Review Article
Childhood Leukemia: A Review
1Department of Chemistry, Sri J.N.M.PG College, Lucknow, U.P, India.
2Department of Chemistry, Dayanand Girls PG College, Kanpur, U.P. India.
*Corresponding Author: D.K. Awasthi, Department of Chemistry, Sri J.N.M.PG College, Lucknow, U.P, India.
Citation: Awasthi D.K, Dixit A. (2023). Childhood Leukemia: A Review, Clinical Case Reports and Studies, BRS Publishers. 2(4); DOI: 10.59657/2837-2565.brs.23.034
Copyright: © 2023 D.K. Awasthi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 08, 2023 | Accepted: May 22, 2023 | Published: May 29, 2023
Abstract
Leukemia is cancer of the blood. It’s the most common form of cancer in childhood. The cancer cells grow in bone marrow and go into the blood. The bone marrow is the soft, spongy centre of some bones. It makes blood cells. When a child has leukemia, the bone marrow makes abnormal blood cells that don’t mature. The abnormal cells are usually white blood cells (leukocytes). The bone marrow also makes fewer healthy cells. The abnormal cells reproduce very quickly. Children with leukaemia can need treatment for up to 3 years.
Keywords: leukemia; bone marrow; blood cells; cancer; leukocyts
Introduction
Leukemia affects the blood cells, most often, the white blood cells. White blood cells normally develop from stem cells in the bone marrow When these cells grow abnormally, leukemia appears. The types of blood cells include:
- Red blood cells (erythrocytes): Red blood cells carry oxygen. When a child has a low level of healthy red blood cells, this is called anemia. A child may feel tired, weak, and short of breath.
- Platelets (thrombocytes): Platelets help with blood clotting and stop bleeding. When a child has low levels of platelets, he or she bruises and bleeds more easily.
- White blood cells (leukocytes): These fight infection and other disease. When a child has low levels of white blood cells, he or she is more likely to have infections.
There are different types of leukemia in children. Most leukemias in children are acute, which means they tend to grow quickly. Some of the types of leukemia that occur in children include:
- Acute lymphocytic (lymphoblastic) leukemia (ALL): This is the most common type of leukemia in children.
- Acute myelogenous (myeloid, myelocytic, non-lymphocytic) leukemia (AML): This is the second most common type of leukemia in children.
- Hybrid or mixed lineage leukemia: This type is rare. It is a mix of ALL and AML.
- Chronic myelogenous leukemia (CML): This type is also rare in children.
- Chronic lymphocytic leukemia (CLL): This type is extremely rare in children.
- Juvenile myelomonocytic leukemia (JMML): This is a rare type of cancer that doesn’t grow quickly (acute) or slowly (chronic).
Children with leukaemia can need treatment for up to 3 years. During this time, it's important they have the opportunity to live as normal a life as possible. Whenever feeling well enough, they should be encouraged to do their usual activities, like having playtime, and going to school or day care. The exact cause of leukemia in children is not known. There are certain conditions passed on from parents to children (inherited) that increase the risk for childhood leukemia. But, most childhood leukemia is not inherited. Researchers have found changes (mutations) in genes of the bone marrow cells. These changes may occur early in a child's life or even before birth. But they may occur by chance (sporadic).
The risk factors for childhood leukemia include:
- Exposure to high levels of radiation
- Having certain inherited syndromes, such as Down syndrome and Li-Fraumeni syndrome
- Having an inherited condition that affects the body's immune system
- Having a brother or sister with leukemia
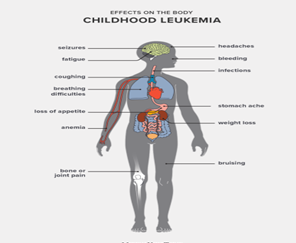
- Symptoms can occur a bit differently in each child.
They can include:
- Pale skin
- Feeling tired, weak, or cold
- Dizziness
- Headaches
- Shortness of breath, trouble breathing
- Frequent or long-term infections
- Fever
- Easy bruising or bleeding, such as nosebleeds or bleeding gums
- Bone or joint pain
- Belly (abdominal) swelling
- Poor appetite
- Weight loss
- Swollen lymph glands (nodes)
The prognosis for leukemia greatly depends on:
- How far the disease has spread
- The type of leukemia
- How well your child responds to treatment
- Genetics
- Age and overall health
- How well your child can tolerate the treatment
- New discoveries in treatment
The outlook and long-term survival are different for every cancer. Getting to a doctor and starting aggressive therapy quickly are key for the best outcome. A child with leukemia needs constant follow-up. Survivors can suffer from side effects of radiation and chemotherapy. Other cancers can occur as well. These may include skin, breast, brain / spine, thyroid gland, bone or other blood cancers. Monitoring for these diseases is crucial. Developing healthy habits like eating right and not smoking is important, too. New methods are being found every day to improve treatment and to decrease side effects from the treatment for this disease.
