Review Article
Clinical Pharmacology of Gentamicin
Professor of Pharmacology, via Sant’Andrea 32, 56127 Pisa, Italy.
*Corresponding Author: Gian Maria Pacifici Professor of Pharmacology, via Sant’Andrea 32, 56127 Pisa, Italy
Citation: Gian Maria Pacifici. (2023). Clinical Pharmacology of Gentamicin, Journal of Clinical Research and Clinical Trials, BRS publishers. 2(2); DOI: 10.59657/2837-7184.brs.23.010
Copyright: © 2023 Gian Maria Pacifici, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 12, 2023 | Accepted: May 15, 2023 | Published: May 22, 2023
Abstract
Gentamicin is an important agent for the treatment of many serious gram-negative bacillary infections. Gentamicin is the aminoglycoside of first-choice because the reliable activity against all but the most resistant gram-negative aerobes. Gentamicin preparations are available for parenteral, ophthalmic, and topical administration. The recommended intramuscular or intravenous dose of gentamicin sulphate for treatment of gram-negative organisms in adults with normal renal function is 5 to 7 mg/kg daily. For patients with renal dysfunction the interval between may be extended. The efficacy and safety of gentamicin have been reported but gentamicin may induce nephrotoxicity and/or ototoxicity in some patients. Gentamicin penetrates in significant amounts into bone, skeletal muscle, wound tissue, ischemic foot ulcers, and in subcutaneous tissue. The pharmacokinetics of gentamicin have been studied in febrile neutropenic adult patients and in healthy adult subjects and gentamicin elimination half-life is about 3 hours. The pharmacokinetics of gentamicin have been investigated in tetraplegic and in paraplegic adult patients and in healthy adult subjects and the elimination half-life of gentamicin is about 2 hours. The prophylaxis, treatment, and trials with gentamicin have been extensively reported. Gentamicin penetrates into the cerebrospinal fluid in significant amounts and treats bacterial meningitis in infants. Gentamicin is poorly transferred across the human placenta and poorly migrates into the breast-milk. The aim of this study is to review gentamicin efficacy and safety, toxicity, diffusion into body-tissues, pharmacokinetics, prophylaxis, treatment, trials, penetration into the cerebrospinal fluid, treatment of bacterial meningitis, transfer across the human placenta, and migration into the breast-milk.
Keywords: breast-milk; cerebrospinal-fluid; efficacy-safety; gentamicin; meningitis; pharmacokinetics; prophylaxis; tissue-concentration; toxicity; treatment; trials
Introduction
Gentamicin is an important agent for the treatment of many serious gram-negative bacillary infections. It is the aminoglycoside of first-choice because of its lower cost and the reliable activity against all but the most resistant gram-negative aerobes. Gentamicin preparations are available for parenteral, ophthalmic, and topical administration. The typical recommended intramuscular or intravenous dose of gentamicin sulfate when used for the treatment of known or suspected gram-negative organisms as a single agent or in combination therapy for adults with normal renal function is 5 to 7 mg/kg daily. For patients with renal dysfunction, the interval between doses may be extended. For patients who are not candidates for extended-interval dosing, a loading dose of 2 mg/kg and then 3 to 5 mg/kg per day, given as divided doses every 8 to 12 hours, is recommended. Dosages at the upper end of this range may be required to achieve therapeutic concentrations for trauma or burn patients, those with septic shock, patients with cystic fibrosis, and others in whom drug clearance is more rapid or the volume of distribution is larger than normal. It should be emphasized that the recommended doses of gentamicin do not always yield desired concentrations. Periodic determination of the plasma concentration of gentamicin is recommended strongly. Gentamicin is absorbed slowly when it is applied topically in an ointment and somewhat more rapidly when it is applied as a cream. When the gentamicin is applied to large areas of denuded body surface, as may be the case in burn patients, plasma concentrations can reaches 4 µg/ml, and 2% to 5% of the drug may appear in the urine [1].
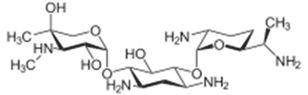
Formulae: Gentamicin molecular structure (molecular weight = 477.603 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “gentamicin efficacy and safety”, “gentamicin toxicity”, “gentamicin tissue concentrations”, “gentamicin pharmacokinetics”, “gentamicin prophylaxis”, “gentamicin treatment”, “gentamicin trials”, gentamicin CSF”, “gentamicin meningitis”, “gentamicin placental transfer”, and “gentamicin breast-milk”. In addition, the book “The pharmacological basis of therapeutics” [1] has been consulted.
Results
Efficacy and safety of gentamicin
Once-daily dose of gentamicin effectively and safety treats malnourished children suffering from serious infections caused by gram-negative pathogens [2]. A single-daily dose of gentamicin effectively and safety treats 89% of hospitalized children with infections caused by gram-negative organisms [3]. Fifty-five adult patients infected by gram-negative pathogens received gentamicin at the standard dose and the cure-rate was 96% suggesting that gentamicin is effective and safe [4]. Gentamicin administered once-daily is efficacy and safe in treatment of adult patients with severe infections caused by gram-negative organisms [5]. Gentamicin is efficacy and safe in treatment of adult patients with severe infections caused by gram-negative bacteria [6].
