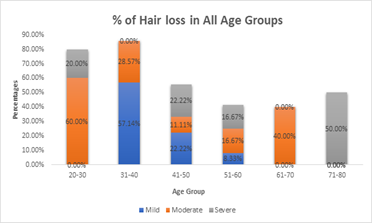Original article
Clinical Audit on Sensor-Controlled Scalp Cooling to Prevent Chemotherapy Induced Alopecia in Cancer Patients at Day Care Oncology in a Tertiary Care Hospital Karachi, Pakistan
1Staff Medical Officer Supervisor at Day Care Oncology Department of Oncology the Aga Khan University Hospital, Karachi, Pakistan.
2Associate professor Section Head of Medical Oncology Department of Oncology the Aga Khan University Hospital, Karachi, Pakistan.
3Medical Officer Day Care Oncology Department of Oncology the Aga Khan University Hospital, Karachi, Pakistan.
4Consultant oncologist Department of Oncology the Aga Khan University Hospital, Karachi, Pakistan.
5Head Nurse Day care/Radiation Oncology Department of Oncology the Aga Khan University Hospital, Karachi, Pakistan.
*Corresponding Author: Arifa Aziz,Staff Medical Officer Supervisor at Day Care Oncology Department of Oncology the Aga Khan University Hospital, Karachi, Pakistan.
Citation: Aziz A, Moosajee M, Shehzad I, Zaki A, Hussain A. (2024). Clinical Audit on Sensor-Controlled Scalp Cooling to Prevent Chemotherapy Induced Alopecia in Cancer Patients at Day Care Oncology in a Tertiary Care Hospital Karachi, Pakistan, International Journal of Medical Case Reports and Reviews, BioRes Scientia Publishers. 3(1):1-6. DOI: 10.59657/2837-8172.brs.24.041
Copyright: © 2024 Arifa Aziz, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 22, 2024 | Accepted: February 12, 2024 | Published: February 16, 2024
Abstract
Background: Scalp cooling has been introduced in May 2023 at Aga Khan University Hospital at chemotherapy unit in day care oncology to prevent chemotherapy induced alopecia, one of the most common and psychologically troubling side effects in both genders and especially in females. Currently available scalp cooling cap systems demonstrate varying results concerning effectiveness and tolerability.
Methods: For this prospective study forty patients including both genders and receiving neoadjuvant, adjuvant and palliative chemotherapy. Effectiveness of a sensor-controlled scalp cooling cap is assessed according to alopecia areata severity scale (AASc) [1]. A Multidimensional Assessment Tool for Clinical use system to measure chemotherapy-induced alopecia. Patients using sensor-controlled scalp cooling cap mostly have diagnosis of breast cancer, gynecological cancers, germ cell tumour and lymphomas. Frequently administered chemotherapeutic drugs were carboplatin, paclitaxel, doxorubicin, cyclophosphamide, docetaxel, bleomycin, and gemcitabine. Clinical assessments, satisfaction questionnaires, and alopecia evaluations feedback form was developed, and these forms will be filled by day care physicians after each chemotherapy cycle.
