Research Article
Comparison Between Mitomycin-C And Ologen Implants in The Treatment of Primary Open Glaucoma by Subscleral Trabeculectomy
- Ahmed N Elsayed 1*
- Hatem Mahmoud 1
- Anas M. Ebrahim 1
- Mohamed Gaber Okasha 1
- Mohamed Gaber Okasha 1
- Ahmed A. Elsayed 1
- Nehad Mohammed Yusef 1
- Ahmed Mohammed Sakr 1
- Ibrahim Hassan Elabd 1
- Abdel Ghany Ali El Gabbar 1
- Mahmoud abdelhalim Ali Ali 2
1Department of Ophthalmology, Faculty of Medicine, Al-Azhar University, Cairo Egypt
2Department of Ophthalmology, Faculty of Medicine, Aswan University, Aswan, Egypt.
*Corresponding Author: Hatem Mahmoud, Department of Ophthalmology, Faculty of Medicine, Al-Azhar University, Cairo Egypt.
Citation: Elsayed A.N., Mahmoud H., Ebrahim A.M., Farag M.H., Okasha M.G. et al. (2024). Comparison Between Mitomycin-C And Ologen Implants in The Treatment of Primary Open Glaucoma by Subscleral Trabeculectomy, Journal of BioMed Research and Reports, BioRes Scientia Publishers. 5(1):1-9. DOI: 10.59657/2837-4681.brs.24.090
Copyright: © 2024 Ahmed Nabil Elsayed Hafiz, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 30, 2024 | Accepted: July 08, 2024 | Published: July 25, 2024
Abstract
Objective: We investigated the efficacy of Mitomycin-C and Ologen Implants in the Treatment of Primary Open Glaucoma by Subscleral Trabeculectomy.
Methods: A randomized approaching comparative interventional research was directed at 45 eyes of 40 participants 25 males and 15 females with primary open-angle glaucoma (POAG), Patients were distributed into three groups, Group A (included 15 eyes) of 13 participants who went through SST with adjuvant intraoperative usage of 0.2 mg/ml MMC, Group B (included 15 eyes) of 14 participants who underwent SST with a collagen matrix implant during surgery. Group C (included 15 eyes) of 13 participants who underwent SST with both a collagen matrix implant and MMC during surgery.
Results: IOP % during the whole follow-up period in all groups, Mean IOP distribution over the follow-up period. A, B, and C also show the IOP (%) during 1 year of follow-up. In all groups, the mean IOP was calculated for 1 year of follow-up.
Conclusion: Based on these findings, we approved that the Ologen implant could be a secure and reliable substitute for MMC in terms of enhancing the long-term efficacy of trabeculectomy.
Keywords: mitomycin-c; ologen implants; primary open glaucoma; subscleral trabeculectomy
Introduction
Trabeculectomy was first performed in 1968 and is currently the greatest known glaucoma management method in the world (Cairns 1968). However, scarring and wound healing can lead to bleb fibrosis and drainage fistula occlusion, which can ultimately result in bleb failure [1,2]. Thus, inhibiting wound development during the wound-curative process should increase patient Success 1 Histological investigations have demonstrated that the proliferation of subconjunctival fibroblasts, which are thought to be a key part of bleb malfunction, happens between the third and fifth postoperative days [3]. Anterior segment optical coherence tomography (AS-OCT) is a noninvasive method that affords cross-sectional pictures of the morphology of the bleb and may help distinguish between functional and nonfunctional blebs and improve the understanding of the healing process [4].
To expand the success of trabeculectomy, antimetabolites such as mitomycin-C (MMC) and 5-fluorouracil (5-FU) are frequently used. Nevertheless, the usage of antimetabolites rises the risk of infection, bleb leakage, and hypotony. 3 A potential long-term intraocular pressure (IOP) regulation method as well as an alternative to the side effects accompanying the administration of antifibrotic agents and control of the wound-healing progression following filtration surgery should be developed [5]. Diverting wound healing away from the scar production decreases this biological process. Biodegradable spacers, such as porous collagen matrices, are employed in medical preparation to avoid ocular tissue union and alter fibroblast migration outlines, diverting wound healing away from the production of scars, to decrease this biological process [6]. Additional results included the amount of postoperative medication given and any issues that occurred during the follow-up period. Scarring at the scleral flap, covering the episcleral, or the conjunctiva-Tenon’s episcleral interface may increase the difficulty of maintaining long-term IOP control following trabeculectomy [7].