Toxicity induced by gentamicin
Gentamicin causes acute renal failure when accumulates in the kidney [7]. Two-hundred-fifty-eight adult patients were treated with gentamicin at standard dose and the auditory toxicity developed in 10% of patients and the nephrotoxicity developed in 26% of patients [8]. Gentamicin administered at standard dose induces acute kidney injury in patients undergoing orthopaedic surgery [9]. Gentamicin administered subconjunctivally is highly toxic to the corneal endothelium and to the anterior chamber structures [10]. In adult patients, a single-dose of gentamicin induces minimal nephrotoxicity and ototoxicity compared to multiple-doses [11]. Gentamicin administered at standard dose to adult patients causes ototoxicity in two-thirds of patients and cochlear toxicity occurs in one-third of patients [12]. Gentamicin administered at a dose of 1.5 mg/kg daily induces ototoxicity and vestibular dysfunction in some patients when the cumulative dose exceeds 17 mg/kg [13]. In adult patients with renal failure gentamicin accumulates in plasma and induces ototoxicity in some patients [14].
Diffusion of gentamicin into body-tissues
Gentamicin penetrates in significant amounts in bone during total hip arthroplasty and knee arthroplasty [15]. The concentration of gentamicin in peripheral subcutaneous tissue is 0.7 times that in skeletal muscle tissue [16]. Concentrations of gentamicin were measured in serum and in wound tissue of 30 patients after the topical administration of 260 mg gentamicin. The median gentamicin concentration was 3.04 µg/ml in the wound tissue and 2.05 µg/ml in serum [17]. Sixteen patients with ischaemic foot ulcers received 284+116 mg of gentamicin intravenously. The median gentamicin concentration in ischemic foot ulcers was 9.4 µg/ml 1 hour after gentamicin administration [18]. Gentamicin was administered intravenously at a dose of 240 mg to 7 healthy volunteers. The peak concentration of gentamicin in subcutaneous tissue was 6.7+2.0 µg/ml and the area under the concentration-time curve was 1,281+390 µg*min/ml [19].
Pharmacokinetics of gentamicin in febrile neutropenic adult patients and in healthy adult subjects
Bianco et al. [20] studied the pharmacokinetics of gentamicin in 34 febrile neutropenic patients, aged 57.7+13.7 years, weighing 68.7+18.6 kg, with a creatinine clearance corrected by surface body area of 70.1+33.8 ml/min/1.73 m2, and in 40 health subjects, aged 57.7+13.6 years, weighing 67.5+12.5 kg, with a creatinine clearance corrected by surface body area of 61.7+23.9 mg/min/1.73 m2. In each patient and in each healthy subject the gentamicin dose was adjusted to provide 1-hour peak concentration of 5 to 10 µg/ml and a trough concentrations < 2>
Table 1: Pharmacokinetic parameters of gentamicin which have been obtained in 34 febrile neutropenic adult patients and in 40 healthy adult subjects. Values are the mean+SD, by Bianco et al. [20].
T1/2 = elimination half-life. TBC = total body clearance. *P-value = 0.02. **P-value = 0.04 (Student t test for umpired data).
This table shows that the elimination half-life of gentamicin is about 3 hours in patients and in healthy subjects indicating that gentamicin is rapidly eliminated. The distribution volume of gentamicin is lower than the water volume. The total body clearance is similar in patients and in healthy subjects. The daily dose of gentamicin is lower in healthy subjects than in patients thus the peak concentration of gentamicin is lower in healthy subjects than in patients.
Pharmacokinetics of gentamicin in tetraplegic and paraplegic adult patients and in healthy adult subjects
Segal et al. [21] investigated the pharmacokinetics of gentamicin in 7 tetraplegic patients, in 7 paraplegic patients, and in 7 healthy subjects who were aged 20 to 60 years and gentamicin was intravenously infused at a dose of 1.5 mg/kg.
Table 2: Pharmacokinetic parameters of gentamicin which have been obtained in 7 tetraplegic patients, in 7 paraplegic patients, and in 7 healthy subjects and gentamicin was intravenously infused at a dose of 1.5 mg/kg. Values are the mean+SD, by Segal et al. [21].
Kel = elimination-rate constant. §Elimination half-life. TBC = total body clearance. *P-value < 0>
This table shows that gentamicin is rapidly eliminated as the mean elimination half-life is about 2 hours in both patients and healthy subjects. The distribution volume is lower that the water volume, the distribution volume expressed as L/kg is larger in the tetraplegic and paraplegic patients than in healthy subjects, and the total body clearance is higher in tetraplegic and paraplegic patients than in healthy subjects.
Prophylaxis with gentamicin
Gentamicin is efficient as quinolones in the prevention of urinary-tract infection in adult patients [22]. A single-dose of intravenous gentamicin is a safe option for perioperative prophylaxis in patients undergoing orthopaedic surgery [23] and prophylaxis with gentamicin-containing collagen implants prevents infections in patients undergoing orthopaedic surgery [24]. Intramuscular gentamicin is a safe and effective prophylactic agent in reducing the incidence of infections in patients undergoing prostatic biopsy [25]. A single-dose combination of metronidazole and gentamicin reduces surgical site infections in patients undergoing uncomplicated appendectomy [26]. Prophylaxis with intravenous gentamicin prevents sternal wound infection in adult patients [27]. Long-term prophylaxis with inhaled gentamicin effectively treats Pseudomonas aeruginosa infection in children with cystic fibrosis [28], and prophylaxis with intravenous gentamicin prevents infections in adult patients undergoing cardiac surgery [29].
