Research Article
Clinical Presentation and Management of Primary Mycotic Aortic Aneurysms: A Single Center Experience and Review of the Literature
- Badreldin Mohamed 1*
- Sami Taha 2
- L. Navaro 1
- M. Cusso 1
- T. Barakat 1
- S. Babikir 1
- D. Taran 1
- C. Marson 1
- T. Ojimba 1
- D. Mirghani 2
- S. Naqvi 2
- R. Eifell 1
1 Department of General and Vascular Surgery, Cumberland Infirmary, Newtown Road, Cumbria, UK.
2 General Surgery, Cumberland Infirmary, Carlisle, UK.
*Corresponding Author: Badreldin Mohamed, Department of General and Vascular Surgery, Cumberland Infirmary, Newtown Road, Cumbria, UK.
Citation: B Mohamed, S Taha, L Navaro, M Cusso, T Barakat, et al. (2023). Clinical Presentation and Management of Primary Mycotic Aortic Aneurysms: A Single Center Experience and Review of the Literature. International Journal of Medical Case Reports and Reviews, BRS Publishers. 2(1); DOI: 10.59657/2837-8172.brs.23.005
Copyright: © 2023 Badreldin Mohamed, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 05, 2022 | Accepted: December 06, 2022 | Published: January 02, 2023
Abstract
Background: Primary mycotic aortic aneurysm (PMAA) is a localized dilation of the aortic wall caused by direct spread of bacteria via the bloodstream to the aortic wall and subsequent development of an aneurysm. The term mycotic aortic aneurysm was first used to describe a patient with four aortic aneurysms which have a mushroom-like appearance, and the term has since been used.
Aim: To conduct a literature review and to report a series of cases of infra-renal mycotic aortic aneurysms, their presentation and subsequent outcome and management.
Methods: Information was gathered retrospectively from medical records for patients who were over the age of 18 and who had presented with PMAAs at Cumberland infirmary o from April 2016 to July 2022.
Results: A total of six patients were assessed in our study, mean age was 57-88 years. Presenting complains were: Back pain (n=2), abdominal pain (n=1), fever (n=4), collapsed (n=2), confusion (n=1) and other clinical presentations were leg pain, feeling unwell and malaise each reported in one patient each. Potential risk factors for PMAA identified were malignancy (n=2), diabetes mellitus (n=2), chronic kidney disease (n=2), immunosuppressive medication (n=2) and alcohol misuse (n=1). Potential sources of infection were retroperitoneal inflammation (n=1), pyelonephritis (n=1), retroperitoneal /psoas infection (n=2), duodenitis (n=1), systemic sepsis (n=1). Management can be surgical intervention such as Endovascular aortic repair (EVAR) (n=2), or axillo-bifemoral bypass (n=2) all of them had post-operative antibiotics or only antibiotics (n=3), however two patients of them died.
Conclusion: PMAAs are rare but they have high risk of morbidity and mortality. The diagnosis is challenging but CT A-abdomen and pelvis remains valuable for diagnosis. Surgery, either bypass or EVAR with antibiotics, is a cornerstone for management, however other alternatives can be considered.
Keywords: primary mycotic aortic aneurysm; mycotic aneurism; bacteraemia; atherosclerotic
Introduction
Primary mycotic aortic aneurysm (PMAA), or infected aortic aneurysm, is a localized dilation of the aortic wall caused by direct spread of bacteria via the bloodstream to the aortic wall [1, 2]. Following infection of the aortic wall, there is subsequent degradation of the aortic intima and then media leading to development of an aneurism [3].
Sir William Osler was the first to coin the term mycotic aneurism in 1885 to describe a patient with a valve vegetation and four aortic aneurysms which, in his eyes had a mushroom-like appearance and not because of their underlying microbial etiology. The term has since been used to describe the pathology [4, 5]. However, Sir Osler wasn’t the first to describe the relationship between infection and aneurysm development as Oggle, et al. reported the relationship between aneurysm development and endocarditis in 1866 and Tufnell, et al. in 1853 reportedly came to a similar conclusion [6].
The following years saw many authors using different terminologies and definitions such as embolomycotic aneurysm [6]. Causes of aneurysm due to infection were not solely endocarditis but also trauma and other infection as reported by Lewis, et al. in 1909, and Blum, et al. in 1962 used the term cryptogenic mycotic aneurysm to describe bacteria invading the endothelium at the site of atherosclerotic disease [6]. The term septic aortic pseudo aneurysm used by Luo, et al. in 2003 for aortic pseudo aneurysm caused by infection with or without bacteraemia, excluding fusiform aneurysms [6].
