Research Article
Clinico-Radiological Spectrum of Non-Compressive Myelopathies
- Shabeer Ahmad Paul ID *
- Gouranga Prasad Mondal
- Ramesh Bhattacharyya
- Kartik Chandra Ghosh
- Sarbajit Das
- Hema Krishna
- Chandrakanta Patra
- Devlina Roy
- Jyoti Kiran
Department of Neurology, Calcutta National Medical College & Hospital, West Bengal, India.
*Corresponding Author: Shabeer Ahmad Paul, Department of Neurology, Calcutta National Medical College & Hospital, West Bengal, India
Citation: Shabeer A Paul, Gouranga P Mondal, R Bhattacharyya, Kartik C Ghosh, S Das. (2023). Clinico-Radiological Spectrum of Non-Compressive Myelopathies. Journal of Neuroscience and Neurological Research, BRS Publishers. 2(1); DOI: 10.59657/2837-4843.brs.23.004
Copyright: © 2023 Shabeer Ahmad Paul, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 07, 2022 | Accepted: December 27, 2022 | Published: January 03, 2023
Abstract
Objective: To study the etiology and clinico-radiological profile of non-compressive myelopathies.
Methods: 40 consecutive admissions of non-compressive myelopathy were studied for clinical and radiological profile. Statistical methods: Descriptive statistics, linear regression and independent t-test using SPSS 20.
Results: Age of the studied population ranged from 2 years to 55 years with a median age of 32.35 years and male:female ratio of 2:1. 87.5% of cases had an acute to subacute presentation. Complete cord syndrome was the most common presentation followed by anterior cord syndrome, central cord syndrome and mixed pattern. LETM was seen in nearly two-thirds of cases. Lesions involving more than two-thirds of the cross section of spinal cord were seen in more than 60% of cases, thereby accounting for significant disability at onset. Most of the lesions were hyperintense on T2 and STIR images and isointense on T1 with only 18.91% showing contrast enhancement. Optic neuritis was recorded with an overall prevalence of 28.26%. EDSS at presentation varied among the subgroups with ADEM, NMOSD and LETM form of ITM having EDSS scores of 9.5,8.0 and 7.5 respectively whereas in MS and Non-LETM form of ITM, EDSS score was 4.0 and 2.5 respectively.
Conclusion: Demyelinating disorders contribute to nearly 80% cases of non-compressive myelopathies with NMOSD being the most common pathology. CSF cell count, cross-sectional involvement of spinal cord, pattern of myelitis and aquaporin-4 seropositivity were found to be predictive of the severity at onset i.e., EDSS at presentation with the initial 3 factors also showing predictability of EDSS at last visit.
Keywords: non-compressive myelopathies; myelitis; clinico-radiological
Introduction
Pathologies that affect the spinal cord are collectively referred to as myelopathies. Etiologies include traumatic, neoplastic, vascular, demyelinating, infectious and others. Onset of symptoms may be acute, subacute or chronic. Early diagnosis and intervention are of paramount importance to achieve a good functional outcome and prevent the long-term disability. Imaging of spinal cord in isolation or including brain in special circumstances is essential part of initial work-up to exclude compressive etiologies which are usually managed surgically. Etiological work-up of non-compressive myelopathies is more extensive ranging from CSF study including oligoclonal bands and IgG index, screening for infectious agents, screening for collagen vascular diseases and vasculitis, antibody testing for inflammatory demyelinating disorders of central nervous system like multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD) or myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease and others.
Despite an exhaustive work-up, etiological diagnosis may not be established in many cases. Diagnostic criteria have been laid down for different diseases like MS, NMOSD and Idiopathic transverse myelitis. Challenges still remain in cases not fulfilling the criteria particularly at the initial presentation and in non-demyelinating disorders. Empirical treatment and a close follow-up are essential in such cases where the actual diagnosis may be revealed in due course of time.
A large number of studies pertaining to the etiological spectrum of non-compressive myelopathies have been carried out in different centres of India. However, considering the fact that significant developments have taken place in last decade or so with regards to research in the field of neuromyelitis optica spectrum disorders (NMOSD), we intended to do this study to see the change in pattern of etiological spectrum of non-compressive myelopathies and study the disease specific clinico-radiological parameters.
Materials and Methods
This study was an observational prospective study conducted in the department of Neurology inpatient department, Calcutta National Medical College & Hospital, Kolkata from Feb 2020 to September 2021. 40 cases admitted with a diagnosis of myelopathy in the inpatient Neurology department were selected with following criteria.
Inclusion criteria:
- Patients with clinical evidence of myelopathy
Exclusion criteria:
- MRI evidence of spinal cord compression
- Diagnosis consistent with motor neuron disease
- Myelopathy associated with degenerative spinocerebellar ataxia.
Results
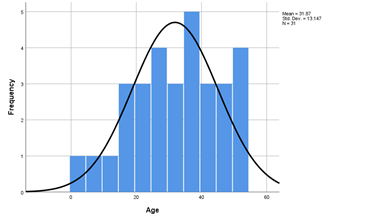
In this study, we evaluated 40 cases of non-compressive myelopathy for their etiology and studied their clinical and radiological parameters. Demyelinating disorders that formed the major proportion of cases were studied separately. The age of patients ranged from 2 years to 55 years with a median age of 32.35 years (S.D -12.799). 62.5% of cases were between 25-50 years, 32.5% were below 25 years and only 5% of cases were above 50 years of age. Figure 1 shows the histogram of age distribution. Male female ratio was 2:1. 72.5 percentage cases of non-compressive myelopathy had acute presentation, 15% had subacute, while as 12.5% had chronic presentation. Symptom duration before presentation ranged from minimum of 1 day in a case of transverse myelitis to a maximum of 1095 days in a case of HSP. Median symptom duration prior to presentation was 7 days (S.D = 182.80).
Figure 1: Histogram showing the age distribution of cases in the study.
Complete cord syndrome was the most common presentation in the study seen in 35% of cases followed by anterior cord syndrome and mixed pattern in 15 percentage each. Table 1 shows the pattern of presentation of myelopathy in the studied cases. NMOSD was the most frequent diagnosis made with an incidence of 30% followed by idiopathic transverse myelitis in 25% and MS in 15%. 10 percentage cases had infectious myelitis including tubercular, zoster and scrub etiology. ADEM was seen in 7.5%. SLE, B12 deficiency, copper deficiency and HSP were seen with a frequency of 1 case each. In one case etiology remained undefined (Figure 2).
