and AB (28.2%), and the most blood group of babies was A (42%). Most of mothers and babies were positive Rh (95.4%, 97.7 %) respectively. In addition, no significant variation was reported among pre term and full-term groups regarding TSB, DSB, TCB forehead, TCB sternum and TCB interscapular (P>0.05), (Table 4).
Table 4: Serum bilirubin and different bilirubin measurements in relation to GA (N=131).
| Variables | GA | t | P-value | 95% CI | |
| Pre term(<37> | Full term(>37 wks) | ||||
| TSB Mean ±SD | 17.62±2.12 | 15.42±2.36 | 0.38 | 0.67 | 0.32- 1.12 |
| TCB Forehead Mean ±SD | 15.91±2.11 | 16.88±3.20 | 0.19 | 0.84 | 0.15- 0.98 |
| TCB Sternum Mean ±SD | 15.26±2.17 | 15.72±2.91 | 0.26 | 0.73 | 0.23- 1.6 |
| TCB Interscapular Mean ±SD | 15.17±2.25 | 15.67±2.79 | 0.18 | 0.81 | 0.14- 1.26 |
TSB: Total serum bilirubin TCB: Transcutaneous bilirubinometer t: student t test CI: Confidence Interval
Furthermore, no significant variation regarding mean different of TSB - TCB Forehead (P>0.05) was found. While, there were significant difference regarding TSB - TCB Sternum and TSB - TCB Interscapular with mean change (0.98±2.39, 1.28±2.62), less than (P<0>
Table 5: Bland Altman of the agreement between serum bilirubin and the bilirubin measurements.
| Variables | Paired Differences | t | P-value | ||
| Mean ±SD | 95% CI | ||||
| Lower | Upper | ||||
| TSB – TCB Forehead | -0.27±2.53 | -0.70 | 0.17 | -1.205 | 0.231 |
| TSB - TCB Sternum | 0.98±2.39 | 0.57 | 1.40 | 4.708 | less than 0.001* |
| TSB - TCB Interscapular | 1.28±2.62 | 0.83 | 1.73 | 5.582 | less than 0.001* |
TSB: Total serum bilirubin TCB: Transcutaneous bilirubinometer t: student t test CI: Confidence Interva
Also, a significant positive correlation was reported between TSB with TCB forehead, TCB sternum and TCB interscapular (P<0>
Table 6: Correlation between serum bilirubin and different bilirubin measurements.
| TSB | TCB Forehead | TCB Sternum | TCB interscapular | ||
| TSB | r | 1 | 0.652 | 0.687 | 0.624 |
| p | --- | less than 0.001 | less than 0.001 | less than 0.001 | |
| TCB Forehead | r | 0.652 | 1 | 0.547 | 0.600 |
| p | less than 0.001 | --- | less than 0.001 | less than 0.001 | |
| TCB Sternum | r | 0.687 | 0.547 | 1 | 0.692 |
| p | less than 0.001 | less than 0.001 | --- | less than 0.001 | |
| TCB interscapular | r | 0.624 | 0.600 | 0.692 | 1 |
| p | less than 0.001 | less than 0.001 | less than 0.001 | --- | |
TSB: Total serum bilirubin TCB: Transcutaneous bilirubinometer r: Correlation coefficient p: P-value
ROC curve analysis showed that TCB Forehead was the best method for detection jaundice, it had sensitivity of 88.90%, specificity of 73.80% at AUC of 0.812 as compared TCB sternum and TCB interscapular that had sensitivity of 77.50%, 66.70%, specificity of 56.90%, 54.10% at AUC of 0.703, 0.641 respectively, (Table 7).