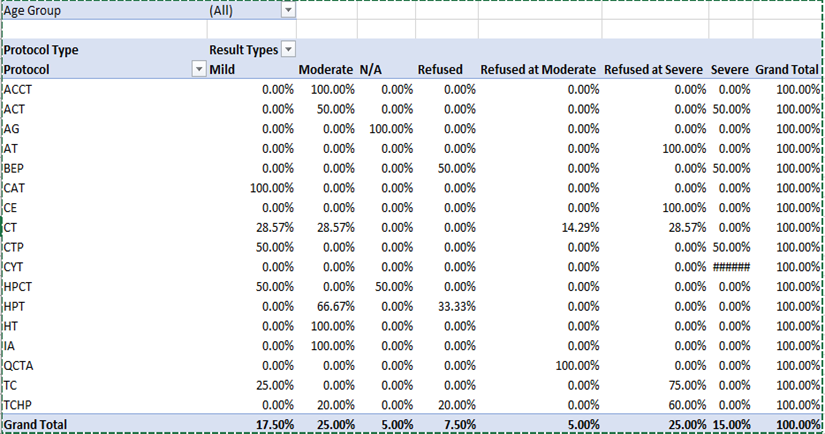
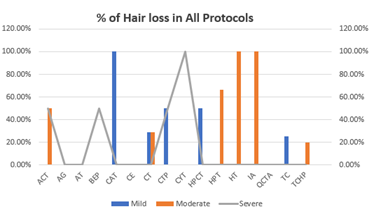
Result: Out of forty patients’ mild hair fall was seen in patients between age of thirty to forty years, moderate hair fall was seen in patients between age of twenty to thirty years and severe hair fall was seen in patients age between fifty to seventy. Above seventy years of age 100 % hair fall noticed in patients irrespective of drugs administered. According to chemotherapeutic drugs, patients receiving carboplatin and paclitaxel have mild hair loss. Patients receiving Adriamycin, cyclophosphamide, ifosphamide had moderate hair loss. Patients receiving docetaxel, cyclophosphamide, bleomycin, etoposide, cisplatin had severe hair fall.
Conclusion: The ability of scalp cooling cap is to prevent chemotherapy-induced alopecia and this cooling cap effectiveness varies with chemotherapeutic drugs and age. Therefore prior to start of chemotherapy patients counselling need to be done regarding partial and complete hair loss and no response according to chemotherapeutic drugs and age.
Keywords: chemotherapy induced alopecia; sensor-controlled scalp cooling cap; breast cancer; ovarian cancer; carboplatin; paclitaxel; young age
Introduction
Sensor controlled scalp cooling cap was introduced for the first time in Pakistan at Aga Khan University Hospital Karachi in May 2023. Aim of using this cooling cap is to prevent chemotherapy induced hair loss in cancer patients. The sensor-controlled scalp cooling cap has been used internationally since last forty years to prevent chemotherapy-induced hair loss [1]. Chemotherapy-induced alopecia is one of the most common and distressing side effects of chemotherapy treatment, that may lead to a negative body image, lower self-esteem, severe depression, anxiety and disturbances in social relationships [2,3,4]. In a few patients it was seen that the fear of hair loss, and the associated distress may even result in refusal to treatment with chemotherapy, although the patients are well informed that once chemotherapy completed the hair will regrow and chemotherapy induced alopecia is not permanent only temporary phase [5]. The results regarding effectiveness of scalp cooling are unpredictable and varies from person to person depending on multiple factors [6]. The aim of our study is to assess the effectiveness and tolerability of scalp cooling cap through feedback-proforma which was prepared by day care oncology physicians.
Primary Objective
To check effectiveness of sensor-controlled scalp cooling cap and to assess the percentage of chemotherapy induced hair fall. Effectiveness of a sensor-controlled scalp cooling cap is assessed according to Alopecia Areata Severity Scale (AASc) [6,7].
Materials And Methods
Effectiveness of a sensor-controlled scalp cooling cap is assessed according to alopecia areata severity scale (AASc) multidimensional international assessment tool for clinical use to assess chemotherapy-induced alopecia.
Study Site
Daycare Oncology chemotherapy unit, Department of oncology at Aga Khan University Hospital, Karachi.
Duration of Study
Patients from 29th May 2023 till 30th December 2023 were included who had given consent for sensor-controlled scalp cooling.
Study Design
Prospective study.
Sample Size
Forty patients.
Inclusion criteria
All adult patients of both genders aged eighteen years and above,diagnosed with cancer and are receiving chemotherapy.
Exclusion criteria
Pediatric population and patients who refused for use of scalp cooling cap in their subsequent sessions of chemotherapy cycles.