Since Osler first described the mycotic aneurysm until now, the term MAA has gone through a series of changes one of them famously being that the actual cause of the mycotic aneurysm was bacterial either from syphilis causing aortitis from occlusion of the terminal vessels or distant emboli of endocarditis. However, after introduction of antibiotics this has changed dramatically as bacteremia of atherosclerotic aorta is the commonest cause of MAA [6-8].
MAA incidence accounts for 1-3% of all arterial aneurysms and is potentially life threatening [9].
Materials & Methods
Information was gathered retrospectively from medical records for patients of either sex who were over the age of 18 years and who had presented with primary mycotic aortic aneurisms (PMAAs) at Cumberland infirmary (Carlisle, United Kingdom) from April 2016 to July 2022.
The aim of the study is to report a series of cases of infra-renal mycotic aortic aneurysms at Cumberland infirmary, their presentation and subsequent outcome and management. Also, aims to conduct a literature review to compile an overview of modern management of PMAAs looking specifically at surgical treatment of PMAAs and associated outcomes in terms of survival, and antibiotic treatment and other factors associated with adverse or favorable outcomes.
We included all patients presented with infrarenal PMAAs to Cumberland infirmary between April 2016 and July 2022. The diagnostic criteria for PMAA were used: PMAA is an aneurysm specific to the aorta as a result of infection, while the diagnosis is based on a combination of clinical presentation (pain, fever, concomitant infection, and patient comorbidities and/or immunosuppressed state), laboratory results (raised inflammatory parameters, including C reactive protein [CRP], white blood cell [WBC] count, and positive blood cultures ), and imaging findings on computed tomography, such as saccular, eccentric, or multilocular aneurysm, peri-aortic mass, peri-aortic gas, and rapid aortic expansion.
Patients who had pre-existing aortic aneurism, suprarenal aortic aneurism or thoracic aneurisms were excluded from this study. Also, patients who had aortic graft infections and aorto-enteric fistulas were excluded from this study as well.
Variables collected included patient age, gender, presenting symptoms, potential source of aortic infection, patient's comorbidities, laboratory and radiological findings and management options.
The need for patient consent and approval from the research council was discounted because the study was observational and did not involve identifying information about individual patients.
SPSS V. 21 (IBM Corp., Armonk, NY, USA) was used for both descriptive and inferential analyses of the data.
A systematic literature search was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement on PubMed, Google and Medline up to July 2022. Mycotic aortic aneurysm and infected aortic aneurysm were used as key search words.
Results
The total number of patients in the study were six patients. Mean patient age was 70 Years, 66.67% were men and 33.33% were female (Table 1).
| Patient | Age | sex |
| Patient one | 53 | Male |
| Patient two | 62 | Female |
| Patient three | 81 | Male |
| Patient four | 86 | Male |
| Patient five | 75 | Female |
| Patient six | 78 | Male |
Table 1: Patient's demographics.
The most common symptoms at presentation were fever and back pain (66.67% and 33.33%, respectively), other presenting symptoms were abdominal pain (right flank pain) (n=1), feeling unwell (n=1), symptoms of lumbosacral radiculopathy (leg pain) (n=1). General malaise and weight loss (n=1) in patient four, gradually deteriorating into uncontrolled sepsis (n=2) as in both patient four and patient six. Potential sources of a contiguous infection to the aorta in our patients were coexisting retroperitoneal source (n=5) or systemic sepsis (n=1). 33.33% had psoas abscess (n=2), 16.67% had pyelonephritis (n=1). Retroperitoneal inflammation and duodenitis implicated as aortitis source in patient one and patient four respectively. History of immune-compromised conditions was an important determinant observed in our study that can predispose to mycotic aneurisms like malignancy (n=2), type two diabetes mellitus (DM2) (n=2), chronic kidney disease (CKD) (n=2), immunosuppressive medication (n=2) both patients are on Imuran for rheumatoid arthritis (RA) management and alcohol misuse (n=1) (Table 2).
| Patient | Patients Presenting symptoms | Potential source for mycotic aneurisms | Patients Co-morbidities |
| Patient one | Back pain, fever-night sweats | Retroperitoneal inflammation | DM2, alcohol consumption |
| Patient two | leg Pain | Left psoas abscess | - |
| Patient three | back pain | Systemic sepsis: Cellulitis | RA on Imuran, CKD, carcinoid tumour |
| Patient four | Nonspecific symptoms, fever, sepsis | Duodenitis | CKD, Bladder cancer |
| Patient five | Abdominal pain, fever | Left psoas abscess | DM2 |
| Patient six | Collapsed, sepsis-fever | Pyelonephritis | RA on Imuran |
Table 2: Clinical presentation, risk factors and co-morbidities of primary mycotic aortic aneurisms patients.