Treatment of bacterial infections with gentamicin
Intra-vesical instillation of gentamicin reduces the episodes of urinary-tract infection in adult patients [30]. Topical gentamicin is an effective treatment of Nagashima-type disease [31]. Topical and intradermal gentamicin suppresses dystrophic epidermolysis bullosa [32]. Subcutaneous gentamicin injection is a well- tolerated and is an effectively agent for treating cutaneous infections in adult patients [33]. Gentamicin is an effective antibiotic for empirical treatment of women with community-onset complicated and acute pyelonephritis [34]. Ten patients with brucellosis were treated with azithromycin and gentamicin and 7 patients (70.0%) had an excellent therapeutic response at the end of therapy [35]. Gentamicin was administered intravenously at a dose of 4 mg/kg daily to adult patients with urinary-tract infection and this treatment cured the infection [36]. Gentamicin was administered intramuscularly at a daily dose of 3 to 27 mg/kg for 7 to 12 days (mean, 10 days) to patients infected by Staphylococcus aureus and this treatment cured the infection in all patients [37]. Gentamicin was administered intravenously at a daily dose of 5 mg/kg for 10 days to 16 patients infected by Staphylococcus aureus and to 6 patients infected by Staphylococcus albus. The minimum inhibitory concentration of Staphylococcus aureus and Staphylococcus albus was 3.1 and 0.78 µg/ml, respectively, and the infection was cured in all patients [38]. Four-hundred-four isolates of Salmonella serovar typhi were obtained from the blood of adult patients. The isolates were sensitive to gentamicin and had a minimum inhibitory concentration of 0.01 to 4 µg/ml and gentamicin eradicates this organism from the blood of all patients [39].
Trials with gentamicin
Eighty-eight adult patients with infected diabetic foot ulcer were treated with intravenous gentamicin at the standard dose and in 46 (52.3%) patients the pathogens were eradicated. Gentamicin was well-tolerated and treats the infection in diabetic foot ulcer in some patients [40]. A trial demonstrated that intra-tympanic gentamicin is an effective and safe treatment of Ménière's disease [41]. Fifteen randomized controlled trials encompassing a total of 6,979 adult patients undergoing surgery were included in the study. Gentamicin-collagen implants were applied and the treatment reduced the surgical site infections in most of patients [42], the implantable gentamicin-collagen sponges significantly reduce the risk of sternal wound infection in patients undergoing cardiac surgery [43], and gentamicin collagen sponges reduced the infection of sternal wound in patients undergoing cardiac surgery [44]. A prospective, randomized, controlled monocentric trial was performed to evaluate the efficacy and safety of 2 grams of ceftriaxone once-daily plus 5 mg/kg gentamicin once-daily in comparison to 2 grams of cefepime thrice-daily plus 5 mg/kg gentamicin once-daily in the treatment of neutropenic fever. The treatment of ceftriaxone plus gentamicin is not inferior to that of cefepime plus gentamicin in controlling neutropenic fever [45]. Once-daily dose of gentamicin with twice-daily dose of clindamycin is efficacious and safe as gentamicin once-daily with clindamycin thrice- daily in treatment of peripartum uterine infection [46]. Amikacin and gentamicin are effective agents against severe infections caused by gram-negative organisms and amikacin and gentamicin have similar ototoxic and nephrotoxic profiles [47].
Penetration of gentamicin into the cerebrospinal fluid (CSF)
Adult patients received gentamicin intravenously at the standard dose and the concentration of gentamicin in serum ranged from 3.9 to 9.4 μg/ml (mean, 4.8+2.0) and the concentration of gentamicin in the CSF was about 2 µg/ml thus gentamicin penetrates into the CSF in significant amounts and treated the meningitis caused by Candida albicans or by Citrobacter freundii [48]. Gentamicin concentration was measured in serum and in the CSF of 8 adult patients suffering from bacterial meningitis and gentamicin concentrations in CSF ranged from 0.4 to 5.7 µg/ml and CSF to serum ratio ranged from 0.058 to 0.70 (mean, 0.26) [49]. Gentamicin was administered intraventricularly at a mean dose of 2.5 mg/kg to 52 infants with meningitis caused by Escherichia coli or by Salmonella species. The concentration of gentamicin in ventricular and lumbar CSF ranged from 10 to 130 µg/ml 1 hour after dosing and ranged from 8 to 85 µg/ml 6 hours after dosing [50]. Gentamicin was administered intraventricularly at a mean dose of 1 mg to 5 children with bacterial meningitis and the mean ventricular concentration of gentamicin was 20 µg/ml 1 hour after dosing and ranged from 5 to 14 µg/ml 36 hours after the administration [51]. Gentamicin concentration was measured in the CSF of 21 adult patients suffering from meningitis caused by gram-negative enteric bacilli who received 3 to 12 doses of 4 mg of gentamicin intrathecally and the intrathecal concentration of gentamicin ranged from 19 to 46 µg/ml 20 hours after dosing [52]. These results indicate that gentamicin penetrates into the CSF in significant amounts.
Treatment of bacterial meningitis with gentamicin in infants
Two-hundred-nine infants, aged ≤ 2 months, suffered from meningitis caused by group B Streptococcus (N = 60), or by Streptococcus pneumoniae (N = 45), or by non-typhoid Salmonella enterica (N = 104). Gentamicin was administered intravenously at the standard dose and treats the infection in all infants [53]. Group B β-haemolytic streptococci and Escherichia coli strains accounted for approximately two thirds of all cases of neonatal meningitis and gentamicin is recommended for the initial empiric therapy of newborns with meningitis caused by these pathogens [54]. Twenty-one infants with purulent meningitis were treated with gentamicin. Two infants died and another one developed hydrocephalus but the remaining 18 infants were cured [55].