Discussion
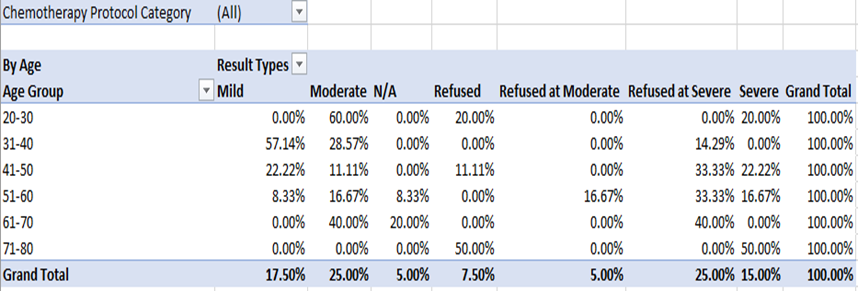
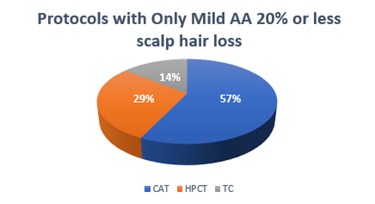
Scalp hypothermia due to vasoconstriction of blood vessels to prevent alopecia to reduce blood circulation to the scalp leading to decreased perfusion of the hair follicles and ultimately to diminish intrafollicular drug uptake and metabolism [7]. Different methods for scalp cooling have been used internationally, one of the methods is use of frozen cryogel in the shape of a helmet and circulating cold air or cold liquid in a cap or helmet but this method is not very comfortable [8-12]. Drawbacks of these reported cooling methods include lack of consistent scalp temperature control, loosely fitted caps, weight of the cap and high workload for the nursing staff to change the frozen cryogel cap every 30 minutes therefore now use of such cap is decrease significantly [13-15]. The modern scalp-cooling system used in this study includes tight-fitting, light, and soft silicone caps not covering the ears and partially cover the forehead. Lowering of the temperature is stepwise and slow, and the temperature of the scalp is measured with three different sensors to maintain a constant hypothermia of the skin surface [16]. This sensor-controlled scalp cooling system showed a high efficacy in preventing chemotherapy-induced alopecia in many updated chemotherapy regimens which was assessed with subjective and objective measurements [17,18]. In our study good response is seen in chemotherapeutic drugs carboplatin and paclitaxel regimen as it causes only mild hair loss with cooling cap as compared to other regimens which potentially contributed towards the moderate and severe hair fall. In our total sample of forty patients, thirty-three patients were receiving carboplatin and paclitaxel regimen along with immunotherapy, out of which seventeen (42.5%) patients refused for cooling cap due to multiple reasons; persistent hair fall, headache and other intolerance issues. The seventeen patients who refused to continue cooling cap, fifteen patients of them (37.5%) received Carboplatin and paclitaxel and two patients (5%) were on other chemotherapeutic drugs. With combined subjective and objective success rates of over 50%, in females age between thirty years to forty years as shown in figure -1, mostly patients were receiving chemotherapy regimen with carboplatin and paclitaxel which suggest that scalp cooling can be recommended in schedules such as paclitaxel weekly and the combination regimens of plus carboplatin as shown in Table-(1,1.1). The combined success rate was significantly higher in the paclitaxel weekly and paclitaxel plus carboplatin regimens when compared to the others chemotherapeutic drugs like docetaxel, doxorubicin cyclophosphamide, hair loss could not be prevented and better not to recommend scalp cooling cap for these chemotherapeutic drugs. Patients receiving docetaxel three cycles at every 3 weeks, as well as Adriamycin and cyclophosphamide four cycles at every 3 weeks showing moderate to severe response in hair fall. Docetaxel, doxorubicin and cyclophosphamide, the success rate of the scalp cooling system was less then 30%. Scalp cooling cap response lies between the moderate range of 60% for younger patients. As young age is a prognostic factor for lower levels of hair loss, despite more then half of the patients in age group of 20 and 30 experienced moderate hair fall as shown in Table-2. This suggests that, despite their young age, the predominant factor contributing to persistent hair loss in individuals undergoing chemotherapy is the specific chemotherapy regimen they are receiving, not withstanding the use of a cooling cap. However, noteworthy observations within this age group cohort indicate that the onset and duration of alopecia were attenuated when considering thirty-three patients receiving paclitaxel / carboplatin three weekly used no head cover till the last cycle, The Scalp cooling intolerance of only 2% in our study reflects the high acceptability of this sensor-controlled cooling cap and is similar to the rate reported by Riddersheim et al. [13]. In international study using another scalp cooling device (Paxman), intolerance to cooling was reported to be 3% [19]. A recent randomized study showed that scalp cooling duration may be shortened without reduction in efficacy, which may make scalp cooling even more acceptable to patients [20-24].