The White Cell Count (WBC) was less than 11*10^9/L in patient one, patient two and patient four, whereas in patient three WBC was 16*10^9/L, in patient five WBC was 20*10^9/L and in patient six WBC was 23*10^9/L. C-Reactive Protein (CRP) less than 100 milligram/liter (mg/L) reported in patient one, patient two, patient three and patient five. CRP of 176 mg/L and 233 mg/L reported in patient three and patient six respectively. Erythrocyte sedimentation rate (ESR) was slightly raised in all patients in our study (Table 3). Among the preoperative blood cultures, two blood cultures (33.33%) grew Staphylococcus aureus (S. aureus), one blood culture (16.67%) grew Citrobacter Younie, and three patients (50.0%) had negative blood culture results. Empirical broad-spectrum antibiotics were given to all patients and adjusted based on the blood culture result. Three patients (50.00%) had long term oral amoxicillin for three months after relevant intravenous antibiotics. Whereas patient five had lifelong doxycycline (Table 4). All patients had Computed tomography angiography (CTA) -abdomen and pelvis and the findings varies from abdominal aortic aneurism, oedema, soft tissue mass or fat stranding as can be seen in Figures 1-6.
| Laboratory findings | Normal range | Patients range |
| White Cell Count (WBC) | (4.0-11.0) 109/Litre (109/L) | 7-23 109/L |
| C-React. Protein (CRP) | (<5> | 22-233 mg/L |
| Erythrocyte sedimentation rate (ESR) | (0-15) millimetre/hour (mm/hr)
| 3-16 mm/hr |
Table 3: Laboratory findings of primary mycotic aortic aneurisms patients.
| Patients | Blood culture result | preoperative antibiotics | Duration of preoperative antibiotics | post-operative antibiotics |
| Patient one | Negative-No growth | Co- amoxiclav | Six weeks | Amoxicillin for three months |
| Patient two | Negative-No growth | Meropenem. | Eight weeks | Amoxicillin for three months |
| Patient three | citrobacter younae | Piperacillin and Co-trimoxazole | one week | patient died |
| Patient four | Negative-No growth | Co-amoxiclav | 12 weeks | patient died |
| Patient five | S. Aureus | Flucloxacillin | Eight weeks | Lifelong doxycycline |
| Patient six | S. Aureus | Meropenem | Six weeks | Amoxicillin for three months |
Table 4: Blood Cultures results and antibiotics management of primary mycotic aortic aneurisms patients.
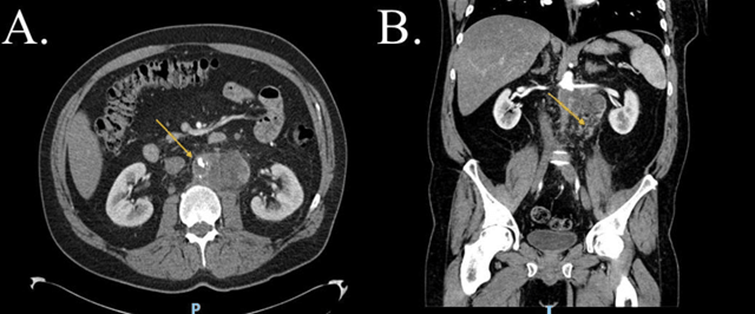
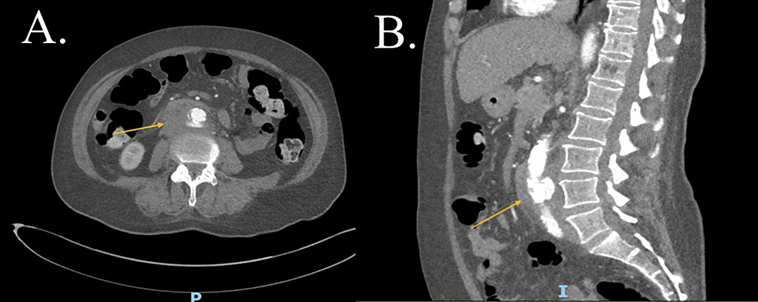
Figure 1: CTA-abdomen and pelvis, cross section view (A) and coronal view (B), showing by yellow arrows a large soft tissue like hypodense lesion in the infrarenal abdominal aorta with thrombosis of MAA in view (A) and Phlegmon in the retroperitoneum in view (B) (patient one). CTA: Computed tomography angiography. MAA: Mycotic aortic aneurism
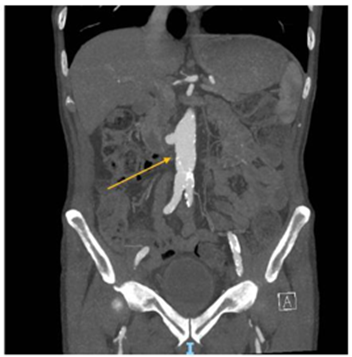
Figure 2: CTA-abdomen and pelvis, cross section view (A) and coronal view (B), showing infra renal MAA and diffuse retroperitoneal inflammatory process (yellow arrows) extends around the left common iliac vessels with possibly developing abscess in view (A) and involving the left psoas in view (B) (patient two). CTA: Computed tomography angiography. MAA: Mycotic aortic aneurism.