Surgical success rates are increased, and postoperative scarring is significantly inhibited by adjuvant antifibrotic medications such as MMC and 5-FU. The risk profile for using MMC after trabeculectomy is low, but several adverse effects, such as endophthalmitis, avascular filtering blebs, cataract formation, and conjunctival thinning, have been documented [8]. Compared to traditional trabeculectomy, this new method (Mitomycin-C and Ologen Implants) demonstrated a non-inferior success rate and fewer surgical problems; nonetheless, the quantity of postoperative needling operations has increased [9]. Biodegradable tissue-engineered implants have emerged as substitutes for traditional trabeculectomy augmentation. It is possible to create structures that are strikingly similar to normal tissue by modeling tissue growth [10]. While solitary surgery improved IOP decline in the short-term follow-up, there was no discernible change in long-term follow-up IOP reduction among the two groups [11-13].
Numerous implants are being produced. The goal of this implantation is to stop the subconjunctival space from collapsing. In studies conducted on animals, the Ologen, second group was used [14]. Moreover, the biodegradable implant decreased the scarring that developed soon after surgery. An obvious trial review showed no differences between postoperative IOP in the study group after trabeculectomy with ologen and in the control group after trabeculectomy without ologen [15].
Methods
A randomized approach is the process of assigning our patients to treatment and control groups, assuming that each patient has an equal chance of being assigned to both three groups. comparative interventional research was explained in 45 eyes of 40 participants 25 males and 15 females with primary open-angle glaucoma (POAG), and operations were performed at Al-Azhar University Hospitals. Indications for surgery were based on an IOP above 21 mmHg on the highest tolerated treatment. Target IOP levels should be decreased to decrease the threat of developing glaucoma, glaucomatous visual field loss, or abnormalities in the optic disc that suggest glaucomatous damage (flame-shaped hemorrhages around the optic nerve head).
Patients were distributed into three groups, Group A (included 15 eyes) of 13 participants who went through SST with adjuvant intraoperative usage of 0.2 mg/ml MMC, it is approved that There is no significant difference in efficacy or safety between the 0.2 mg/mL and 0.4 mg/mL concentration. Group B (included 15 eyes) of 14 participants who underwent SST with a collagen matrix implant during surgery. Group C (included 15 eyes) of 13 participants who underwent SST with both a collagen matrix implant and MMC during surgery. This study conformed to the guidelines presented in the Declaration of Helsinki. Written informed agreement was gained from all patients previously involved in the research. This study was approved by the ethics committee of the Faculty of Medicine, Al-Azhar University, Cairo, Egypt. Informed consent was obtained from all participants.
The Inclusion Criteria: (1) were over the age of eighteen years, (2) had a POAG, (3) had insufficient IOP control (IOP) >21 mmHg or increasing visual field degradation on maximally accepted treatment, (4) had suggestions for surgery established on IOP values accompanying through a great possibility of development of glaucoma, glaucomatous visual field loss, or deviated in the optic disc indicative of glaucomatous injury with a lower target IOP.
The Exclusion Criteria: (1) clinically significant cataracts for which combined surgery was recommended, (2) congenital or juvenile glaucoma, (3) a history of prior surgical or laser methods, (4) a known allergy to MMC, (5) pregnant or nursing women (6) uncontrolled diabetes and hypertension or any extra health condition that increases the danger of side effects.
Preoperative Assessment: Each patient underwent a thorough ophthalmological examination and a history, and the quantity and kinds of medications used for the three months before trabeculectomy were documented. Best corrected visual acuity (BCVA). Before or during the procedure, there should be no history of conjunctival tissue involvement during intraocular or extraocular surgery. slit-lamp analysis. The optic disc was evaluated along with the fundus. Goldman’s applanation tonometry was used to record IOP twice on different days. Angle grading via Schaeffer's method and gonioscopy with the Goldmann 3-mirrors contact goniolens.