Table 1: Syndromic presentation of myelopathy in the studied population.
| Cord syndrome | Frequency | Percent |
| Anterior | 8 | 20 |
| Central | 4 | 10 |
| Complete | 14 | 35 |
| Conus-cauda | 1 | 2.5 |
| Mixed pattern | 6 | 15 |
| Posterior | 2 | 5 |
| Posterolateral | 2 | 5 |
| Tract specific pattern | 3 | 7.5 |
| Total | 40 | 100 |
Figure 2: Prevalence of different etiologies of non-compressive myelopathy
Since demyelinating diseases constituted the maximum proportion of our cases, clinical and radiological variables were also studied separately for this group. Higher prevalence was seen in males with a male to female ratio of 1.8:1. Preceding febrile illness was noted in 22.5% of cases and prior history of vaccination was found in 6.45% of cases. Longitudinally extensive transverse myelitis (LETM) was more common pattern seen in 67.7% of cases of demyelinating causes of myelitis. Short transverse myelitis (STM) was seen in 22.6% and MRI imaging was normal in 3 cases (9.7%). Number of segments affected ranged from 0 to 9. The length of lesion in abnormal MRI patients ranged from 1 vertebral segment to 14 vertebral segments with mean length being 3.52 (SD = 2.75). In majority of the cases (61.3%), lesion involved more than two thirds of the spinal cord cross sectional area. In 22.6%, it was between one-third to two-third while as in 6.5% of cases, it was less than one-third. Dorsal region was the most common site affected in demyelinating subgroup (Figure 4 & 5). However, in MS subgroup, cervical region was the most common site of involvement. Table 2 shows the distribution of lesions in the spinal cord. Hyperintensity on T2 and STIR images was the most common finding seen in 94.59% (n = 35/37) lesions in demyelinating subgroup. T1 hypointensity was seen in 8.10% (n = 3/37) and cord swelling was noted in 5.4% (n = 2/37) each. Contrast enhancement was seen in 18.91% (n = 7/37) cases (Table 3). Optic neuritis had a cumulative prevalence of 28.26% including attacks at onset and in relapses VEP abnormality was reported with a prevalence of 35.5% which was higher than the incidence of optic neuritis reflecting the subclinical involvement in rest of the cases. Most common abnormality on VEP study was delayed P100 latency. Figure 3 shows the distribution of VEP abnormalities in the demyelinating subgroup. Brain lesions were detected in 38.70% at onset. Subcortical region was the most frequent affected site followed by brainstem, periventricular and cerebellum (Table 4). CSF cell count in demyelinating group ranged from 0 to 45 with a mean of 21.13 (SD = 11.275) with a mean lymphocyte count of 82.90%. CSF protein ranged from 47 mg/dl to 174 mg/dl with a mean of 70.79 (SD = 23.241). CSF OCB was positive in 16.12% (n = 5) cases, 3 of which had multiple sclerosis. CSF IgG index was high in 6.45% (n = 2) cases. Table 5 depicts the descriptive statistics of different variables in the study population.
Table 2: Site of spinal cord involvement in the demyelinating subgroup.
| Spinal cord region | Frequency | Percent |
| Cervical | 10 | 32.22 |
| Cervicodorsal | 6 | 19.35 |
| Dorsal | 12 | 38.7 |
| Dorsolumbar | 1 | 3.22 |
| Lumbosacral | 1 | 3.22 |
| Cervicodorsolumbar | 1 | 3.22 |
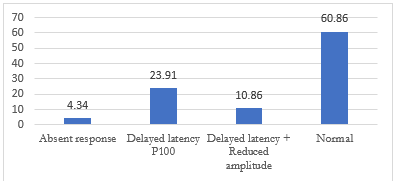
Figure 3: VEP abnormalities in the demyelinating subgroup
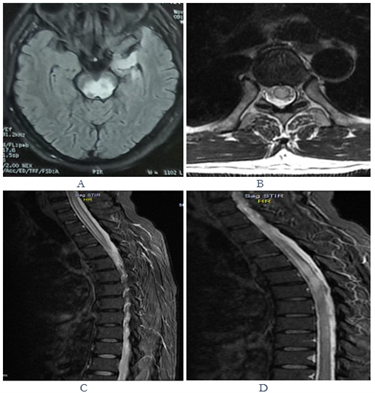
Figure 4: Axial T2 FLAIR MRI showing hyperintense lesion involving tectal and tegmental portions of midbrain and left anteromedial temporal lobe in a case of seropositive NMOSD (a); axial T2WI of MRI spine at the level of D3 shows hyperintense signal change sparing only a thin peripheral rim of cord (b); sagittal T2WI of dorsal cord shows a hyperintense lesion spanning D1 to D8 vertebral level (c & d).
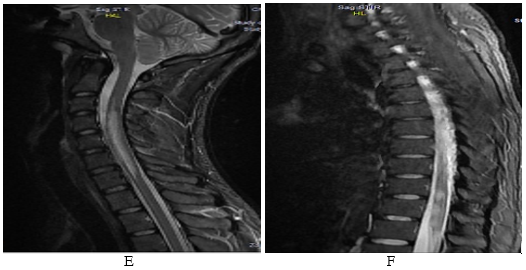
Figure 5: A long segment hyperintense lesion extending from C3 to C7 vertebral level on T2W sagittal image of cervical spine in a case of seronegative NMOSD (e); another demyelinating lesion in the same patient in the lower dorsal region on sagittal T2W dorsal spine MRI (f).