Table 7: ROC curve of different bilirubin measurements for detection of jaundice.
| Test Result Variable(s) | Area | Std. Error | Asymptotic Sig. | Sens. | Spec. | Asymptotic 95% CI | |
| Lower Bound | Upper Bound | ||||||
| TCB Forehead | 0.812 | 0.050 | 0.002* | 88.90 | 73.80 | 0.713 | 0.911 |
| TCB Sternum | 0.703 | 0.087 | 0.042* | 77.50 | 56.90 | 0.533 | 0.873 |
| TCB interscapular | 0.641 | 0.102 | 0.160 | 66.70 | 54.10 | 0.441 | 0.841 |
TSB: Total serum bilirubin TCB: Transcutaneous bilirubinometer Sens.: Sensitivity Spec.: Specificity CI: Confidence Interval
Discussion
Jaundice or neonatal hyperbilirubinemia is defined as TBS concentration > 3-5 mg/dl. This disease is a benign and common disorder that appears in neonates owing to enzymatic immaturity and is detected more commonly in preterm neonates [9]. Measuring TSB is considered the most used diagnostic approach globally; however, the procedure is expensive, invasive, and time-consuming. Another option is to measure TcB, an affordable, painless, non-invasive, and quick tool that minimizes blood collection in neonates [10]. The present study showed that, the mean gestational age was 37.44±0.98 and birth weight was 2.95±2.07. Moreover, the most common sex was male (64.9%) then female (35.1%). The sociodemographic characteristics of the present study patients were similar to that reported by other researches; Abbas et al., [11], Oyapero et al., [12], and Slusher et al., [13]. Male gender was predominant. Other previous studies demonstrated that higher bilirubin levels presented in males than females [12, 14]. These outcomes suggest an increased susceptibility of male neonate to marked jaundice. In contrast, different results reported by other study Sabzehei et al., [15]. Also, Abbas et al., [11] discovered that the mean birth weight was (2.62± 1.16) and most neonates were of gestational age ≥37 weeks. This finding was compatible with other study by Oyapero, et al., [12] who found that birth weights of most neonate were between 2.5 and 2.99 kg and they reported that high incidence of neonatal jaundice in term neonates of 37 and 39 weeks. Other studies found that term neonates of low birth weight and low gestational age were more susceptible to jaundice and most be investigated for jaundice before discharge [16, 17].
This study showed that, regarding age of jaundice 1st appear, the most common was 3 days (38.2%) then 2 days (33.6%) followed by 4 days (12.2%), 1day (8.4%), 2 days (3.8%), (7, 8) days (1.5%) and 9 days (10.8%). In this concern, a study by Alcock and Liley, [18] noted that breast fed neonates usually have jaundice between 24–72 h of age, reaches the peak at the age of 5–15 days and disappears by the age of three weeks. These neonates were found to have higher levels of bilirubin. Additionally, Abbas et al., [11] reported that, the neonate age at admission for most patients was ≥ 3 days of life. This agreed with Oyapero et al., [12] who reported high bilirubin level after 72 hr. of neonatal birth. Other study showed that mean age of hospital admission was 4.4±2.4 (0-9) days [19]. Shah et al., [20] reported that the mean age of neonate hospital admission was 2.92 and 2.97 days respectively. The current study showed that, the mean HB was 15.05±2.40. Our result was in agreement with the result obtained by Akgul et al., [19] who founded that the mean serum hemoglobin level was 15.6±2.3g/dl. Also, Abbas et al., [11] noted that, the mean initial serum hemoglobin level of this study was 17.54±2.24. In contrast a study by Thakkar and Shah, [21] reported low hemoglobin level (9.0mg/dl).