Postoperative Evaluation: There were 5 postoperative and follow-up visits within 1 year of surgery: 1 day, 7 days, 90 days, 6 months, and one year after surgery. The following parameters were recorded: IOP using a calibrated Goldmann applanation tonometer.
Results
Group A
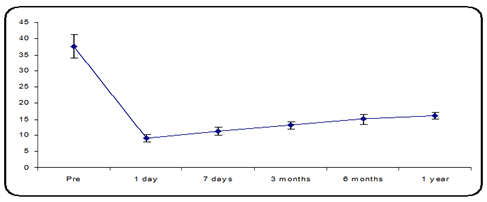
The mean preoperative IOP was 37.47 ± 3.56 mmHg in group A, (15 eyes of 13 participants) and the mean IOP decreased from 37.47 ± 3.56 mmHg to 9.07 ± 1.22 mmHg on a postoperative day one 11.40 ± 1.18 mmHg 7 days postoperative and 13.13 ± 1.06 mmHg 3 months postoperative, 16.04 ± 1.49 mmHg at 6 months postoperative and 16.13 ± 0.99 mmHg 1- year postoperative follow-up, there was a statistically significant variance, p-value less than 0.05 (Figure 1).
Figure 1: Mean IOP distribution over the follow-up period.
Group B
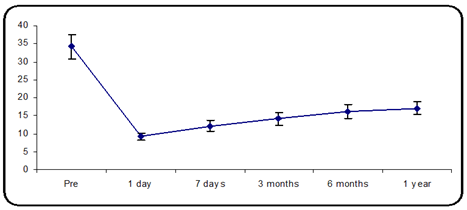
The mean preoperative IOP was 34.13 ± 3.36 mmHg in group B (15 eyes of 13 participants). The mean IOP decreased from 34.13 ± 3.36 mmHg to 9.27 ± 0.96 mmHg on postoperative day one and 12.13 ± 1.55 mmHg at 7 days postoperative, 14.20 ± 1.74 mm Hg at 3 months postoperative, 17.4 ± 1.94 mmHg at 6 months postop. and 17.1 ± 1.68 mmHg 1 year postop. follow-up. There was a statistically significant variance in the mean IOP, p-value less than 0.05 (Figure 2).
Figure 2: Mean IOP distribution during the follow-up period.
Group C
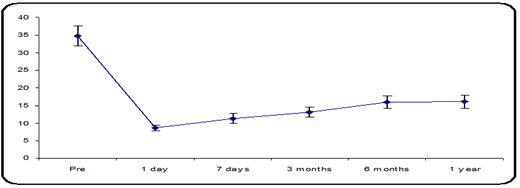
The mean preoperative IOP was 34.73 ± 2.82 mmHg in group C (15 eyes of 13 participants). The mean IOP decreased from 34.73 ± 2.82 mmHg to 8.73 ± 0.80 mmHg on postoperative day one and 11.33 ± 1.35 mmHg at 7 days postoperative, 13.07 ± 1.44 mmHg at 3 months postop., and 13.93 ± 1.83 mmHg at 6 months postoperative and 16.08 ± 1.83 mm hg 1- year postop. follow-up. There was a statistically significant variance in the mean IOP, p-value less than 0.05 (Figure 3).
Figure 3: Mean IOP distribution during the follow-up period.
Table 1: IOP (%) during the whole follow-up period in all groups.