Transfer of gentamicin across the human placenta
Gentamicin was administered intravenously at a dose of 80 mg followed by an intravenous infusion of 18.5 mg per hour to 6 women with a gestational age of 18 to 23 weeks undergoing elective abortion. Maternal and foetal concentrations of gentamicin were obtained 30 min after the loading dose and hourly during the infusion period and the foetal weight ranged from 238 to 515 grams [56].
Table 3: Gentamicin concentrations in the maternal serum, foetal serum, foetal urine, amniotic fluid, and maternal urine which have been obtained in 6 mothers and 5 foetuses. Values are the minimum, maximum, mean+SD, by Kauffman et al. [56].
This table shows that the mean maternal and umbilical cord serum concentration of gentamicin is 3.85 and 1.08 µg/ml, respectively, suggesting that gentamicin is poorly transferred crosses the human placenta.
A single intramuscular dose of 40 mg of gentamicin was administered to 37 pregnant women before delivery. Maternal and umbilical cord concentration of gentamicin was determined in the serum at delivery. The mean peak concentration of gentamicin was 3.65 µg/ml in the maternal serum 30 min after dosing and the mean peak umbilical cord serum was 1.25 µg/ml 60 to 120 min after dosing. The peak concentration in the umbilical cord serum corresponds to 34.5% of that in the maternal serum indicating that gentamicin is poorly transferred crosses the human placenta [57].
Migration of gentamicin into the breast-milk
Ten lactating women received a single intramuscular dose of 80 mg of gentamicin in the early postpartum period. Average gentamicin concentration is 157 ng/ml 1 hour after the dose, 156 ng/ml 3 hours after the dose, 137 ng/ml 6 hours after the dose, and 130 ng/ml 12 hours after the dose [58]. Gentamicin-impregnated beads were implanted into the femur of one mother with osteomyelitis and the breast-milk concentration of gentamicin ranged from 70 to 190 ng/ml at various times after the administration [59]. Ten lactating women received prophylactic gentamicin intramuscularly at a dose of 80 mg thrice-daily. On the 4th day of treatment, the concentration of gentamicin in the breast-milk was 420 ng/ml 1 hour after the dose, 480 ng/ml 3 hours after the dose, 490 ng/ml 5 hours after the dose, and 410 ng/ml 7 hours after the dose [60]. These results indicate that gentamicin poorly migrates into the breast-milk.
Discussion
Gentamicin is an important agent for the treatment of many serious gram-negative bacillary infections and gentamicin is the aminoglycoside of first-choice because of its lower cost and the reliable activity against all but the most resistant gram-negative aerobes. Gentamicin preparations are available for parenteral, ophthalmic, and topical administration. The typical recommended intramuscular or intravenous dose of gentamicin sulphate when used for the treatment of gram-negative organisms as a single agent or in combination therapy for adults with normal renal function is 5 to 7 mg/kg daily given. For patients who are not candidates for extended-interval dosing a loading dose of 2 mg/kg and then 3 to 5 mg/kg per day given as divided doses every 8 to 12 hours is recommended. In patients with renal dysfunction the interval between doses may be extended and periodic determination of the plasma concentration of gentamicin is recommended strongly [1]. The efficacy and safety of gentamicin have been reviewed. Once-daily gentamicin dose effectively and safe treats malnourished children infected by gram-negative organisms [2] and a single-daily dose of gentamicin effectively treats 89% hospitalized children infected by gram-negative organisms [3]. When gentamicin is administered at the standard dose to adult patients infected by gram-negative pathogens the cure-rate is 96% [4], gentamicin administered once-daily effectively and safe treats adult patients infected by gram-negative organisms [5], and gentamicin effectively and safe treats infections caused by gram-negative bacteria in adult patients [6]. These results indicate that gentamicin effectively and safety treats children and adult patients infected by gram-negative organisms. The nephrotoxicity and the ototoxicity caused by gentamicin have been reviewed. Gentamicin causes acute renal failure when accumulates in the kidney [7], following standard dose of gentamicin the auditory toxicity occurs in 10% of patients and the nephrotoxicity occurs in 26% of patients [8], and gentamicin induces acute kidney injury in patients undergoing orthopaedic surgery [9]. Gentamicin administered subconjunctivally causes toxicity in the corneal endothelium and in the anterior chamber structures [10]. A single-dose of gentamicin induces minimal nephrotoxicity and ototoxicity compared to multiple-doses [11], and gentamicin administered at the standard dose causes ototoxicity in two-thirds of patients and cochlear toxicity in one-third of patients [12]. The cumulative dose of gentamicin exceeding 17 mg/kg induces ototoxicity and vestibular dysfunction in some patients [13] and in patients with renal failure gentamicin accumulates in plasma and induces ototoxicity [14]. These results indicate that gentamicin may induce nephrotoxicity and/or ototoxicity in some patients. The diffusion of gentamicin into body-tissues has been reviewed. Gentamicin penetrates in significant amounts in bone during total hip arthroplasty and knee arthroplasty [15] and gentamicin concentration in peripheral subcutaneous tissue is 0.7 times that in skeletal muscle tissue [16]. Following the topical administration of 260 mg of gentamicin the median concentration of gentamicin is 3.04 µg/ml in wound tissue and 2.05 µg/ml in serum [17], gentamicin was administered intravenously at a dose of 284+116 mg to patients with ischaemic foot ulcer and the concentration of gentamicin in ischemic foot ulcer is 9.4 µg/ml 1 hour after dosing [18], and gentamicin was administered intravenously at a dose of 