Table 3: MRI characteristics of demyelinating lesion
| MRI characteristics of lesions | Frequency | |
| T2 | Hyperintensity | 35 |
| Normal | 2 | |
| T1 | Hypointensity | 3 |
| Normal | 34 | |
| Contrast | Contrast enhancing | 7 |
| Non enhancing | 30 | |
| Cord swelling | Cord swelling | 2 |
| No cord swelling | 35 | |
Table 4: Distribution of brain lesions in demyelinating subgroup
| Site involved | Frequency | Percent |
| Brainstem | 7 | 22.58 |
| Cerebellum | 4 | 12.9 |
| Periventricular | 5 | 16.12 |
| Subcortical | 8 | 25.8 |
| Juxtacortical | 2 | 6.45 |
| Internal capsule | 1 | 3.22 |
| Cortical | 1 | 3.22 |
| Corpus callosum | 2 | 6.45 |
Table 5: Descriptive statistics of different variables in the study
| Variable | Minimum | Maximum | Mean | Std. Deviation |
| Age | 2 | 54 | 31.87 | 13.147 |
| Symptom duration | 1 | 365 | 22.06 | 64.752 |
| Follow up duration | 3 | 36 | 12.29 | 7.52 |
| CSF cell count | 0 | 45 | 21.13 | 11.275 |
| CSF lymphocyte (%) | 0 | 100 | 82.9 | 21.439 |
| CSF sugar | 56 | 96 | 76.68 | 9.745 |
| CSF protein | 47 | 174 | 70.7968 | 23.24192 |
The overall incidence of AQP4 seropositivity in our study was 26.92% and specifically in the NMOSD group it was 50%. Among those who were seronegative, 3 cases were found to be positive for MOG antibody. 62.5% of cases in our study presented with a severe disability at onset with EDSS score more than 6. The median EDSS scores at presentation and on last visit were 7.25 and 3.5 respectively. EDSS at presentation varied among different subgroups of myelitis. ADEM, NMOSD and LETM form of idiopathic transverse myelitis presented with the highest disability at onset with median EDSS of 9.5, 8.0 and 7.5 respectively. Myelitis in MS group had lesser disability at presentation with median EDSS of 4.5. Median EDSS at last visit was 5.5 in NMOSD, 5.0 in LETM form of ITM and 3.5 in MS. However, these results were not statistically significant. Out of 12 cases of NMOSD, Rituximab was used as maintenance therapy in 7 cases while as Azathioprine was used in the rest along with an initial steroid overlap. In the MS group, 2 patients received teriflunomide and 3 received dimethyl fumarate and one patient received rituximab. ITM cases were managed with pulse steroids followed by 4-6 weeks of oral steroids. The follow up of cases ranged from 3 months to 36 months with mean follow-up of 12.29 months. A total of 14 relapses were seen with maximum relapses occurring in the NMOSD subgroup. Optic neuritis and myelitis featured among the relapses in NMOSD whereas brain involvement and optic neuritis were the presentation of relapses in MS subgroup.
Different clinical, laboratory and radiological parameters were studied for their relation with EDSS at presentation and the EDSS on last visit. CSF cell count, cross sectional involvement of cord, pattern of myelitis (LETM vs Non-LETM) and AQP4 seropositivity were found to be predictive of the disability at onset i.e., EDSS at presentation. The final outcome i.e., EDSS on last visit was found to have significant correlation with pattern of myelitis, AQP4 seropositivity, cross sectional involvement of cord and EDSS at presentation. (Table 6a,6b,7a,7b).
Table 6a: Linear regression results of variables with EDSS at presentation
| Variables | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | |
| B | Std. Error | Beta | |||
| Age | -0.012 | 0.029 | -0.079 | -0.428 | 0.672 |
| CSF cell count | 0.092 | 0.029 | 0.509 | 3.185 | 0.003 |
| CSF protein | 0.017 | 0.016 | 0.195 | 1.069 | 0.294 |
| Cross sectional involvement | 1.207 | 0.313 | 0.583 | 3.863 | 0.001 |
Table 6b: Linear regression results of variables with EDSS at last visit
| Variables | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | |
| B | Std. Error | Beta | |||
| Age | -0.023 | 0.036 | -0.115 | -0.625 | 0.537 |
| CSF cell count | 0.079 | 0.04 | 0.347 | 1.99 | 0.056 |
| CSF protein | 0.014 | 0.02 | 0.123 | 0.666 | 0.511 |
| Cross sectional involvement | 1.105 | 0.439 | 0.423 | 2.516 | 0.018 |
| EDSS at presentation | 0.91 | 0.162 | 0.721 | 5.61 | 0.001 |
Table 7a: Independent t test results of different variables studied for comparing the means with regards to EDSS at presentation
| t-test for Equality of Means | ||||||
| Variables | t | df | Sig. (2-tailed) | 95% Confidence Interval of the Difference | ||
| Lower | Upper | |||||
| Sex | Equal variances assumed | 0.209 | 29 | 0.836 | -1.40343 | 1.72361 |
| Equal variances not assumed | 0.205 | 21.775 | 0.84 | -1.4634 | 1.78357 | |
| Brain involvement | Equal variances assumed | -1.313 | 29 | 0.199 | -2.49605 | 0.54429 |
| Equal variances not assumed | -1.175 | 16.049 | 0.257 | -2.73616 | 0.78441 | |
| Optic neuritis | Equal variances assumed | 0.365 | 29 | 0.718 | -1.58135 | 2.26801 |
| Equal variances not assumed | 0.388 | 8.192 | 0.708 | -1.68983 | 2.37649 | |
| VEP abnormalities | Equal variances assumed | 1.121 | 29 | 0.271 | -0.77042 | 2.63998 |
| Equal variances not assumed | 1.303 | 16.742 | 0.21 | -0.58016 | 2.44973 | |
| AQP4 seropositivity | Equal variances assumed | 2.473 | 29 | 0.02 | 0.34648 | 3.65948 |
| Equal variances not assumed | 4.417 | 27.087 | 0.001 | 1.0727 | 2.93326 | |
| Pattern of myelitis | Equal variances assumed | 3.569 | 29 | 0.001 | 1.01239 | 3.73047 |
| Equal variances not assumed | 3.191 | 13.774 | 0.007 | 0.77511 | 3.96774 | |
Table 7b: Independent t test results of different variables studied for comparing the means with regards to EDSS at last visit
| t-test for Equality of Means | ||||||
| Variables | t | df | Sig. (2-tailed) | 95% Confidence Interval of the Difference | ||
| Lower | Upper | |||||
| Sex | Equal variances assumed | -0.697 | 29 | 0.491 | -2.62307 | 1.28974 |
| Equal variances not assumed | -0.68 | 21.624 | 0.504 | -2.703 | 1.36967 | |
| Brain involvement | Equal variances assumed | -0.944 | 29 | 0.353 | -2.84002 | 1.04616 |
| Equal variances not assumed | -0.992 | 27.117 | 0.33 | -2.75174 | 0.95788 | |
| Optic neuritis | Equal variances assumed | -0.799 | 29 | 0.431 | -3.34579 | 1.46579 |
| Equal variances not assumed | -0.757 | 7.165 | 0.473 | -3.86341 | 1.98341 | |
| VEP abnormalities | Equal variances assumed | 0.225 | 29 | 0.823 | -1.95219 | 2.43589 |
| Equal variances not assumed | 0.222 | 11.903 | 0.828 | -2.13609 | 2.61979 | |
| AQP4 seropositivity | Equal variances assumed | 2.055 | 29 | 0.049 | 0.01066 | 4.30482 |
| Equal variances not assumed | 1.833 | 8.477 | 0.102 | -0.53016 | 4.84563 | |
| Pattern of myelitis | Equal variances assumed | 2.43 | 29 | 0.022 | 0.35259 | 4.09979 |
| Equal variances not assumed | 2.496 | 19.058 | 0.022 | 0.36013 | 4.09225 | |
| Relapse | Equal variances assumed | -0.296 | 29 | 0.769 | -2.25484 | 1.68466 |
| Equal variances not assumed | -0.31 | 26.825 | 0.759 | -2.17501 | 1.60483 | |
Discussion
Non compressive myelopathies comprise of a wide spectrum of disorders including infectious, demyelinating, nutritional, paraneoplastic, radiational and others. The prognosis and the therapeutic outcome vary considerably across this spectrum. Therefore, it is of paramount importance to establish the etiology of non-compressive myelopathy. Onset of the disease helps to guide the evaluation as most of the infectious and demyelinating disorders have acute to subacute onset, while as chronic causes include degenerative, nutritional and some infections like HIV, HTLV and syphilis.