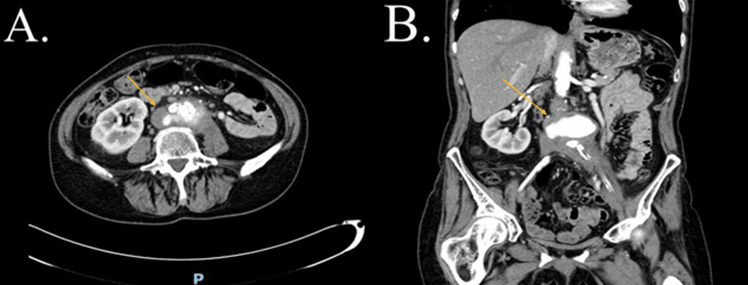
Figure 3: CTA-abdomen and pelvis, cross section view (A) and sagittal view (B), showing by yellow arrows bulging of the posterior wall of MAA with localize point of thinning of the surrounding soft tissue thickening around the aorta in view (A) reaching toward the left paravertebral region of the left psoas muscle in view (B) indicates impending rupture (patient three). CTA: Computed Tomography Angiography. MAA: Mycotic aortic aneurism.
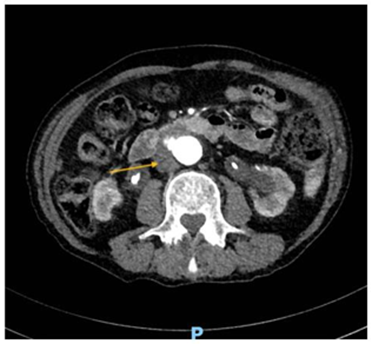
Figure 4: CTA-abdomen and pelvis, cross section view showing periortic soft tissue thickening interpose between MAA and third part of the duodenum (yellow arrow) (patient four). CTA: Computed Tomography Angiography. MAA: Mycotic aortic aneurism.
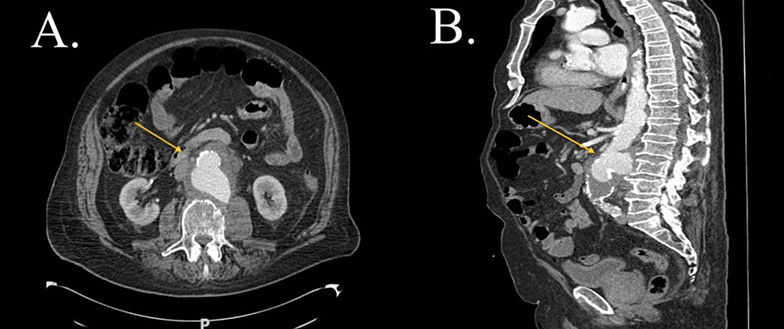
Figure 5: CTA-abdomen and pelvis, cross section view (A) and sagittal view (B), showing by yellow arrows retroperitoneal inflammatory process centered on the aneurismal infrarenal aorta in view (A), with multiple foci of apparent mural irregularity and interruption or elevation of calcified atheroma of MAA view (B) (patient five). CTA: Computed Tomography Angiography. Mycotic aortic aneurism.
Figure 6: CTA-abdomen and pelvis, coronal view showing MAA with retroperitoneal inflammation and periortic soft tissue thickening (yellow arrow) (patient six). CTA: Computed tomography angiography. MAA: Mycotic aortic aneurism.