240 mg to healthy volunteers and the peak concentration of gentamicin in subcutaneous tissue is 6.7+2.0 µg/ml [19]. These results indicate that gentamicin diffuses in body-tissues in significant amounts. The pharmacokinetics of gentamicin have been studied by Bianco et al. [20] in febrile neutropenic patients and in healthy subjects and the elimination half-life of gentamicin is about 3 hours in both patients and healthy subjects. The pharmacokinetics of gentamicin have been investigated by Segal et al. [21] in tetraplegic and in paraplegic patients and in healthy subjects and the elimination half-life of gentamicin is about 2 hours in both patients and in healthy subjects. These results indicate that gentamicin is rapidly eliminated. The prophylaxis with gentamicin has been reviewed. Gentamicin effectively prevents urinary-tract infection as quinolones [22], a single-dose of intravenous gentamicin effectively prevents infections in patients undergoing orthopaedic surgery [23], and gentamicin-containing collagen implants prevents infection in patients undergoing orthopaedic surgery [24]. Intramuscular gentamicin prevents infection in patients undergoing prostatic biopsy [25], single-dose combination of metronidazole and gentamicin reduces surgical site infections in patients undergoing appendectomy [26], long-term prophylaxis with inhaled gentamicin effectively treats Pseudomonas aeruginosa infection in children with cystic fibrosis [28], and the prophylaxis with intravenous gentamicin prevents the infections in patients undergoing cardiac surgery [29]. These results indicate that gentamicin effectively prevents different infections. The treatment of bacterial infections with gentamicin has been reviewed. Intra-vesical instillation of gentamicin reduces the episodes of urinary-tract infection in adult patients [30]. Topical gentamicin effectively treats Nagashima-type disease [31] and topical and intradermal gentamicin suppresses dystrophic epidermolysis bullosa [32]. Subcutaneous injection of gentamicin effectively treats cutaneous infections in adult patients [33] and gentamicin effectively treats women with community-onset complicated and acute pyelonephritis [34]. Azithromycin and gentamicin treat brucellosis in 70% of patients [35]. Gentamicin administered intravenously to adult patients at a dose of 4 mg/kg daily treats urinary-tract infection [36], gentamicin administered intravenously at a dose of 3 to 27 mg/kg daily for 10 days treats patients infected by Staphylococcus aureus [37], and gentamicin administered intravenously at a dose of 5 mg/kg daily treats patients infected by Staphylococcus aureus and patients infected by Staphylococcus albus [38]. Gentamicin eradicates Salmonella serovar typhi from the blood of patients [39]. These results indicate that gentamicin treats different infections. The trials with gentamicin have been reviewed. Gentamicin administered intravenously at the standard dose cures some patients with diabetic foot ulcers [40] and intra-tympanic gentamicin effectively treats Ménière’s disease [41]. Gentamicin collagen implants reduce surgical site infections in most patients [42] and gentamicin collagen sponges reduce sternal wound infection in patients undergoing cardiac surgery [44]. Two grams of ceftriaxone once-daily plus 5 mg/kg gentamicin once-daily is comparable to 2 grams of cefepime thrice-daily plus 5 mg/kg gentamicin once-daily in treatment of neutropenic fever [45], once-daily gentamicin with twice-daily clindamycin is efficacious and safe as gentamicin once-daily with clindamycin thrice-daily in treatment of peripartum uterine infection [46], and amikacin and gentamicin effectively treat infections caused by gram-negative organisms [47]. These results indicate that trials with gentamicin effectively treat different infections. The penetration of gentamicin into the cerebrospinal fluid has been reviewed. Adult patients received gentamicin intravenously at the standard dose and gentamicin concentration ranged from 3.9 to 9.4 µg/ml (mean, 4.8+2.0) in serum, the gentamicin concentration in the cerebrospinal fluid was about 2 µg/ml, and gentamicin treats the meningitis caused by Candida albicans a or by Citrobacter freundii [48], and in adult patients with bacterial meningitis the cerebrospinal fluid to serum ratio of gentamicin ranged from 0.058 to 0.70 (mean, 0.26) [49]. Gentamicin was administered intraventricularly at a mean dose of 2.5 mg/kg to infants with meningitis caused by Escherichia coly or by Salmonella species and gentamicin concentration in ventricular and lumbar cerebrospinal fluid ranged from 10 to 130 µg/ml 1 hour after dosing and from 8 to 85 µg/ml 6 hours after dosing [50]. Gentamicin was administered intraventricularly at a dose of 1 mg to children with bacterial meningitis and the mean ventricular concentration of gentamicin was 20 µg/ml 1 hour after dosing and ranged from 5 to 14 µg/ml 36 hours after dosing [51]. Gentamicin was administered intrathecally at a dose of 4 mg for 3 to 12 doses to adult patients with meningitis caused by gram-negative enteric bacilli and the gentamicin concentration in the cerebrospinal fluid ranged from 19 to 46 µg/ml 20 hours after dosing [52]. These results indicate that gentamicin penetrates into the cerebrospinal fluid in significant amounts. The treatment of bacterial meningitis with gentamicin has been reviewed. Gentamicin treats the meningitis caused by group B Streptococcus, or by Streptococcus pneumoniae, or by non-typhoid Salmonella enterica in infants [53], treatment with gentamicin is recommended in newborns with meningitis caused by group B β-haemolytic streptococci and Escherichia coli [54], and gentamicin treats infants with purulent meningitis [55]. These results indicate that gentamicin treats the meningitis caused by different pathogens in infants. Gentamicin is poorly transferred across the human placenta [56, 57] and poorly migrates into the breast-milk [58-60].