Age distribution of our cases ranged from 2 years to 55 years with a median age of 32.35 years. 62.5% of cases were between 25-50 years, 32.5% were below 25 years and only 5% of cases were above 50 years of age. This indicates a higher prevalence of non-compressive myelopathies in younger and middle-aged population compared to elderly. Kamble et al reported median age of 33 years in his study with a range of 6-65 years. Prabhakar et al reported the mean age of presentation to be 34.45 years ranging from 14-82 years [1].
Male female ratio in our case study was 2:1. In the demyelinating subgroup, the ratio was 1.6:1. The male female ratio in the study by Kamble et al was reported as 1.5:1 [2]. Mishra et al reported a higher female to male ratio 1.8:1 in their study of spectrum of demyelinating disorders of spinal cord [3]. Female preponderance for demyelinating diseases is only known in multiple sclerosis and relapsing NMOSD [4]. Low prevalence of MS in our study group could explain the unexpected higher male preponderance of demyelinating disorders of spinal cord.
87.5% of cases had an acute to subacute presentation. Only 12.5% had a chronic presentation. Symptom duration before presentation ranged from minimum of 1 day in a case of acute transverse myelitis to a maximum of 1095 days in a case of HSP. Das et al in a similar study found 31.5% of non-compressive myelopathy presenting within 2 weeks of symptom onset, 40% presenting within 2 to 4 weeks and 29.5% presenting within 1 to 6 months [5]. Kayal et al reported 63.6% of cases of non-compressive myelopathy having acute to subacute presentation and 36.4% having chronic presentation. The higher proportion of cases having acute to subacute presentation reflects the dominance of myelitis cases in our study population.
Clinical presentation varied depending upon the pattern of involvement of spinal cord. Complete cord syndrome was however, the most common presentation seen in 35% of cases. Anterior cord syndrome was seen in 20%, central cord in 10%, tract specific pattern in 7.5%, posterior and posterolateral cord syndrome in 5 percentage each, conus-cauda pattern in 2.5%. Mixed pattern was seen in 15% that also included segmental neuromyotonia and sensory loss in one lower limb. Prabhakar et al in a similar study reported 81 percentage cases having a symmetrical form of myelitis and asymmetric myelitis in 19% [1]. Pyramidal tract involvement was reported in all cases of non-compressive myelopathy by das et al, alone in 29.6 percentage cases and in combination with other tract involvement in rest of the cases [5].
In our study, NMOSD was the most common diagnosis seen in 35% followed by ITM - idiopathic transverse myelitis in 20% and multiple sclerosis in 15%. Infectious myelitis was seen in 10% that included 2 cases of tubercular myelitis without any spine involvement, 1 case of zoster myelitis and 1 case of scrub typhus associated myelitis. Other diagnoses included ADEM in 7.5%, HSP, B12 deficiency, copper deficiency, SLE and undefined etiology in 2.5 percentage each. These findings are in consistency with the results from other similar studies carried out in India. NMOSD, MS, ADEM, ITM, Infections, SLE and spinal cord ischemia were the causes of acute myelopathy in a study by Kayal et al [6]. In the same study, infections, nutritional, radiation, paraneoplastic, sarcoidosis and AV fistula were reported among the causes of chronic myelopathy. However, our results are different from somewhat older studies like that of das et al where demyelinating etiology was reported in 19.5% only possibly because of the lack of established criteria for NMOSD at that time [5].
Preceding history of a febrile illness was noted in 22.5% of cases in the demyelinating subgroup. History of vaccination was reported in 6.45%(n=3) cases, one of them having received covid vaccination preceding his illness. Association between preceding viral illness or vaccination and demyelinating forms of myelitis has been reported in literature with a variable incidence. More frequently, however, in idiopathic transverse myelitis it has been reported to range from 30-60% [7-9]. There are many case reports in literature citing an association between Covid-19 vaccination and myelitis [10].
Analyzing the data separately for demyelinating subgroup, we found NMOSD to be the most common demyelinating form of myelitis with an incidence of 45.16%. The incidence of ITM, MS and ADEM was 25.80%, 19.35% and 9.67% respectively. A study by Li et al in a chinese population revealed NMOSD to be the cause in 40.3%, MS in 16.4% and ITM in 43.3% [11]. Studies from African countries like Ethiopia and Niger have reported low prevalence of demyelinating diseases as a cause of non-compressive myelopathy [12,13]. Indian studies, however, have reported high incidence of NMOSD compared to MS and other etiologies. Mishra et al reported an incidence of 55% for NMOSD, 10% for MOG-associated disease and 5% for MS while studying the demyelinating disorders of spinal cord at a tertiary care center [3].
The data in our study showed that dorsal cord was the most common site of involvement in demyelinating group of myelopathies with an incidence of 38.7% followed by cervical 32.22% and cervicodorsal 19.35%. On comparing the results between MS and NMOSD, cervical region was more commonly involved in MS with a frequency of 50%, whereas dorsal with or without cervical cord involvement was more frequent in NMOSD with a frequency of 75%. These results are consistent with similar studies worldwide. Li et al reported the incidence of dorsal cord involvement to be 74.1% in NMO associated transverse myelitis, 88.1% in relapsing form of LETM and 54.5% in MS related acute transverse myelitis. Interestingly, this study was carried out before 2015 NMOSD criteria, therefore term NMOSD was not used in this study [11]. In a study of patients presenting with LETM, Choudhary et al found the dorsal region to be involved with a frequency of 93% and cervical with 50%. The incidence of MS in their study was reported to be 9.37% [14].