The surgical treatment was Endovascular Aneurysm Repair (EVAR) (n=2), patient two along with EVAR had femoral-femoral crossover bypass but represented four weeks post operation with lower limb paralysis, and CTA-abdomen and pelvis showed blocked EVAR and femoral-femoral crossover graft and underwent urgent axillo-bifemoral bypass, however the post-operative course complicated by spinal cord ischemia. Axillo-bifemoral bypass (n=2) is the other surgical intervention observed in our study group. Three patients had been treated with antibiotics, however two patients died because one patient had ruptured PMAA, and the second patient was discussed at the Multidisciplinary team meeting (MDT) and planned for EVAR but also had ruptured PMAA and died. The third patient (patient six) was not fit for surgical intervention and treated with long-term antibiotics, (Table 5).
| patient | Operative intervention | post-operative outcome | post-operative management |
| Patient one | Axillo-bifemoral bypass | improved | Oral Antibiotics |
| Patient two | EVAR &Crossover (after four weeks- Axillo-bifemoral bypass) | Spinal cord ischemia | Oral Antibiotics |
| Patient three | Antibiotics- no operative intervention | Died | Died |
| Patient four | Awaiting EVAR- no operative intervention | Died | Died |
| Patient five | EVAR | improved | lifelong oral Antibiotics |
| Patient six | Antibiotics Only- no operative intervention | Improved | Oral Antibiotics |
Table 5: Operative intervention and post-operative management of PMAAs patients.
Discussion
The etiology of the primary aortic infections has been classified as the following: (1) Mycotic aneurysm from distal sources classically was endocarditis forming septic like embolization. (2) Infection of pre-existing atheromatous plaque causing damage of the layers forming the arterial wall. (3) Infected arterial aneurysms caused by trauma, secondary infection and subsequently forming pseudo aneurysms. Can be either iatrogenic through arterial canulation or catheterization or through incidental intra-arterial injection in drug abuse. (4) Contiguous inflammation causing aortitis from surrounding structures as vertebral body osteomyelitis, pancreatitis, retroperitoneal or psoas Abscess or lung, gastrointestinal or urinary tract infection. (5) Infection of preformed aneurism [10]. In our study the potential causes of primary mycotic aortic aneurisms (PMAAs) are spreading infection from surrounding retroperitoneal structures like patient two, patient five and patient six, retroperitoneal inflammation like patient one and patient four or systemic sepsis as in patient three, however patient three had also duodenitis which might predispose to aortitis as well.
The diagnosis of PMAAs can be made based on many diagnostic criteria Sörelius, et al, summarized them into three categories: (1) clinical factors such as abdominal pain, fever, source of infection or immune compromise or significant medical history. (2) Investigation factors either laboratory as elevated inflammatory markers or positive blood cultures or radiological findings. (3) Intra operative findings [6].
However, Sörelius, et al. on a systemic review of 963 patients found the symptoms of the Mycotic aortic aneurisms (MAAs) are pain, either abdominal or back pain 684/887 Patients (w), Fever 603/899 Patients (g), Shock 101/667 Patients () and 382/862 Patients (44%) ruptured, whereas in our patients, fever was the commonest presenting symptoms then back pain [11]. Young-wook Kim, et al. reported that Pain can be in the abdomen but also might occur in the back or the flank and usually vague, therefore it is a nonspecific clue for diagnosis [10]. The risk factors for developing MAAs are various, but the main risk factor is pre-existing atherosclerosis which damages the intima thus making it possible for bacterial colonization and secondary wall degeneration [12]. Pre-existing vascular anomalies as Abdominal aortic aneurisms also increase the risk of Mycotic aneurisms [9]. Immunity compromise is another potential risk factor for PMAAs such as malignancy, diabetes mellitus, alcohol misuse, use of immunosuppressive medication and HIV infection [9]. In our patients, all of them were immunocompromise apart of patient two.
Leukocytosis and erythrocyte sedimentation rate (ESR) are sensitive but not specific in diagnosis of infected aneurysm, 66.67% of our patient had leukocytosis but in all of them ESR was not significantly raised [6]. Microbiology species implicated in MAAs as Sörelius, et al reported are Salmonella species (33.4%), Staphylococci (15.6%), Streptococci (10.4%), and Escherichia coli (3.1%). Over the following years the percentage of Salmonella as a causative microorganism of MAAs has decreased and Staphylococcus aureus (S.aureus) increased this is partially because of more use of antibiotics as management of endocarditis and also arterial trauma increased from 10% to 54% by 2010 from either arterial Catheterization or intravenous drug abuse [10]. The causative pathogen may vary with the pathology, in mycotic aneurism it is Gram (+) cocci, in microbial arteritis it is Salmonella, in infection of existing aneurysm it is Staphylococcus aureus, and in infection of post-traumatic existing aneurysm it is Staphylococcus aureus, others polymicrobial [10]. In our patients, one patient grew Citrobacter Younie in the blood culture and two patients (33.33%) grew S. aureus in their blood cultures.