The current research showed that, the most blood group of mothers was A and AB (28.2%), and the most blood group of babies was A (42%). Most of mothers and babies were positive Rh (95.4%, 97.7 %) respectively. However, Kalakheti et al., [22] and Heier et al., [23] found a risk factor of neonatal hyperbilirubinemia among the maternal blood group ‘O’ Positive that necessitates treatment; also, they reported that ‘O’ Positive mothers who gave birth to ABO-incompatible neonates has the double risk to develop jaundice that necessitates treatment along with 5-10 times higher risk of exchange transfusion. Also, Mohamed et al., [24] noted that mothers with an AB positive blood group (6.2%) were the least frequent in this research, while mothers with an O positive blood group (46.1%) were the most frequent. Our study disagreed with Abbas et al., [11] who found that, according to ABO blood groups, high prevalence of neonatal jaundice occurs in patients with blood group O (41.3%). Blood group B (22.7%) were less prevalent than blood group A (34.7%). This disparity between our results and those of other research may be attributable to the demographic differences between our Egyptian patients and those of other researches, our small number of populations, and the retrospective nature of the prior investigations. Future extensive long-term research will be required to validate this controversy. In this research, no significant variation was reported regarding mean different of TSB - TCB Forehead (P>0.05). While, there were significant difference regarding TSB - TCB Sternum and TSB - TCB Interscapular with mean change (0.98±2.39, 1.28±2.62), (P<0>2 mg/dL.
Other investigations utilizing other transcutaneous bilirubinometers have also revealed significant variations between sternum and forehead TSB and TcBs. [25, 26, 27, 28, 29]. This may be due to deficiency of subcutaneous fat in the sternal region and light exposure to the forehead. At the interscapular area, TcB overestimated in 50 (22%) cases while TSB underestimated in 9 (4%) cases by >2 mg/dl. In the present study, a significant positive correlation was reported between TSB with TCB sternum, TCB forehead, and TCB interscapular (P<0 xss=removed xss=removed xss=removed xss=removed>
In this study, ROC curve analysis showed that TCB interscapular was the best method for detection serum bilirubin, it had sensitivity of 88.90%, specificity of 73.80% at AUC of 0.812 as compared TCB sternum and TCB forehead that had sensitivity of 77.50%, 66.70%, specificity of 56.90%, 54.10% at AUC of 0.703, 0.641 respectively. In the same line, in a study by Agrawal et al. [5] reported that forehead TcB sensitivity in identifying study subjects requiring phototherapy was 79.2%. however, better sensitivity was reported at sternum (87.1%). they also found that, the highest sensitivity in identifying study subjects requiring phototherapy (87.6%) was reported with interscapular TcB and it incorrectly identified 12.4% of those requiring phototherapy. Also, Knüpfer et al. [25] reported that forehead transcutaneous had a sensitivity of 86.8%. Additionally, Yaser et al. [29] reported that forehead transcutaneous had a sensitivity of 80.3%. They determined that sternum TcB sensitivity in detecting the necessity to start phototherapy was 69.7%. However, the highest sensitivity in identifying study subjects requiring phototherapy (87.6%) was reported with interscapular TcB, and it incorrectly identified 12.4% of those requiring phototherapy. And interscapular TcB obtained 93.9% sensitivity, though comparable, these values were lower. The disparity may be due to racial and ethnic diversity.
Conclusion
TcB measurement is a good approach for identifying hyperbilirubinemia requiring therapy in neonates (pre term and full term). TcB reduces neonatal and parental distress, the number of blood samples, and cost of medical care. This research demonstrates TcB validity (using three sites: sternum TcBS, forehead TcBF, and interscapular TcBI sites) as a tool for screening preterm neonates for neonatal jaundice, as it showed a strong and significant correlation and predictive accuracy between TSB and levels of TcBS, TcBF, and TcBI in preterm newborns. The forehead site had the lowest false negative rate and the highest sensitivity.
Declarations
Acknowledgments: The authors would like to express their gratitude to the participants who participated in the study and the data collecting team.
Data Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure: The authors declare no conflicts of interest related to this work.
Funding: This research did not receive any fund.
Informed consent: was obtained from the parents (or legal representative).
References
- Aynalem, S., Abayneh, M., Metaferia, G., et al, (2020). Hyperbilirubinemia in Preterm Infants Admitted to Neonatal Intensive Care Units in Ethiopia. Global Pediatric Health, 7:1-8.