Table 1
Table- 1.1
Table-2
Figure-1
Figure-2
Figure-3
Results
Out of forty patients, thirty-three patients were receiving carboplatin and paclitaxel along with immunotherapy. Seventeen (42.5%) patients refused cooling cap due to multiple issues, in which 15 patients (37.5%) were on carboplatin and paclitaxel and two (5%) were on other chemotherapeutic drugs. Out of forty patients whose age is seventy or above had 100 % hair fall irrespective of drugs administered, in patients age between 20 to 30 years moderate hair fall seen and in patients between age 30 to 40 years mild hair fall seen. According to chemotherapeutic drugs carboplatin and paclitaxel have mild hair loss. Patients receiving Adriamycin, cyclophosphamide, ifosphamide had moderate hair loss. Patients receiving docetaxel and cyclophosphamide, bleomycin, etoposide, and cisplatin had severe hair fall.
Conclusion
Sensor controlled scalp cooling cap systems has been designed to prevent chemotherapy-induced alopecia showing variable results according to age and chemotherapeutic drugs. Our research showed that the effectiveness of sensor-controlled scalp cooling system, depends on the chemotherapeutic drugs. Carboplatin and paclitaxel showing good response and considering the patient’s age good response also seen in younger age. Therefore prior to start of chemotherapy patients counselling need to be done regarding partial and complete hair loss and no response according to the chemotherapeutic drugs and age.
Conclusion
Sensor controlled scalp cooling cap systems has been designed to prevent chemotherapy-induced alopecia showing variable results according to age and chemotherapeutic drugs. Our research showed that the effectiveness of sensor-controlled scalp cooling system, depends on the chemotherapeutic drugs. Carboplatin and paclitaxel showing good response and considering the patient’s age good response also seen in younger age. Therefore prior to start of chemotherapy patients counselling need to be done regarding partial and complete hair loss and no response according to the chemotherapeutic drugs and age.
Declarations
Conflict of interest
None
Acknowledgement
Authors would like to thank Dr Zehra Fadoo Professor and Chair of Department of Oncology, Mr. Noman Siddiqui Senior manager, Mrs. Nousheen Aftab Nursing Manager and Salman Fahim Associate. Suhana Fatima Shahid (Final year medical student) Karachi Medical and Dental College.
References
- King BA, Mesinkovska NA, Craiglow B, et al. (2022). Development of the alopecia areata scale for clinical use: Results of an academic-industry collaborative effort. J Am Acad Dermatol, 86(2):359-364.
Publisher | Google Scholor - King BA, Mesinkovska NA, Craiglow B, et al. (2022). Development of the alopecia areata scale for clinical use: Results of an academic-industry collaborative effort. J Am Acad Dermatol, 86(2):359-364.
Publisher | Google Scholor - Edelstyn GA, Macdonald M, Macrae KD. (1977). Doxorubicin-induced hair loss and possible modification by scalp cooling. The Lancet, 310:253-254.
Publisher | Google Scholor - Hesketz PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D.(2004).Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Cancer Care, 12:543-549.
Publisher | Google Scholor - Mols F, van den Hurk CJ, Vingerhoets AJ, Breed WP. (2009). Scalp cooling to prevent chemotherapy-induced hair loss: practical and clinical considerations. Support Care Cancer, 17(2):181-189.