Conclusion
In conclusion, gentamicin is an important agent for the treatment of many serious gram-negative bacillary infections. The intramuscular or intravenous dose of gentamicin sulphate is 5 to 7 mg/kg daily in patients with normal renal function and the interval between doses may be expanded in patients with renal dysfunction. The efficacy and safety of gentamicin have been reviewed but gentamicin may induce nephrotoxicity and/or ototoxicity in some patients and gentamicin diffuses into body-tissues in significant amounts. The pharmacokinetics of gentamicin have been studied in febrile neutropenic patients and in healthy subjects and the elimination half-life is about 3 hours in patients and adult patients and the pharmacokinetics of gentamicin have been investigated in tetraplegic and in paraplegic patients and in healthy subjects and the elimination half-life of gentamicin is about 2 hours in patients and adult subjects. The prophylaxis, treatment, and trials with gentamicin have been reviewed. Gentamicin penetrates into the cerebrospinal fluid in significant amounts and treated bacterial meningitis in infants. Gentamicin is poorly transferred across the human placenta and poorly migrates into the breast-milk. The aim of this study is to review the clinical pharmacology of gentamicin.
Conflict of interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- MacDougal C. (2018). Aminoglycosides. In The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, Brunton Hilal-dandan LL, Knollmann BC, editors. Mc Graw Hill, 13th Edition, USA, New York. 1039-1047.
Publisher | Google Scholor - Khan AM, Ahmed T, Alam NH, Chowdhury AK, Fuchs GJ. (2006). Extended-interval gentamicin administration in malnourished children. J Trop Pediatr. 52(3):179-184.
Publisher | Google Scholor - Tiwari S, Rehan HS, Chandra J, Mathur NN, Singh V. (2009). Efficacy and safety of a single daily dose of gentamicin in hospitalized Indian children: a quasi-randomized trial. J Antimicrob Chemother. 64(5):1096-1101.
Publisher | Google Scholor - Buabeng KO, Mackenzie AR, Laing RB, Cook I, Jappy B, Gould IM. (1999). Assessment of the efficacy, safety and quality of gentamicin use in Aberdeen Royal Infirmary. J Antimicrob Chemother. 44(6):843-845.
Publisher | Google Scholor - Gilbert DN, Lee BL, Dworkin RJ, Leggett JL, Chambers HF, Modin G, et al. (1998). A randomized comparison of the safety and efficacy of once-daily gentamicin or thrice-daily gentamicin in combination with ticarcillin-clavulanate. Am J Med. 105(3):182-191.
Publisher | Google Scholor - Gentry LO. (1978). Efficacy and safety of cefamandole plus either gentamicin or tobramycin in therapy of severe gram-negative bacterial infections. J Infect Dis. 137(1):144-149.
Publisher | Google Scholor - Zager RA. (1992). Gentamicin effects on renal ischemia/reperfusion injury. Circ Res. 70(1):20-28.
Publisher | Google Scholor - Smith CR, Lipsky JJ, Laskin OL, Hellmann DB, Mellits ED, Longstreth J, et al. (1980). Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med. 302(20):1106-1109.
Publisher | Google Scholor - Srisung W, Teerakanok J, Tantrachoti P, Karukote A, Nugent K. (2017). Surgical prophylaxis with gentamicin and acute kidney injury: a systematic review and meta-analysis. Ann Transl Med. 5(5):100.
Publisher | Google Scholor - Koban Y, Genc S, Bilgin G, Cagatay HH, Ekinci M, Gecer M, et al. (2014). Toxic Anterior Segment Syndrome following Phacoemulsification Secondary to Overdose of Intracameral Gentamicin. Case Rep Med. 2014:143564.
Publisher | Google Scholor - Saleh P, Abbasalizadeh S, Rezaeian S, Naghavi-Behzad M, Piri R, Pourfeizi HH. (2016). Gentamicin-mediated ototoxicity and nephrotoxicity: A clinical trial study. Niger Med J. 57(6):347-352.
Publisher | Google Scholor - Esterhai JK Jr, Bednar J, Kimmelman CP. (1986). Gentamicin-induced ototoxicity complicating treatment of chronic osteomyelitis. Clin Orthop Relat Res. 20(9):185-188.
Publisher | Google Scholor - Gailiunas P Jr, Dominguez-Moreno M, Lazarus M, E Lowrie G, Gottlieb MN, Merrill JP. (1978). Vestibular toxicity of gentamicin. Incidence in patients receiving long-term hemodialysis therapy. Arch Intern Med. 138(11):1621-1624.
Publisher | Google Scholor - Meyers RM. (1970). Ototoxic effects of gentamicin. Arch Otolaryngol. 92(2):160-162.
Publisher | Google Scholor - Torkington MS, Davison MJ, Wheelwright EF, Jenkins PJ, Anthony I, Lovering AM, et al. (2017). Bone penetration of intravenous flucloxacillin and gentamicin as antibiotic prophylaxis during total hip and knee arthroplasty. Bone Joint J. 99(3):358-364.
Publisher | Google Scholor - Zammit MC, Fiorentino L, Cassar K, Azzopardi LM, LaFerla G. (2011). Factors affecting gentamicin penetration in lower extremity ischemic tissues with ulcers. Int J Low Extrem Wounds. 10(3):130-137.
Publisher | Google Scholor - Friberg O, Jones I, Sjöberg L, Söderquist B, Vikerfors T, Källman J. (2003). Antibiotic concentrations in serum and wound fluid after local gentamicin or intravenous dicloxacillin prophylaxis in cardiac surgery. Scand J Infect Dis. 35(4):251-254.