Publisher | Google Scholor - Das, S. and van Landeghem, F.K., (2019). Clinicopathological spectrum of bilirubin encephalopathy/kernicterus. Diagnostics, 9(1):24-36.
Publisher | Google Scholor - Olusanya, B.O., Kaplan, M. and Hansen, T.W., (2018). Neonatal hyperbilirubinaemia: a global perspective. The Lancet Child & Adolescent Health, 2(8):610-620.
Publisher | Google Scholor - Pillai, A., Pandita, A., Osiovich, H. et al, (2020). Pathogenesis and management of indirect hyperbilirubinemia in preterm neonates less than 35 weeks: moving toward a standardized approach. Neoreviews, 21(5):298-307.
Publisher | Google Scholor - Agrawal, G., Garg, K., Sitaraman, S., & et al. (2019). Comparison of diagnostic accuracy of different sites for transcutaneous bilirubin measurement in early preterm infants. The Indian Journal of Pediatrics, 86:32-37.
Publisher | Google Scholor - Hynes, S., Moore, Z., Patton, D., et al, (2020). Accuracy of transcutaneous bilirubin versus serum bilirubin measurement in preterm infants receiving phototherapy: a systematic review. Advances in Neonatal Care, 20(6):118-126.
Publisher | Google Scholor - Sarici, S.U., Ozcan, M., Akpinar, M., et al, (2021). Transcutaneous Bilirubin Levels and Risk of Significant Hyperbilirubinemia in Early-Term and Term Newborns. Journal of Obstetric, Gynecologic & Neonatal Nursing, 50(3):307- 315.
Publisher | Google Scholor - Ho, S.R., Lin, Y.C. and Chen, C.N., (2021). The Impact of Phototherapy on the Accuracy of Transcutaneous Bilirubin Measurements in Neonates: Optimal Measurement Site and Timing. Diagnostics, 11(9):1729-1740.
Publisher | Google Scholor - Mahram, M., Oveisi, S., & Jaberi, N. (2015). Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Medica Iranica.
Publisher | Google Scholor - Srinivas, G. L., Cuff, C. D., Ebeling, M. D., et al. (2016). Transcutaneous bilirubinometry is a reliably conservative method of assessing neonatal jaundice. The Journal of Maternal-Fetal & Neonatal Medicine, 29(16):2635-2639.
Publisher | Google Scholor - Abbas, S. H., Nafea, L. T., & Abbas, R. S. (2020). Prevalence of ABO Incompatibility and its effect on Neonates Hyperbilirubinemia. Research Journal of Pharmacy and Technology, 13(1):141-146.
Publisher | Google Scholor - Oyapero, O., Disu, A. E., & Njokanma, F. O. (2018). Clinical and sociodemographic correlates of neonatal jaundice at a tertiary health facility in Lagos, Nigeria. Advances in Human Biology, 8(2):117.
Publisher | Google Scholor - Slusher, T. M., Angyo, I. A., Bode-Thomas, F., et al. (2004). Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics, 113(6):1636-1641.
Publisher | Google Scholor - Firouzi, M., Yazdanmehr, R., Eliasy, H., et al. (2018). The prevalence of the ABO hemolytic disease of the newborn and its complications in an Iranian population. Iranian Journal of Pediatric Hematology and Oncology, 8(1):37-47.
Publisher | Google Scholor - Sabzehei, M. K., Basiri, B., Gohari, A., et al. (2015). Etiologies of prolonged unconjugated hyperbilirubinemia in neonates admitted to neonatal wards. Iranian Journal of Neonatology, 6(4):37-42.
Publisher | Google Scholor - Newman, T. B., Escobar, G. J., Gonzales, V. M., et al. (1999). Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics, 104(S6):1198-1203.
Publisher | Google Scholor - Burgos, A. E., Schmitt, S. K., Stevenson, D. K., et al. (2008). Readmission for neonatal jaundice in California, 1991–2000: trends and implications. Pediatrics, 121(4): e864-e869.