| Group A | Group B | Group C | One-Way ANOVA Test | |||
| F | P-value | |||||
| Pre | Mean ± SD | 37.47 ± 3.56 | 34.13 ± 3.36 | 34.73 ± 2.82 | 4.455 | 0.018 |
| Range | 32-45 | 28-40 | 30-40 | |||
| 1 day | Mean ± SD | 9.07 ± 1.22 | 9.27 ± 0.96 | 8.73 ± 0.80 | 1.069 | 0.353 |
| Range | 6-11 | 8-11 | 8-10 | |||
| 7 days | Mean ± SD | 11.40 ± 1.18 | 12.13 ± 1.55 | 11.33 ± 1.35 | 1.578 | 0.218 |
| Range | 9-13 | 10-16 | 10-14 | |||
| 3 months | Mean ± SD | 13.13 ± 1.06 | 14.20 ± 1.74 | 13.07 ± 1.44 | 2.926 | 0.065 |
| Range | 11-15 | 12-18 | 11-15 | |||
| 6 months | Mean ± SD | 16.04 ± 1.49 | 17.4 ± 1.94 | 16.8 ± 1.83 | 1.885 | 0.161 |
| Range | 13-21 | 13-21 | 11-21 | |||
| 1 year | Mean ± SD | 16.13 ± 0.99 | 17.1 ± 1.68 | 16.07 ± 1.83 | 2.804 | 0.069 |
| Range | 15-18 | 15-20 | 11-17 | |||
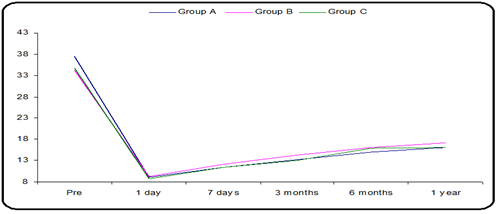
Figure 4: Mean IOP distribution during the follow-up period in all groups.
Table 2: IOP % during 1 year of follow-up in all groups.
| Group A Mean ± SD of difference | Group B Mean ± SD of difference | Group C Mean ± SD of difference | One-Way ANOVA Test | ||
| F | P-value | ||||
| 1 day | 27.4 ± 4.2 | 24.87 ± 3.02 | 26.00 ± 3.23 | 3.932 | 0.027 |
| 7 days | 26.1 ± 4.0 | 22.00 ± 2.85 | 23.40 ± 2.85 | 6.061 | 0.005 |
| 3 months | 24.3 ± 4.1 | 19.93 ± 4.20 | 21.67 ± 2.87 | 5.103 | 0.010 |
| 6 months | 21.4 ± 4.63 | 17.03 ± 4.32 | 18.75 ± 3.26 | 4.252 | 0.021 |
| 1 year | 21.3 ± 3.9 | 16.47 ± 4.32 | 20.67 ± 2.74 | 7.497 | 0.002 |
IOP % during the whole follow-up period in all groups, Mean IOP distribution over the follow-up period. A, B, and C also show the IOP (%) during 1 year of follow-up. In all groups, the mean IOP was calculated for 1 year of follow-up (Table 1,2. Figure 4).
Complications
Regarding complications, four patients in group A had a shallow anterior chamber, which had spontaneously changed by the end of the first week. There were also four patients with hypotony in group A. However, Groups B and C did not lead to any recorded complications, such as hypotony or shallow AC. No signs of hyphemia were recorded in any of the groups.
Discussion
Surgical treatment for glaucoma most commonly involves SST, first performed by Cairns in 1968. Supplemental antimetabolites were utilized to improve the achievement scale of such surgery because episcleral fibrosis and subconjunctival scarring lower the achievement ratio [16]. Ologen is a wound modulator it does not inhibit the scarring process by acting as an antifibrotic agent [17]. Several reports have revealed that using MMC, an antitumor antibiotic derived from streptomycin capacitors, intraoperatively significantly improves success rates and postoperative IOP control. It causes cell death by preventing DNA-dependent RNA synthesis [18]. Time and dose depend on how well it inhibits proliferation. When MMC is injected intraoperatively during glaucoma filtration surgery, patient outcomes improve, and patients at risk of failure have a lower IOP [19]. The development of cataracts, along with this and to improve the outcomes of antiglaucoma surgery additional treatments including growth factor inhibition, corticosteroids, and amniotic membranes are used [20].
Through opening sizes ranging from 10 to 300 mm, the Ologen implant is a porous material, The matrix inhibits the formation of scars and new infections while promoting better remodeling of the regenerating tissue [21]. Recently, Lommatzsch et al. reported on the usage of a collagen matrix implant as a human bevacizumab store in patients undergoing MMC trabeculectomy [22]. Surgery for trabeculectomy can account for the initial decrease in IOP in our groups. Because MMC suppresses human fibroblasts for a prolonged period but does not permanently harm them, the behavior of the IOP in this group can be explained. Throughout a 1-year follow-up period, the study by Hatem et al. aimed to evaluate the outcomes of using Ologen versus MMC as an adjuvant treatment in SSTs. For both groups, there was no statistically significant variation in terms of preoperative IOP, type of glaucoma, number of antiglaucoma medications taken, or age. These variables also did not affect the surgical outcome. The IOP was found to be substantially lower at follow-up visits than before surgery in each group in this investigation [23].