In our study, Longitudinally-extensive transverse myelitis (LETM) was the most common pattern seen in 67.7% of cases of demyelinating causes of myelitis. Short transverse myelitis (STM) was seen in 22.6% and MRI imaging was normal in 3 cases (9.7%). The length of lesion in abnormal MRI patients ranged from 1 vertebral segment to 14 vertebral segments with mean length being 3.52 (SD=2.75). Incidence of LETM in the study by Li et al was 54.54% and 70% in the study by Mishra et al. Kayal et al reported an overall incidence of longitudinally extensive lesions in all forms of non-compressive myelopathy to be 63.8% including demyelinating and non-demyelinating etiologies. Debette et al reported 75% of the spinal lesions in NMO and 11.4% in MS extending more than 2 vertebral segments. In a study of LETM, Choudhary et al reported 43.8% of lesions showing involvement of more than 10 segments of spinal cord.
In 61.3% of the cases in our study, lesions involved more than 2/3rd of the cross section of spinal cord, accounting for significant disability at presentation. In 22.6 percentage cases, cord involvement was between 1/3rd to 2/3rd and 6.5% had less than 1/3rd involvement. As per the literature, MS lesions usually involve less than half of the cross-sectional area whereas NMOSD lesions are known to involve more than half of the cross-sectional area of spinal cord [15]. ITM, however, can have any range of involvement. In our case study, most of the ITM lesions showed a morphology similar to NMOSD lesions both with regard to the length of lesion as well as the cross-sectional involvement.
Patients presenting with first episode of LETM form of ITM are known to progress to NMOSD on follow up. In our case study, 3 cases of LETM at first presentation fulfilled the criteria for NMOSD. Out of the remaining 21 cases of LETM, 7 showed progression to NMOSD on follow up. Among the non-LETM group, only 1/11 case progressed to NMOSD. These findings match with the results from the study by Li et al who showed that 37.5 percentage cases of LETM progressed to NMOSD on follow up [11]. In a study by Sechi et al, 14% of cases initially diagnosed as ITM-LETM were diagnosed to have NMOSD on follow up by demonstrating seropositivity for aquaporin-4 or MOG antibody [16].
Hyperintense signal on T2WI and STIR images was the most common abnormality found in 94.59% (n = 35/37) lesions in demyelinating subgroup. T1 hypointensity was seen in 8.10% (n = 3/37) and cord swelling was noted in 5.4% (n = 2/37) each. Contrast enhancement was seen in 18.91% (n = 7/37) cases, all of them in the NMOSD subgroup. Dorsal spine was the most common affected site in demyelinating causes of myelopathy as well as in NMOSD. However, in MS cases, cervical site was more common. Various studies have shown dorsal cord to be the commonly affected site in NMOSD and cervical cord in MS. Dumrikarnlert et al in a study of spinal imaging of demyelinating diseases showed cervicothoracic to be the most common affected site in NMOSD, dorsal cord in ITM and cervical cord in MS. In the same study, LETM was seen in 96% of seropositive NMOSD, 67% of seronegative NMOSD and 59% of ITM. The same author reported 63% and 78 percentage cases of seropositive and seronegative NMOSD having 30-70% cross sectional involvement of spinal cord and 59% and 58% of cases in ClS and MS group having less than 30% cross sectional involvement of spinal cord [17]. Pekcewik et al reported 49 percentage cases of LETM having an etiology other than NMOSD including infectious, vascular, paraneoplastic, sarcoidosis and others. However, bright spotty lesion on T2 axial weighted image was found to be the most distinctive finding in NMOSD with a frequency 86.1%, T1 hypointense lesions with a frequency of 70.3% contrast enhancement with a frequency of 65.1%. The author also reported that NMO lesions constituted 58.4% of the lesions involving more than 50% of the spinal cord cross section. Cervical and dorsal cord involvement was noted with equal frequency in their study while as cervical site was reported to be more frequent in MS [18].
Optic neuritis had a cumulative prevalence of 28.26% including attacks at onset and in relapses. Optic neuritis was found to be proportionately higher in MS (42.85%) than NMOSD (38.46%). Bilateral optic neuritis was seen in 2 cases in NMOSD and none in MS. VEP abnormalities were seen in those with clinical evidence of optic neuritis and even in visually asymptomatic patients. The overall prevalence of VEP abnormalities in the demyelinating group was 39.11% that included prolonged P100 latencies in 23.91%, prolonged P100 latency with reduced amplitude in 10.86% and absent waveforms in 4.34%. In an earlier study before the development of well-established NMOSD criteria, Pandit et al in a study from South India reported MS to be the most common etiology in optic neuritis representing 49% of patients. NMO contributed to 5% of the cases only [19]. Ambika et al found the incidence of AQP4 antibody seropositivity to be 20% in a study of optic neuritis [20]. Choudhary et al whose study focused on LETM form of myelitis only reported the incidence of optic neuritis to be 34%, with NMOSD contributing to the bilk of cases as expected [14]. Wingerchuk et al and Papais et al have reported nearly 50 percentage cases of NMOSD presenting with optic neuritis, 20 percentage being bilateral [21,22]. Severe visual deficit has been found to occur with a frequency of 80% in NMOSD [23]. In a comparative study of NMOSD and MS, Bukhari et al reported bilateral optic neuritis to be more common in MS and longer P100 latency in MS as compared to NMOSD [24]. Naismith et al reported the sensitivity of VEP study to be higher than OCT in detecting clinical as well as subclinical optic neuritis [25].
Concomitant brain lesions were seen in 12 cases. Subcortical region was the most common site with incidence of 25.8% followed by brainstem with an incidence of 22.58%. Other sites included periventricular in 16.12%, cerebellum in 12.9%, corpus callosum and juxtacortical in 6.5 percentage each, internal capsule and cortex in 3.22 percentage each. Periventricular, callosal, juxtacortical and cerebellar lesions occurred mostly in MS; whereas in ADEM and NMOSD lesions were more prevalent in subcortical and brainstem location. These findings are consistent with the usual distribution patterns of demyelinating lesions reported in literature [26].
CSF cell count in demyelinating group cases ranged from 0 to 45 with a mean of 21.13 and a mean lymphocyte count of 82.90%. CSF protein ranged from 47 mg/dl to 174 mg/dl with a mean of 70.79. CSF OCB was positive in 5/31 cases of myelitis, 3 of which had multiple sclerosis. CSF IgG index was high in 2 cases of MS and was normal in other groups. These results match with the overall pattern of CSF findings in demyelinating disorders. Mild pleocytosis is known to occur in all demyelinating diseases with usual cell count being less than 50/microliter. CSF protein has been reported to be raised up to 100 mg/dl in 50% of NMOSD and MS cases. However, OCB and elevated IgG index are reported to be more frequent in MS than other demyelinating disorders [27-29]. Dumrikarnlert et al reported incidence of OCB positivity to be 50% in MS and 12.5% in NMOSD [17].