Publisher | Google Scholor - Alcock, G. S., & Liley, H. (2002). Immunoglobulin infusion for isoimmune haemolytic jaundice in neonates (Cochrane Review). Cochrane Library Systematic Reviews, 3(2):1-23.
Publisher | Google Scholor - Akgül, S., Korkmaz, A., Yiğit, S., et al. (2013). Neonatal hyperbilirubinemia due to ABO incompatibility: does blood group matter. Turk J Pediatr, 55(5):506-9.
Publisher | Google Scholor - Shah, A., Shah, C. K., & Shah, V. (2012). Study of hematological parameters among neonates admitted with neonatal jaundice. Journal of Evolution of Medical and Dental Sciences, 1(3):203-208.
Publisher | Google Scholor - Thakker, B., & Shah, A. A. (2014). Haemolytic conditions of newborn-A laboratory study. NHL Journal of Medical Sciences, 3(2).
Publisher | Google Scholor - Kalakheti, B. K., Singh, R., Bhatta, N. K., et al. (2009). Risk of neonatal hyperbilirubinemia in babies born to ‘O’positive mothers: A prospective cohort study. Kathmandu University Medical Journal, 7(1):11-15.
Publisher | Google Scholor - Heier, H. E., Fugelseth, D., Lindemann, R., et al. (1996). Maternal blood group 0 as a risk factor of neonatal hyperbilirubinemia requiring treatment. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke, 116(1):34-36.
Publisher | Google Scholor - Mohamed, M., Ibrahim, N. R., Ramli, N., et al. (2022). Comparison between the Transcutaneous and Total Serum Bilirubin Measurement in Malay Neonates with Neonatal Jaundice. The Malaysian Journal of Medical Sciences: MJMS, 29(1):43.
Publisher | Google Scholor - Knüpfer, M., Pulzer, F., Braun, L., et al. (2001). Transcutaneous bilirubinometry in preterm infants. Acta Paediatrica, 90(8):899-903.
Publisher | Google Scholor - Schmidt, E. T., Wheeler, C. A., Jackson, G. L., et al. (2009). Evaluation of transcutaneous bilirubinometry in preterm neonates. Journal of Perinatology, 29(8):564-569.
Publisher | Google Scholor - De Luca, D., Zecca, E., Corsello, M., et al. (2008). Attempt to improve transcutaneous bilirubinometry: a double-blind study of Medick BiliMed versus Respironics BiliCheck. Archives of disease in childhood. Fetal and neonatal edition; 93(2):F135-139. 10.1136/adc.2007.121053
Publisher | Google Scholor - Willems, W. A., Berg, L. V. D., Wit, H. D., et al. (2004). Transcutaneous bilirubinometry with the Bilicheck® in very premature newborns. The Journal of Maternal-Fetal & Neonatal Medicine, 16(4):209-214.
Publisher | Google Scholor - Yaser, A., Tooke, L., & Rhoda, N. (2014). Interscapular site for transcutaneous bilirubin measurement in preterm infants: a better and safer screening site. Journal of Perinatology, 34(3):209-212.
Publisher | Google Scholor - Sajjadian, N., Shajari, H., Saalehi, Z., et al. (2012). Transcutaneous bilirubin measurement in preterm neonates. Acta Medica Iranica, 765-770.
Publisher | Google Scholor - Tan, K. L., & Mylvaganam, A. (1988). Transcutaneous bilirubinometry in preterm very low birth weight infants. Acta Pædiatrica, 77(6):796-801.
Publisher | Google Scholor - Szabo, P., Wolf, M., Bucher, H. U., et al. (2004). Assessment of jaundice in preterm neonates: comparison between clinical assessment, two transcutaneous bilirubinometers and serum bilirubin values. Acta Paediatrica, 93(11):1491-1495.
Publisher | Google Scholor