Computed Tomography Angiography (CTA)- abdomen and pelvis has a sensitivity of 92-96% and specificity of 93-100% for diagnosing PMAAs. CTA- abdomen and pelvis might point to the source of the infection and the plan for construction. Findings on the CTA- abdomen and pelvis scan suggested infection or infected aneurysm are saccular or multilocular in appearance, or rapid expansion of existing aneurysm, a change in size or contour with adjacent normal aorta. Gas shadow intravascular or around the aorta, perivascular soft tissue mass, stranding or possible source of infection as psoas abscess or osteomyelitis of vertebral body. Disruption of the aortic calcification or contrast extravasation indicates imminent rupture [12]. Rapid expansion of aneurism reported in patient three and eventually had contrast extravasation, which was also reported in patient four. Retroperitoneal stranding as possible source of infection reported in 66.67% of the reported patients in this study.
Magnetic resonance angiography (MRA) is another diagnostic alternative but of limited value because longer examination time and invasive aortography [13]. Can be used if the clinical suspicion and/or mycotic aneurism cannot be ruled out with non-invasive tests [13]. The disadvantages are it is limited to the vessel's lumen only and a potential risk of emboli disruption in an already inflamed arterial wall [13]. Leucocyte scintigraphy can be used but is not as sensitive or specific as CTA in diagnosis of infected aneurysm [14]. Positron-emission tomography (PET)-CT had proven value in locating the source of infection in pyrexia of unknown origin with high sensitivity to medium or large vessel vasculitis or graft infection [15]. It has been used for diagnosis of MAAs, and also can assess the aneurysmal histopathogical wall instability or detect synchronous lesions, can also assess the response to the treatment specially the antibiotics and can differentiate if the aneurysm is infected or sterile, none of the latter three studies used in our patients [15, 16].
Treatment of PMAAs is either surgical intervention with antibiotics or antibiotics only. PMAAs should be repaired irrespective of the aneurysm size but depends on the patient’s hemodynamic stability. Stable patients should undergo to surgery at a delayed date giving time to allow the maximum benefit of the antibiotics. However, unstable patient or patients who had no remission of the symptoms such as pain or fever should be offered an urgent date for surgery [6, 17].
Surgical management of PMAA can be either open or endovascular approach. Open approach involves resection of the aneurysm and debridement of the surrounding infected tissues [18]. Revascularization then can be done either in situ or extra anatomical through uninfected tissue planes. The commonest approach is axillo-bi-femoral bypass with ligation of the aorta, the disadvantages of this approach is aortic stump rupture and graft patency [11, 18-19]. In our study two patients underwent axillo-bi-femoral bypass without procedure related complications.
In-situ aortic reconstruction can be done with aortic graft or autologous vein. The advantages of this approach, over the extra anatomical option, is better graft patency and superior hemodynamic stability [10, 20]. However, reported complications of in situ reconstruction by Sörelius, et al. are anastomotic dehiscence and bleeding (1%) and higher rate of graft infection by putting new graft in the infected surgical field [10, 20]. Graft infection can be reduced by implanting antibiotics releasing beads in the tissue around the graft, cover the graft with the omentum, using rifampicin- bonded graft, silver coated Dacron graft, or by use of autogenous vein graft or allograft [11].
Aortic reconstruction with femoral veins is another alternative management option for primary aortic infection. Ilse J.E, et al. reported that 62% (n=18) have been cured after surgical and antimicrobial treatment for both graft and primary aortic infection with one relapse of infection in 12 months’ time, low morality rate or venous hypertension [21]. Nonetheless, this approach needs longer operating time, incisions for harvesting the femoral/popliteal vein and it has low risk of complications as Young-wook Kim, et al. reported that incidence of DVT is 15%, pulmonary embolism is 2.4% and compartment syndrome is 12% [10, 22].