The overall incidence of AQP4 seropositivity in NMOSD cases in our study was found to be 26.92% and in NMOSD subgroup was 50%. AQP4 antibody seropositivity was found to be 68.75% in demyelinating cord diseases by Li et al and the incidence ranged from 10% in MS related transverse myelitis to 90% in NMO related transverse myelitis [11]. Mishra et al reported an overall incidence of AQP4 antibody seropositivity to be 28% [3].
The median EDSS at presentation in our cases was 7.5 and at follow up was 3.5. EDSS in MS and STM form of ITM was found to be lower than other groups. Mean EDSS at presentation for ADEM, NMOSD and ITM-LETM was recorded to be 9.5, 8.0 and 7.5 respectively. Median EDSS on follow up in NMOSD was 5.5 in NMOSD, 5.0 in LETM and 3.5 in MS. The data from the study conducted by Sepulveda et al in LETM cases showed mean EDSS of 7.0 at presentation indicating that LETM forms of transverse myelitis present with a high disability at onset [30]. Cobo Calvo et al who used the modified Rankin scale (mRS) to grade the disability in their study found that mean mRS was 2.8 in idiopathic transverse myelitis and 2.45 in MS related myelitis [31]. Studies on outcome of myelitis on follow up have consistently shown a higher disability in the NMOSD and LETM form of ITM. Median EDSS of 4.0 in the NMOSD group and 2.0 on follow up was reported by Khalilidehkordi et al [32]. Similar results were reported by Dumrikarnlert et al [17]. Sepulveda et al reported median EDSS of 3.75 and 3.0 in the relapsing and monophasic forms of transverse myelitis on follow up [30]. Higher disability in MS subgroup in our study compared to other studies can be attributed to fewer in MS subgroup and most of them having a high lesion burden.
All episodes of myelitis whether at onset or relapse were treated with intravenous methylprednisolone in a dose of 1000 mg/day for 5 days. Among the NMOSD group, Rituximab was used as maintenance therapy in 58.33% (n=7/12) and azathioprine was used in the rest. Rituximab is considered as the first line maintenance therapy in NMOSD considering its potential in achieving the maximum reduction in annualized relapse rate compared to oral agents [33,34]. However, in developing nations, oral agents continue to be used as first line agents in many health centres. Singh et al in their study of 106 NMOSD patients used Azathioprine in 88 cases while as rituximab was used in only 8 cases [35]. Idiopathic transverse myelitis does not warrant maintenance therapy unless it is recurrent. Maintenance therapy of at least 2 years is recommended for patients who have two or more relapses [36].
MS is found to have an overall low prevalence in India. We recruited 6 cases of myelitis secondary to MS. DMF was used as maintenance agent in 50% (n=3/6) cases, teriflunomide in 2 and rituximab in one patient. Decision regarding selection of disease modifying agent in MS always takes into consideration the severity of the disease, availability of the agent and patient preference. With the development of oral drugs, injectable agents have become less acceptable to the patients.
14 relapse events were recorded in patients on follow up that ranged from 3 months to 36 months. Myelitis and optic neuritis were the presentation of relapses in NMOSD whereas brain involvement and optic neuritis were more common in MS relapses. Most of the relapses occurred prior to start of maintenance therapy. Choudhry et al in a study of LETM, reported that more than 50% of patients in the NMOSD group had relapses while as in the other causes of LETM including MS, it was only 11. Since many of our patients did not complete 1 year of follow up, it was not deemed reasonable to calculate the annualized relapse rate. In a large retrospective study, Singh et al showed that 25% of NMOSD cases relapsed within 4.1 months, 50% relapsed within 17.3 months and 75% relapsed within 41.6 months with optic neuritis and LETM representing the majority of the relapses [35].
In our study, we found that CSF cell count, cross-sectional involvement of spinal cord, pattern of myelitis and aquaporin-4 seropositivity were found to be predictive of the severity at onset i.e., EDSS at presentation. EDSS at onset, cross-sectional involvement of spinal cord and pattern of myelitis were found to be predictive of long-term disability i.e., EDSS at last visit. E Li et al found age and presence of longitudinally extensive lesions on MRI as predictive of disability [11]. Debette et al reported association of an unfavourable functional outcome with the initial bad functional score, centrally located lesions on MRI, etiology being NMO or systemic disease and with increasing age [37]. Age at onset and EDSS score at onset were similarly reported to be predictive of disability in a study of NMOSD patient by drulovic et al in addition to several other factors which included time from onset to diagnosis, time from onset to maintenance treatment, length of spinal lesion and area postrema onset [38]. In another study, Sepulveda et al while studying the prognostic factors in LETM, reported EDSS at onset to be the only significant factor associated with the final outcome. The author also concluded that length of spinal lesion was predictive of the relapses in future [39].
Among the non-demyelinating causes of non-compressive myelopathy, we found 2 cases of tuberculous myelitis, 1 case of zoster myelitis, 1 case of scrub myelitis and 1 case each of SLE, B12 deficiency, copper deficiency and HSP. In one of the cases, etiology remained undefined. This patient didn’t fulfil the criteria for ITM as the progression of symptoms continued beyond 2 months. MRI showed a T2 hyperintense lesion extending 4 vertebral segments. Complete evaluation for demyelinating, infectious, nutritional and systemic diseases proved negative.
Tuberculous myelitis in our cases was confirmed by positive CBNAAT assay for MTB DNA. One of the patients had concomitant pulmonary tuberculosis as well though, that was diagnosed 1 month prior to myelopathy. Spinal cord involvement in tuberculosis is most of the times secondary to involvement of spine and arachnoid involvement. However, cases have been reported of tuberculosis presenting as transverse myelitis [40]. Diagnosis is usually established by demonstrating the presence of acid-fast bacilli or MTB DNA in CSF by various methods. However, it can still be considered in cases of ITM where the diagnosis of tuberculosis is not proven but symptoms still show a progressive course despite pulse steroids but after ruling out all other known causes of myelopathy. Feng et al conducted a study of ITM patients who didn’t respond to intravenous steroids and in whom clinical suspicion of tuberculosis was made. After ruling out all other causes, antitubercular therapy was started in these patients. At 24 months follow up, 73.13% of cases were demonstrated to have improvement [41].
Patient with zoster myelitis presented within 4 weeks of onset of zoster rash that was in the T10 dermatome. Patient presented with motor symptoms and sensory abnormalities for vibration and proprioception. The patient was immunocompetent and had not received any immunosuppressant medication prior to illness. Patient had clinical improvement with acyclovir therapy. Devinsky et al reported all cases of zoster myelitis to be immunocompromised in a study involving both clinical and pathological diagnosis. The author also reported that symptoms were mostly ipsilateral to the presence of rash and 4 cases had evidence of zoster vasculitis on autopsy [42].