Eosinophils can decrease infection and fix injured tissue, as evidenced by recent descriptions of them as significant regulators of local immunity and remodeling/healing in both healthiness and illness [24]. The extent of Lowered IOP varied between groups during the entire follow-up visit in our study, with statistically significant differences. There were no potential-specific side effects, such as allergies or implant translocations, that we could find in our study during postoperative follow-up visits Notably, in the MMC group, hypotony was highly prevalent in the early postoperative period (less than 7 days). 4 out of 15 instances. There were no discernible differences of any kind among the three groups. In the combined or Ologen groups, there was no hypotony one week after surgery. Rosentreter et al. stated the onset of endophthalmitis in one patient with Ologen implantation and trabeculectomy. However, in these cases, we looked at, no such issue [21]. The two groups of patients had comparable rates of postoperative complications, preventing any distinctions between the two adjuvants from being identified [25]. Hatem et al. discovered that throughout the follow-up period, the use of a shallow anterior chamber with MMC caused greater problems in 4 patients than in 2 patients.
Rosenteter et al. found no evidence of any potential complication, such as allergies or implant translocations; however, hypotony was prevalent in the early postoperative phase, occurring seven days after surgery, in the Ologen group. However, only 20% of patients had a shallow anterior chamber. There was no discernible difference between the MMC and Ologen groups. One week after surgery, the Ologen patients showed no signs of hypotony, while 30% of the patients in the MMC group showed signs of hypotony [21].
Conclusion
Based on these findings, we approved that the Ologen implant could be a secure and reliable substitute for MMC in terms of enhancing the long-term efficacy of trabeculectomy surgery while avoiding the negative consequences connected to the application of supplementary therapy, such as MMC. For example, the Ologen implant might be recommended when antimetabolite-related dangers must be minimized. It might also be helpful in circumstances requiring IOP lowering and optimal safety. To enhance the functional outcome, additional adjustments to the size of the Ologen implant, surgical method, and molecular makeup will be needed.
Declarations
Ethics Approval and Consent to Participate
This study conformed to the guidelines presented in the Declaration of Helsinki. Written informed agreement was gained from all patients previously they were involved in the research. Written informed consent was obtained from all participants
Competing Interests
The authors declared no potential competing of interest concerning this article's research, authorship, and publication.
Data Availability
All the datasets used/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of Interest
All authors declare no conflict of interest.
Authors’ Contributions
All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work, all authors read and approved the final manuscript
Acknowledgments
The authors would like to thank their university hospital, doctors, nurses, and staff who have taken care of the patients during treatment. Furthermore, they would like to thank their patients who voluntarily contributed to our study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Skuta GL, Parrish RK. (1987). wound healing in glaucoma filtering surgery. Surv Ophthalmol, 32:149-170.
Publisher | Google Scholor - Picht G, Grehn F. (1998). Classification of filtering blebs in trabeculectomy: biomicroscopy and functionality. Curr opin Ophthalmol, 9:2-8.
Publisher | Google Scholor - Jampel HD, Mcguigan LJ, Dunkelberger GR, et al. (1988). Cellular proliferation after experimental glaucoma filtration surgery. Arch Ophthalmol, 106:89-94.
Publisher | Google Scholor - Cillino S, Casuccio A, Di Pace, et al. (2016). Biodegradable collagen matrix implant versus mitomycin-C in trabeculectomy: Five-year follow-up. BMC Ophthalmol, 16-24.
Publisher | Google Scholor - Chen HS, Ritch R, Krupin T, et al. (2006). Control of filtering bleb structure through tissue bioengineering: an animal model. Invest Ophthalmol & Visual Science, 47:5310-5314.
Publisher | Google Scholor - Lu J.L, Hall L, Liu J. (2018). Improving Glaucoma Surgical Outcomes with Adjunct Tools. J. Curr. Glaucoma Pract, 12:19-28.
Publisher | Google Scholor - Wilkins M, Indar A, Wormald R. (2005). Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst, Rev. CD002897.