Allografts another alternative for aortic reconstruction usually obtained from multi organ donor, prepared with antibiotics and cryopreserved at tissue bank [23]. Brown KE, et al. reported that the mortality rate of using allograft is 7.0% compared to prosthetic graft 13.2%, and they concluded on the midterm follow up allografts are resistant to reinfection, thrombosis or development of aneurism [24]. However, on late follow up allografts are associated with aneurismal dilation, graft rupture or calcification subsequently amputation [24]. On a long-term study for allograft implantation (6-101 months) for both aortic graft infection and MAAs found that three years survival is 67%, mortality rate is 17.8% (20% for primary aortic infection), no amputation, recurrent/persistent infection [25]. No aortic reconstruction done in our patients.
Endovascular aneurysm repair (EVAR) is another alternative for mycotic aortic aneurysm management, Kan CD, et al. reported that survival rate is 89% and 82% of 30 days and two years subsequently, however no long-term results yet, but poor prognosis is associated with persistent infection [26]. If patient presented with fever or rupture, EVAR would be a suitable temporary measure to hemodynamically stabilize the patient, but If fever persists open approach can be considered. Antibiotics prior and after procedure might avoid infection of the aortic stent graft [26]. Same findings were supported by Yudong Luo, et al. as he reviewed 14 patients who underwent EVAR; seven years survival rate was 75%, relapse of infection occurred in six patients (42%) [27]. In our study, two patients had EVAR for PMAAs, the post-operative course for one patient was uneventful, and the other patient complicated by blocked EVAR and femoral-femoral crossover graft as well as spinal cord ischemia which needed urgent axillo-bifemoral bypass.
Antibiotic therapy should ideally be started as soon as the primary aortic infection is suspected after relative cultures are obtained [6]. Broad spectrum antibiotics should be covering empirical initial treatment to cover streptococcal and staphylococcal species as well as gram negatives; treponemal, mycobacterial and fungal aneurism [9, 10]. Antibiotics for two to four weeks preoperatively is associated with better outcomes if the patient is stable, no clear recommendation of post-operative antibiotics but should continue for at least six weeks, with most recommendations of about three to six months and longer in immunocompromised patients [9-11]. Antibiotics can be stopped if the inflammatory markers are back to normal, cultures are sterile or no clinical features of sepsis [9, 11]. Lifelong antibiotics were recommended if source of the infection is not controlled, prosthetic vascular bypass or the patient has salmonella infection [9]. In our study, three patients had long term antibiotics for three months and one patient had lifelong antibiotics.
The limitations of the study were the limited number of published literatures related to the investigation topic and relatively the small study cohort, both might negatively affect the power of the study and increase the margin of error. Also, the study was retrospective study which is subjected to selection bias.
Conclusion
Primary mycotic aortic aneurisms are rare, but they have high risk of morbidity and mortality. The diagnosis is challenging, therefore high index of clinical suspicion is crucial for early diagnosis. Computed tomography angiography- abdomen and pelvis remains valuable for diagnosis.
Antibiotic therapy should ideally be started as soon as the mycotic aortic aneurism is suspected after relative cultures are obtained. Surgery, either bypass or endovascular aneurysm repair with antibiotics, is a cornerstone for Primary mycotic aortic aneurisms management, however other alternatives can be considered if the patients deemed to be not fit for surgical intervention.
Multi-institutional collaborations are also essential for increased knowledge on optimal surgical care and antibiotic treatment needed to reduce the risk of perioperative complications and to establish standard protocols for perioperative antibiotics management.
References
- Sommerville RL, Allen EV, Edwards JE. (1959). Bland and infected arteriosclerotic abdominal aortic aneurysms: a clinicopathologic study. Medicine, 38:207-221.
Publisher | Google Scholor - Soravia-Dunand VA, Loo VG, Salit IE. (1999). Aortitis due to salmonella: report of 10 cases and comprehensive review of the literature. Clin Infect Dis, 29:862-868.
Publisher | Google Scholor - Stengel A, Wolferth CC. (1923). Mycotic (bacterial) aneurysms of intravascular origin. Arch Intern Med, 31:527-554.
Publisher | Google Scholor - Osler W. (1885). The Gulstonian Lectures, on Malignant Endocarditis. Br Med J, 1:467-470.
Publisher | Google Scholor - Jarrett F, Darling RC, Mundth ED, et al. (1975). Experience with infected aneurysms of the abdominal aorta. Arch Surg, 110:1281-1286.
Publisher | Google Scholor - Karl Sörelius, Pietro G di Summa. (2018). On the Diagnosis of Mycotic Aortic Aneurysms . Clinical Medicine Insights: Cardiology, 12:1-8.
Publisher | Google Scholor - Arkhurst GF, Dekcer JP. (1955). Bacterial aortitis and mycotic aneurysm of the aorta: a report of twelve cases. Am J Pathol, 31:821-835.