Scrub typhus myelitis diagnosis was established by high O. tsutsugamushi titres in the serum by indirect fluorescence antibody assay. Patient presented with febrile illness starting with weakness of lower limbs on the third day of fever. MRI showed a long segment myelitis extending 6 vertebral segments in the dorsal region. Patient was treated for scrub typhus and showed complete recovery within 2 weeks of treatment. Myelitis due to scrub typhus is rare and is cited as case reports only in the literature [43-45].
We detected one case of myelopathy due to B12 deficiency and one due to copper deficiency. Both of these cases presented with posterolateral cord syndrome. B12 deficiency case showed improvement with B12 therapy and at last visit had a mild proprioceptive deficit only. B12 deficiency was attributed to his strict vegetarian dietary habit as no other cause could for B12 deficiency could be established. Copper deficiency patient was treated with oral copper therapy for 3 months and showed near complete resolution of his deficit. B12 deficiency is reported as not an infrequent cause of chronic non-compressive myelopathy in studies from India, considering the strict vegetarian dietary habits of many communities. Prabhakar et al reported the incidence of B12 deficiency related myelopathy to be 14.03% [1]. The incidence was reported to be 7.2% by kayal et al [6]. Copper deficiency related myelopathy is very rare and only case reports are found in literature [46,47]. The main causes of copper deficiency have been reported to be bariatric surgery, excessive consumption of zinc and malabsorption syndromes. Low ceruloplasmin levels have been detected in adult patients presenting with hypocupremic myelopathy [47]. None of these causes was detected in our patient.
Myelitis due to SLE was reported in one of the cases in our study. The patient was a middle-aged male and was found to have renal manifestations as well. He was found to be positive for ANA and anti dsDNA antibody. MRI showed T2 hyperintense lesion spanning 2 vertebral segments in the dorsal spine. Patient was treated with pulse methylprednisolone and was referred to nephrology department for further management. Myelitis is considered to be an uncommon manifestation of SLE. High ANA titres have been reported in SLE related myelitis. Longitudinally extensive transverse myelitis has found to be more common manifestation than short transverse myelitis in the reported cases [48,49].
One case was diagnosed with HSP. The patient presented with progressive spastic paraparesis over years with no history of any family member being affected in 3 generations. Evaluation for any secondary cause was negative. Family declined to go for a genetic study. Prabhakar et al reported an incidence of 5.2% for HSP in a study of non-compressive myelopathies [1].
Conclusion
Demyelinating diseases constitute the major proportion of non-compressive myelopathies. Despite the availability of assays for AQP4 antibody and MOG antibody and the revised criteria for NMOSD, still a significant number of cases continue to be labelled as idiopathic transverse myelitis. NMOSD and LETM(ITM) being the most common cause of myelitis in the study population contributed to significant long-term disability. CSF cell count, cross sectional involvement of cord, pattern of myelitis (LETM vs Non-LETM) and AQP4 seropositivity were found to be predictive of the disability at onset i.e., EDSS at presentation. The final outcome i.e., EDSS on last visit was found to have significant correlation with pattern of myelitis, AQP4 seropositivity, cross sectional involvement of cord and EDSS at presentation.
Study limitations
The number of cases in our study was small that could impact the comparative data analysis. The follow up was short and in many of the cases was less than 1 year which could bias the assessment of annualized relapse rates. Lack of serological tests for AQP4 and MOG antibody due to non-affordability in some cases could be a factor in delayed diagnosis and delayed start of maintenance therapy.
Declarations
Study Funding: None.
Declaration of Competing Interest:
The authors have no conflict of interest.
Acknowledgments: None
Author contributions:
G.P. Mondal and S. A. Paul developed the concept. R. Bhattacharyya designed the study. C. Patra, D. Roy and J. Kiran collected the data. S. Das and H. Krishna contributed to the analysis and interpretation of data. K.C. Ghosh conducted literature review and was involved in discussion. S. A. Paul analyzed the data and performed the statistical analysis.
Declaration Of Conflict of Interests
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
References
- Prabhakar S. et al. (1999). Non-compressive myelopathy: clinical and radiological study. Neurol India. 47:294-299.
Publisher | Google Scholor - Kamble S, Sardana V, Maheshwari D, Bhushan B. & Ojha P. (2019). Etiological Spectrum of Non-compressive Myelopathies in Tertiary Care Centre. J Assoc Physicians India. 67:14-16.
Publisher | Google Scholor - Mishra S, R, B. & M, G. (2020). Spectrum of spinal cord demyelination: A retrospective study in a tertiary care hospital of eastern India. IP Indian Journal of Neurosciences. 6:124-127.
Publisher | Google Scholor - Wingerchuk D. M. (2009). Neuromyelitis optica: effect of gender. Journal of the neurological sciences 286:18-23.
Publisher | Google Scholor - Das K. et al. (1999). Profile of non-compressive myelopathy in eastern India: a 2-year study. Acta Neurol Scand 99:100-105.
Publisher | Google Scholor - Kayal A. K. et al. (2017). Etiological profile of noncompressive myelopathies in a tertiary care hospital of Northeast India. Ann Indian Acad Neurol, 20:41-50.
Publisher | Google Scholor - Jeffery D. R., Mandler R. N. & Davis L. E. (1993). Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol. 50:532-535.
Publisher | Google Scholor - Paine R. S. & Byers R. K. (1953). Transverse myelopathy in childhood. AMA Am J Dis Child. 85:151-163.
Publisher | Google Scholor - Altrocchi P. H. (1963). ACUTE TRANSVERSE MYELOPATHY. Arch Neurol 9, 111-119.
Publisher | Google Scholor - Gao J.J. et al. (2021). Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines (Basel) 9:1008.
Publisher | Google Scholor - Li R. et al. (2011). Acute transverse myelitis in demyelinating diseases among the Chinese. J Neurol 258:2206-2213.
Publisher | Google Scholor - Assadeck, H. et al. (2019). Inflammatory demyelinating diseases of the central nervous system in Niger. Rev Neurol (Paris), 175:261-268.
Publisher | Google Scholor - Fidèle N. J. & Amanuel A. (2016). Spectrum of nontraumatic myelopathies in Ethiopian patients: hospital-based retrospective study. Spinal Cord 54:604-608.
Publisher | Google Scholor - Choudhary A, Bhargava A, Khichar S & Pradhan S. Etiological spectrum, clinico-radiological profile and treatment outcomes of longitudinally extensive transverse myelitis - A prospective study from Northwest India. J Neuroimmunol 351:577456.