Publisher | Google Scholor - Klink T, Schrey S, Elsesser U, et al. (2008). Interobserver variability of the Wurzburg bleb classification score. Ophthalmologica, 222: 408-413.
Publisher | Google Scholor - Theilig T, Rehak M, Busch C, et al. (2020). Comparing the efficacy of trabeculectomy and XEN gel microstent implantation for the treatment of primary open-angle glaucoma: a retrospective monocentric comparative cohort study. Sci Rep-UK, 10:19337.
Publisher | Google Scholor - Okuda T, Higashide T, Fukuhira Y, et al. (2009). A thin honeycomb-patterned film as an adhesion barrier in an animal model of glaucoma filtration surgery. J Glaucoma, 18:220-226.
Publisher | Google Scholor - Fea AM, Bron AM, Economou MA, et al. (2020). European study of the efficacy of a cross-linked gel stent for the treatment of glaucoma. J Cataract Refract Surg, 46:441-444.
Publisher | Google Scholor - Lim SY, Betzler BK, Yip LWL, et al. (2021). Standalone XEN45 Gel Stent implantation versus combined XEN45-phacoemulsification in the treatment of open angle glaucoma-a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol, 259:3209-3219.
Publisher | Google Scholor - Yang X, Zhao Y, Zhong Y, et al. (2022). The efficacy of XEN gel stent implantation in glaucoma: a systematic review and meta-analysis. BMC Ophthalmol, 22:305.
Publisher | Google Scholor - Tsurumaru N, Arai M, Teruya K, et al. (2009). Seprafilm as a new antifibrotic agent following trabeculectomy in rabbit eyes. Jpn j Ophthalmol, 53:164-170.
Publisher | Google Scholor - Aptel F, Dumas S, Denis P. (2009). Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant. Eur J Ophthalmol, 19:223-230.
Publisher | Google Scholor - Bindlish R, Condon G.P, Schlosser J.D, et al. (2002). Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology, 109:1336-1341.
Publisher | Google Scholor - Singh K, Bhattacharyya M, Mutreja A, et al. (2018). Trabeculectomy with subconjunctival collagen implant in Indian eyes: Long-term results. Indian J Ophthalmol, 66:1429-1434.
Publisher | Google Scholor - Lekha M.A, Dinesh S, Robert C, et al. (2006). Mitomycin: clinical applications in ophthalmic practice. Drugs, 66:321-340.
Publisher | Google Scholor - Fontana H, Nouri-Mahdavi K, Lumba J, et al. (2006). Trabeculectomy with Mitomycin-C: Outcomes and risk factors for failure in phakic open-angle glaucoma. Ophthalmology, 113:930-936.
Publisher | Google Scholor - Siriwardena D, Khaw PT, King AJ, et al. (2002). Overton BM, Migdal C Cordeiro, MF. Human anti transforming growth factor beta (2) monoclonal antibody- a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology, 109:427-431.
Publisher | Google Scholor - Rosenteter A, Schild AM, Jordan JF, et al. (2010). A prospective randomized trial of trabeculectomy using mitomycin C vs an Ologen implant in open angle glaucoma. Eye, 24:1449-1457.
Publisher | Google Scholor - Lommatzsch C, Heinz C, Rothaus K, et al. (2020). Trabeculectomy with Collagen Matrix Implant as Bevacizumab Depot: Clinical Results on Efficacy and Safety. Klin. Monatsblatter Augenheilkd, 237:62-70.
Publisher | Google Scholor - Hatem M. Marey, Sameh S. Mandour, Amin F. Ellakwa. (2013). Subscleral Trabeculectomy with Mitomycin-C Versus Ologen for Treatment of Glaucoma. Journal of ocular pharmacology and therapeutic, 29(3).
Publisher | Google Scholor - Coden ME, Berdnikovs S. (2020). Eosinophils in wound healing and epithelial remodeling: Is coagulation a missing link? J Leukoc Biol, 108:93-103.
Publisher | Google Scholor - Cillino S, Di Pace F, Casuccio A, et al. (2008). Deep sclerectomy vs trabeculectomy with low-dosage mitomycin C: four-year follow-up. Ophthalmologica, 222:81-87.
Publisher | Google Scholor