Publisher | Google Scholor - Burgmann H, Georgopoulos A, Graninger W, Koppensteiner R, Maca T, Minar E, et al. (1996). Tissue concentration of clindamycin and gentamicin near ischaemic ulcers with transvenous injection in Bier's arterial arrest. Lancet. 348(9030):781-783.
Publisher | Google Scholor - Lorentzen H, Kallehave F, Kolmos HJ, Knigge U, Bülow J, Gottrup F. (1996). Gentamicin concentrations in human subcutaneous tissue. Antimicrob Agents Chemother. 40(8):1785-1789.
Publisher | Google Scholor - Bianco TM, Dwyer PN, Bertino JS Jr. (1989). Gentamicin pharmacokinetics, nephrotoxicity, and prediction of mortality in febrile neutropenic patients. Antimicrob Agents Chemother. 33(11):1890-1895.
Publisher | Google Scholor - Segal JL, Gray DR, Gordon SK, Eltorai IM, Khonsari F, Patel J. (1985). Gentamicin disposition kinetics in humans with spinal cord injury. Paraplegia. 23(1):47-55.
Publisher | Google Scholor - Chazan B, Zelichenko G, Shental Y, Edelstein H, Raz R. (2010). Antimicrobial prophylaxis for transrectal ultrasound guided biopsy of prostate: a comparative study between single dose of Gentamicin vs. Ofloxacin. Int Soc Inf Dis. 14(1):199-200.
Publisher | Google Scholor - Dubrovskaya Y, Tejada R, Bosco J 3rd, Stachel A, Chen D, Feng M, et al. (2015). Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: Evaluation of nephrotoxicity. SAGE Open Med. 3:2050312115612803.
Publisher | Google Scholor - Knaepler H. (2012). Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection in orthopaedic surgery. Int J Surg. 10(1):15-20.
Publisher | Google Scholor - Ho HSS, Ng LG, Tan YH, Yeo M, Cheng CWS. (2009). Intramuscular gentamicin improves the efficacy of ciprofloxacin as an antibiotic prophylaxis for transrectal prostate biopsy. Ann Acad Med Singap. 38(3):212-216.
Publisher | Google Scholor - Kasatpibal N, Nørgaard M, Sørensen HT, Schønheyder HC, Jamulitrat S, Chongsuvivatwong V. (2006). Risk of surgical site infection and efficacy of antibiotic prophylaxis: a cohort study of appendectomy patients in Thailand. BMC Infect Dis. 6:111.
Publisher | Google Scholor - Friberg 1, Dahlin L-G, Levin K-A, Magnusson A, Granfeldt H, Källman J, et al. (2006). Cost effectiveness of local collagen-gentamicin as prophylaxis for sternal wound infections in different risk groups. Scand Cardiovasc J. 40(2):117-125.
Publisher | Google Scholor - Heinzl B, Eber E, Oberwaldner B, Haas G, Zach MS. (2002). Effects of inhaled gentamicin prophylaxis on acquisition of Pseudomonas aeruginosa in children with cystic fibrosis: a pilot study. Pediatr Pulmonol. 33(1):32-37.
Publisher | Google Scholor - Mercieri M, Mercieri A, Tritapepe L, Ruggeri M, Arcioni R, Repetto M, et al. (1999). High-dose aprotinin with gentamicin-vancomycin antibiotic prophylaxis increases blood concentrations of creatinine and cystatin C in patients undergoing coronary artery bypass grafting. Br J Anaesth. 82(4):531-536.
Publisher | Google Scholor - Stalenhoef JE, van Nieuwkoop C, Menken PH, Bernards ST, Elzevier HW, van Dissel JT. (2019). Intravesical Gentamicin Treatment for Recurrent Urinary Tract Infections Caused by Multidrug Resistant Bacteria. J Urol. 201(3):549-555.
Publisher | Google Scholor - Wang S, Yang Z, Liu Y, Zhao M-T, Zhao J, Zhang H, et al. (2021). Application of topical gentamicin-a new era in the treatment of genodermatosis. World J Pediatr. 17(6):568-575.
Publisher | Google Scholor - Woodley DT, Cogan J, Hou Y, Lyu C, Marinkovich MP, Keene D, et al. (2017). Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J Clin Invest. 127(8):3028-3038.
Publisher | Google Scholor - Dizdar OS, Ozer O, Erdem S, Gunal AI. (2017). Subcutaneous gentamicin injection around the cuff in treatment of resistant exit site infection in peritoneal dialysis patients: a pilot study. Ther Clin Risk Manag. 13(7):909-914.
Publisher | Google Scholor - Wie S-H, Kim HW, Chang U-I. (2014). Effects of gentamicin monotherapy for the initial treatment of community-onset complicated non-obstructive acute pyelonephritis due to Enterobacteriaceae in elderly and non-elderly women. Clin Microbiol Infect. 20(11):1211-1218.
Publisher | Google Scholor - Solera J, Beato JL, Martínez-Alfaro E, Segura JC, de Tomas E. (2001). Azithromycin and gentamicin therapy for the treatment of humans with brucellosis. Clin Infect Dis. 32(3):506-509.
Publisher | Google Scholor - Gilbert DN, Eubanks N, Jackson J. (1977). Comparison of amikacin and gentamicin in the treatment of urinary tract infections. Am J Med. 62(6):924-929.
Publisher | Google Scholor - Chambers WB, Pallagrosi AU. (1977). Gentamicin in the treatment of staphylococcal infections. J Int Med Res. 5(6):442-449.
Publisher | Google Scholor - Richards F, McCall C, Cox C. (1971). Gentamicin treatment of staphylococcal infections. JAMA. 215(8):1297-1300.