Publisher | Google Scholor - Paus R, Haslam IS, Sharov AA, Botchkarev VA. (2013). Pathobiology of chemotherapy-induced hair loss. Lancet Oncol, 14(2):e50-e59.
Publisher | Google Scholor - King BA, Malinovskaya NA, Craiglow B, et al. (2022). Development of the alopecia areata scale for clinical use: Results of an academic-industry collaborative effort. J Am Acad Dermatol, 86(2):359-364.
Publisher | Google Scholor - Adams L, Lawson N, Maxted KJ, Symonds RP. (1992). The prevention of hair loss from chemotherapy by the use of cold-air scalp-cooling. Eur J Cancer Care, 1:16-18.
Publisher | Google Scholor - Hillen HF, Breed WP, Botman CJ. (1990). Scalp cooling by cold air for the prevention of chemotherapy-induced alopecia. Neth J Med, 37(5-6):231-235.
Publisher | Google Scholor - Katsimbri P, Bamias A, Pavlidis N. (2000). Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer, 36:766-777.
Publisher | Google Scholor - Lemenager M, Lecomte S, Bonneterre ME, Bessa E, Dauba J, Bonneterre J. (1997). Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer, 33(2):297-300.
Publisher | Google Scholor - Symonds RP, McCormick CV, Maxted KJ. (1986). Adriamycin alopecia prevented by cold air scalp cooling. Am J Clin Oncol, 9(5):454-457.
Publisher | Google Scholor - Ridderheim M, Bjurberg M, Gustavsson A. (2003). Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer, 11:371-377.
Publisher | Google Scholor - Sato K, Inoue K, Saito T, et. al. (2003). Multicenter phase II trial of weekly paclitaxel for advanced or metastatic breast cancer: the Saitama Breast Cancer Clinical Study Group (SBCCSG-01). Jpn J Clin Oncol, 33(8):371-376.
Publisher | Google Scholor - Chevallier B, Fumoleau P, Kerbrat P, et. al. (1995). Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol, 13:314-322
Publisher | Google Scholor - Martin M, Pienkowski T, Mackey J, et.al. (2005). Adjuvant docetaxel for node-positive breast cancer. N Engl J Med, 352(22):2302-2313.
Publisher | Google Scholor - Roché H, Fumoleau P, Spielmann M, et al. (2006). Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol, 24(36):5664-5671.
Publisher | Google Scholor - Schaffrin-Nabe D, Schmitz I, Josten-Nabe A, von Hehn U, Voigtmann R. (2015). The Influence of Various Parameters on the Success of Sensor-Controlled Scalp Cooling in Preventing Chemotherapy-Induced Alopecia. Oncol Res Treat, 38(10):489-495.
Publisher | Google Scholor - Van den Hurk CJ, Peerbooms M, van de Poll-Franse LV, Nortier JW, Coebergh JW, Breed WP. (2012). Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients - results of the Dutch Scalp Cooling Registry. Acta Oncol, 51(4):497-504.
Publisher | Google Scholor - Komen MM, Breed WP, Smorenburg CH, et al. (2016). Results of 20- versus 45-min post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer, 1-7.
Publisher | Google Scholor - Friedrichs K, Carstensen MH. (2014). Successful reduction of alopecia induced by anthracycline and taxane containing adjuvant chemotherapy in breast cancer - clinical evaluation of sensor-controlled scalp cooling. Springer plus, 3:500.
Publisher | Google Scholor - Auvinen PK, Mähönen UA, Soininen KM, Paananen PK, Ranta-Koponen PH, Saavalainen IE, Johansson RT. (2010). The effectiveness of a scalp cooling cap in preventing chemotherapy-induced alopecia. Tumori, 96(2):271-275.
Publisher | Google Scholor - Pignata S, Scambia G, Ferrandina G, et al. (2011). Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial. J Clin Oncol, 29(27):3628-3635.
Publisher | Google Scholor - Fisher B, Brown AM, Dimitrov NV, et al. (1990). Two months of doxorubicin cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen- nonresponsive tumors: results from the Nation Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol, 8:1483-1496.
Publisher | Google Scholor