Publisher | Google Scholor - Marrodan M, Gaitán M. I. & Correale J. (2020). Spinal Cord Involvement in MS and Other Demyelinating Diseases. Biomedicines 8:130.
Publisher | Google Scholor - Sechi E. et al. (2019). Aquaporin-4 and MOG autoantibody discovery in idiopathic transverse myelitis epidemiology. Neurology 93:414-420.
Publisher | Google Scholor - Dumrikarnlert C. et al. (2017). The characteristics of spinal imaging in different types of demyelinating diseases. Journal of the neurological sciences 372:138-143.
Publisher | Google Scholor - Pekcevik Y. et al. (2016). Differentiating neuromyelitis optica from other causes of longitudinally extensive transverse myelitis on spinal magnetic resonance imaging. Mult Scler 22:302-311.
Publisher | Google Scholor - Pandit L. et al. (2012). Optic neuritis: experience from a south Indian demyelinating disease registry. Neurol India 60:470-475.
Publisher | Google Scholor - Ambika S, Balasubramanian M, Theresa L, Veeraputhiran A & Arjundas D. (2015). Aquaporin 4 antibody [NMO Ab] status in patients with severe optic neuritis in India. Int Ophthalmol 35:801-806.
Publisher | Google Scholor - Wingerchuk D. M, Hogancamp W. F, O’Brien P. C & Weinshenker B. G. (1999). The clinical course of neuromyelitis optica (Devic’s syndrome. Neurology. 53:1107-1114.
Publisher | Google Scholor - Papais-Alvarenga R. M. et al. (2008). Clinical course of optic neuritis in patients with relapsing neuromyelitis optica. Arch Ophthalmol. 126:12-16.
Publisher | Google Scholor - Beck R. W. et al. (1992). A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 326:581-588.
Publisher | Google Scholor - Bukhari W. et al. (2020). The clinical profile of NMOSD in Australia and New Zealand. Journal of Neurology. 267.
Publisher | Google Scholor - Naismith, R. T. et al. (2009). Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 73:46-52.
Publisher | Google Scholor - Filippi M. et al. (2019). Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain. 142:1858-1875.
Publisher | Google Scholor - Sellner J. et al. (2010). EFNS guidelines on diagnosis and management of neuromyelitis optica. European journal of neurology. 17:1019-1032.
Publisher | Google Scholor - Jarius S. et al. (2011). Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. Journal of the neurological sciences. 306:82-90.
Publisher | Google Scholor - Jarius S. et al. (2020). Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: Results from 163 lumbar punctures in 100 adult patients. Journal of neuroinflammation. 17.
Publisher | Google Scholor - Sepulveda M. et al. (2018). Epidemiology of NMOSD in Catalonia: Influence of the new 2015 criteria in incidence and prevalence estimates. Mult Scler 24:1843-1851.
Publisher | Google Scholor - Cobo Calvo Á. et al. (2013). Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurology 13:135.
Publisher | Google Scholor - Khalilidehkordi E. et al. (2020). Relapse Patterns in NMOSD: Evidence for Earlier Occurrence of Optic Neuritis and Possible Seasonal Variation. Frontiers in neurology, 11.
Publisher | Google Scholor - Bedi, G. S. et al. (2011). Impact of rituximab on relapse rate and disability in neuromyelitis optica. Mult Scler. 17:1225-1230.
Publisher | Google Scholor - Nikoo Z, Badihian S, Shaygannejad V, Asgari N. & Ashtari F. (2017). Comparison of the efficacy of azathioprine and rituximab in neuromyelitis optica spectrum disorder: a randomized clinical trial. Journal of neurology. 264:2003-2009.
Publisher | Google Scholor - Singh N. et al. (2021). Clinical Features, Gender Differences, Disease Course, and Outcome in Neuromyelitis Optica Spectrum Disorder. Ann Indian Acad Neurol. 24:186-191.
Publisher | Google Scholor - Krishnan C, Kaplin A. I, Deshpande D. M, Pardo C. A. & Kerr D. A. (2004). Transverse Myelitis: pathogenesis, diagnosis and treatment. Front Biosci 9:1483-1499.
Publisher | Google Scholor - Debette S. et al. (2009). Long-term outcome of acute and subacute myelopathies. J Neurol. 256:980-988.
Publisher | Google Scholor - Drulovic J. et al. (2019). Long-term outcome and prognosis in patients with neuromyelitis optica spectrum disorder from Serbia. Multiple sclerosis and related disorders, 36.
Publisher | Google Scholor - Sepúlveda M. et al. (2013). Analysis of prognostic factors associated with longitudinally extensive transverse myelitis. Mult Scler 19:742-748.
Publisher | Google Scholor - Sahu S. K, Giri S. & Gupta N. (2014). Longitudinal extensive transverse myelitis due to tuberculosis: a report of four cases. J Postgrad Med. 60:409-412.
Publisher | Google Scholor - Feng Y. et al. (2011). Mycobacteria Infection in Incomplete Transverse Myelitis Is Refractory to Steroids: A Pilot Study. Clinical and Developmental Immunology, e501369.
Publisher | Google Scholor - Devinsky O, Cho E. S, Petito C. K. & Price R. W. (1991). Herpes zoster myelitis. Brain. 114(3):1181-1196.
Publisher | Google Scholor - Mahajan S. K. et al. (2016). Scrub typhus with longitudinally extensive transverse myelitis. Journal of Vector Borne Diseases 53:84.
Publisher | Google Scholor - Lee K. L, Lee J. K, Yim Y. M, Lim O. K & Bae K. H. (2008). Acute transverse myelitis associated with scrub typhus: case report and a review of literatures. Diagnostic Microbiology and Infectious Disease. 60:237-239.
Publisher | Google Scholor - Ryu H.-S. et al. (2020). Acute transverse myelitis following scrub typhus: A case report and review of the literature. J Spinal Cord Med 43:548-551.
Publisher | Google Scholor - Plantone, D. et al. (2015). Copper deficiency myelopathy: A report of two cases. J Spinal Cord Med 38:559-562.
Publisher | Google Scholor - Kumar N, Crum B, Petersen R C, Vernino S. A & Ahlskog J. E. (2004). Copper Deficiency Myelopathy. Archives of Neurology. 61:762-766.
Publisher | Google Scholor - Hryb J. P. et al. (2016). Myelitis in systemic lupus erythematosus: clinical features, immunological profile and magnetic resonance imaging of five cases. Spinal Cord Ser Cases 2:16005.
Publisher | Google Scholor - Oiwa H. et al. (2017). A report of three cases with lupus myelitis. Eur J Rheumatol 4:148-150.
Publisher | Google Scholor