Publisher | Google Scholor - Mandal S, Mandal MD, Pal NK. (2009). In vitro activity of gentamicin and amikacin against Salmonella enterica serovar Typhi: a search for a treatment regimen for typhoid fever. East Mediterr Health J. 15(2):264-268.
Publisher | Google Scholor - Uçkay I, Kressmann B, Malacarne S, Toumanova A, Jaafar J, Lew D, et al. (2018). A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC Infect Dis. 18(1):361.
Publisher | Google Scholor - Bremer HG, van Rooy I, Pullens B, Colijn C, Stegeman I, van der Zaag-Loonen HJ, et al. (2014). Intratympanic gentamicin treatment for Ménière's disease: a randomized, double-blind, placebo-controlled trial on dose efficacy - results of a prematurely ended study. Trials. 15:328.
Publisher | Google Scholor - Chang WK, Srinivasa S, MacCormick AD, Hill AG. (2013). Gentamicin-collagen implants to reduce surgical site infection: systematic review and meta-analysis of randomized trials. Ann Surg. 258(1):59-65.
Publisher | Google Scholor - Kowalewski M, Pawliszak W, Zaborowska K, Navarese EP, Szwed KA, Kowalkowska ME, et al. (2015). Gentamicin-collagen sponge reduces the risk of sternal wound infections after heart surgery: Meta-analysis. J Thorac Cardiovasc Surg. 149(6):1631-1640.
Publisher | Google Scholor - Mavros MN, Mitsikostas PK, Alexiou VG, Peppas G, Falagas ME. (2012). Gentamicin collagen sponges for the prevention of sternal wound infection: a meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg. 144(5):1235-1240.
Publisher | Google Scholor - Cornely OA, Bethe U, Seifert H, Breuer K, Schütt-Gerowitt H, Salzberger B, et al. (2002). A randomized monocentric trial in febrile neutropenic patients: ceftriaxone and gentamicin vs cefepime and gentamicin. Ann Hematol. 81(1):37-43.
Publisher | Google Scholor - Mitra AG, Whitten MK, Laurent SL, Anderson WE. (1997). A randomized, prospective study comparing once-daily gentamicin versus thrice-daily gentamicin in the treatment of puerperal infection. Am J Obstet Gynecol. 177(4):786-792.
Publisher | Google Scholor - Smith CR, Baughman KL, Edwards CQ, Rogers JF, Lietman PS. (1977). Controlled comparison of amikacin and gentamicin. N Engl J Med. 296(7):349-353.
Publisher | Google Scholor - Faillace WJ, Tan P. (2000). Serum and Cerebrospinal Fluid Vancomycin and Gentamicin Concentrations during Ventriculoperitoneal Shunt Surgery: An Observational Study. J Pharmacol Technol. 16(4):155-160.
Publisher | Google Scholor - Brückner O, Alexander M, Martens F. (1980). Gentamicin concentrations in cerebrospinal fluid of patients with inflamed and uninflamed meninges (author's transl). Infection. 8(2):86-89.
Publisher | Google Scholor - McCracken GH Jr, Mize SG, Threlkeld N. (1980). Intraventricular gentamicin therapy in gram-negative bacillary meningitis of infancy. Report of the Second Neonatal Meningitis Cooperative Study Group. Lancet. 1(8172):787-791.
Publisher | Google Scholor - Pickering LK, Ericsson CD, Ruiz-Palacios G, Blevins J, Miner ME. (1978). Intraventricular and parenteral gentamicin therapy for ventriculitis in children. Am J Dis Child. 132(5):480-483.
Publisher | Google Scholor - Rahal JJ Jr, Hyams PJ, Simberkoff MS, Rubinstein E. (1974). Combined intrathecal and intramuscular gentamicin for gram-negative meningitis. Pharmacologic study of 21 patients. N Engl J Med. 290(25):1394-1398.
Publisher | Google Scholor - Swann O, Everett DB, Furyk JS, Harrison EM, Msukwa MT, Heyderman RS, et al. (2014). Bacterial meningitis in Malawian infants <2 months of age. Pediatr Infect Dis J. 33(6):560-565.
Publisher | Google Scholor - Kimberlin DW. (2002). Meningitis in the Neonate. Curr Treat Options Neurol. 4(3):239-248.
Publisher | Google Scholor - Zoumboulakis D, Anagnostakis D, Arseni A, Nicolopoulos D, Matsaniotis N. (1973). Gentamicin in the treatment of purulent meningitis in neonates and infants. Acta Paediatr Scand. 62(1):55-58.
Publisher | Google Scholor - Kauffman RE, Morris JA, Azarnoff DL. (1975). Placental transfer and fetal urinary excretion of gentamicin during constant rate maternal infuse. Pediatr Res. 9(2):104-107.
Publisher | Google Scholor - Yoshioka H, Monma T, Matsuda S. (1972). Placental transfer of gentamicin. J Pediatr. 80(1):121-123.
Publisher | Google Scholor - Ito T. (1970). Absorption and excretion of gentamicin in new-born infants. Jpn J Antibiot. 23(3):187-192.
Publisher | Google Scholor - Boda A. (1990). Gentamycin concentration in the milk of a mother after treatment by implantation of a Septopal chain. Orv Hetil. 131(41):2263-2265.
Publisher | Google Scholor - Celiloglu M, Celiker S, Guven H, Tuncok Y, Demir N, Erten O. (1994). Gentamicin excretion and uptake from breast milk by nursing infants. Obstet Gynecol. 84(2):263-265.
Publisher | Google Scholor