Publisher | Google Scholor - Reddy DJ, Shepard AD, Evans JR, Wright DJ, Smith RF, Ernst CB. (1991). Management of infected aortoiliac aneurysms. Arch Surg, 126:873-879.
Publisher | Google Scholor - M Fisk, L F Peck, K Miyagi, et al. (2012). Mycotic aneurysms: a case report, clinical review and novel imaging strategy. J Med, 105:181-18810.
Publisher | Google Scholor - Young-wook Kim. (2010). Infected Aneurysm: Current Management. Ann Vasc Dis, 3:7-15.
Publisher | Google Scholor - Karl Sörelius, Jacob Budtz-Lilly, Kevin Mani, et al. (2019). Systematic Review of the Management of Mycotic Aortic Aneurysms. Eur J Vasc Endovasc Surg, 58:426-435.
Publisher | Google Scholor - Kyriakides C, Kan Y, Kerle M, et al. (2004). 11-year experience with anatomical and extra-anatomical repair of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg, 27:585-589.
Publisher | Google Scholor - Lopes RJ, Almeida J, Dias PJ, et al. (2009). Infectious thoracic aortitis: a literature review. Clin Cardiol, 32:488-490.
Publisher | Google Scholor - Walsh DW, Ho VB, Haggerty MF. (1997). Mycotic aneurysm of the aorta: MRI and MRA features. J Magn Reson Imaging, 7:312-315.
Publisher | Google Scholor - Hen P, Lamki L, Raval B. (1994). Indium-111 leukocyte appearance of Salmonella mycotic aneurysm. ClinNucl Med, 19:646-648.
Publisher | Google Scholor - Mochizuki Y, Fujii H, Yasuda S, et al. (2001). FDG accumulation in aortic walls. Clin Nucl Med, 26:68-69.
Publisher | Google Scholor - Clough RE, Black SA, Lyons OT, et al. (2009). Isendovascular repair of mycotic aortic aneurysms a durable treatment option?. Eur J Vasc Endovasc Surg, 37:407.
Publisher | Google Scholor - Moneta GL, Taylor LM, Yeager RA, et al. (1998). Surgical treatment of infected aortic aneurysm. Am J Surg, 175:396-399.
Publisher | Google Scholor - Woon CY, Sebastian MG, Tay KH, et al. (2008). Extra-anatomic revascularization and aortic exclusion for mycotic aneurysms of the infrarenal aorta and iliac arteries in an Asian population. Am J Surg, 195:66-72.
Publisher | Google Scholor - Dubois M, Daenens K, Houthoofd S, et al. Fourneau I. (2010). Treatment of mycotic aneurysms with involvement of the abdominal aorta: single-centre experience in 44 consecutive cases.Eur. J Vasc Surg, 40:450-456.
Publisher | Google Scholor - Ilse J E Kouijzer, Michel F P Van der Jagt, Chantal P Bleeker-Rovers, et al. (2022). Outcome in Patients after Autologous Femoral Vein Reconstruction for Primary Aortic Infection and Aortic Graft Infection: A Case Series. ann Vasc Surg, 83:240-250.
Publisher | Google Scholor - Clagett GP, Valentine RJ, Hagino RT. (1999). Autogenous aortoiliac femoral reconstruction from superficial femoral popliteal veins: feasibility and durability. J Vasc Surg, 25:255-270.
Publisher | Google Scholor - Erhelst R, Lacroix V, Vraux H, et al. (2000). Use of cryopreserved arterial homografts for management of infected prosthetic grafts: a multicentre study. Ann Vasc Surg, 14:602-607.
Publisher | Google Scholor - Brown KE, Heyer K, Rodriguez H, et al. (2009). Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J Vasc Surg, 49:660-666.
Publisher | Google Scholor - Lesèche G, Castier Y, Petit M, et al. (2001). Long-term results of cryopreserved arterial allograft reconstruction in infected prosthetic grafts and mycotic aneurysms of the abdominal aorta. J Vasc Surg, 34:616-622.
Publisher | Google Scholor - Kan CD, Lee HL, Yang YJ. (2007). Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systemic review. J Vasc Surg, 46:906-912.
Publisher | Google Scholor - Yudong Luo, Jiechang Zhu, Xiangchen Dai, et al. (2018). Endovascular treatment of primary mycotic aorticaneurysms: a 7-year single-center experience. Journal of International Medical Research, 46(9) 3903-3909.
Publisher | Google Scholor