The term five-year survival rate means the percentage of patients who live at least five years after their cancer is diagnosed. With acute leukemias, these patients are probably cured. It is very rare for leukemia to return later than this. Current five-year survival rates are based on large numbers of children who were treated more than five years ago. These rates really can’t predict what will happen in your child’s case. Every child and every cancer are different. Also, because treatments change all the time, the survival rates from treatment done five or more years ago may not truly reflect today’s survival rates.
The exact causes of leukaemia in children are not known, but it is likely that several factors are involved. Factors that may put some children at higher risk of genetic damage that can lead to leukaemia include:
- infections: delayed exposure to common childhood infections or an abnormal response by the child’s immune system to these infections.
- radiation: exposure to large doses of ionising radiation (energy from x-rays and radioactive materials) before birth or in the early years.
- chemicals: exposure to high levels of certain chemicals, such as benzene.
- congenital disorders: like Down syndrome and Fanconi anaemia.
Figure 1
Tests for leukaemia
There are several tests that can be done to confirm a diagnosis of leukaemia and to work out which type it is:
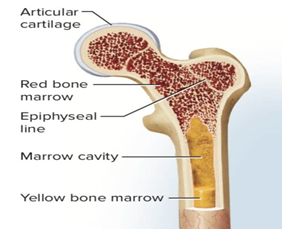
Bone marrow biopsy: A syringe is used to take a sample of bone marrow, usually from the hip bone, for examination under the microscope and genetic testing.
Figure 2
Lumber Puncture: sometimes called a spinal tap. A needle is put into the space between the bones of the lower back and fluid from around the spinal cord is removed for examination under the microscope to look for abnormal white blood cells.
Treatment of childhood leukaemia
The main treatment given to children with leukaemia is chemotherapy (a combination of medicines), usually as tablets or injections. Radiotherapy may also be used to kill cancer cells in the brain, and in some cases, a stem cell or bone marrow transplant may also be necessary.
Antibiotics to prevent infections immunotherapy to use the body's own immune system to fight cancer cells targeted therapy, medicine that targets cancer cells but with fewer side effects than chemotherapy blood products to restore the normal contents of the blood. When a child has leukemia, the bone marrow, for an unknown reason, begins to make faulty white blood cells. Normally, the body can regulate the production of cells by sending signals for when to stop producing more. Leukemia cells do not respond to the body’s signals. These cells go on reproducing themselves, even when there’s no more space in the bone marrow.
The bone marrow doesn’t just make infection fighting white blood cells. It also makes red blood cells and platelets. Red blood cells carry oxygen to all parts of the body. Platelets help with blood clotting to stop bleeding.
In leukemia, the abnormal white cells reproduce very quickly and do not fight infection well. These faulty white blood cells, called blasts, crowd the bone marrow. This can mean that not enough red blood cells or platelets are made. All this trouble in the bone marrow results in the symptoms of leukemia. These may include tiredness and problems with infections and bruising or bleeding. Bone pain can also occur as the bone marrow expands.
Leukemia is the most common form of cancer in childhood. It affects approximately 3,000 children each year in the United States. Leukemia accounts for about 30 percent of childhood cancers. While leukemia can occur at any age, it is most commonly seen in children between 2 and 6 years old. The disease occurs slightly more often in males than in females. It is most commonly seen in Caucasian children. Most childhood leukemias are caused by chance mutations in the genes of white blood cells. Except for rare genetic cases, little is known about the causes of these diseases. Scientists are hard at work trying to learn how these mutations happen.
Role of Immune System
The immune system plays a key role in protecting the body from diseases. A fault in the immune system may increase the risk for getting leukemia. Things like getting certain viruses or other infections can lower immunity. Toxins in the environment or exposure to chemicals may also make the immune system weaker.
Certain conditions may increase a child’s risk of developing leukemia
Down syndrome, Fanconi anemia, Neurofibromatosis, Shwachman-Diamond Syndrome, Ataxia telangiectasia (Louis-Bar syndrome), Bone marrow disorders (myelodysplasia), Pre-birth X-ray exposure, Significant radiation exposure Chemotherapy in the past, Certain genetic conditions, Leukemia is cancer of the blood. The cancer cells develop in the bone marrow and go into the blood. Other tissue and organs that may be affected include the lymph nodes, liver, spleen, thymus, brain, spinal cord, gums, and skin. When a child has leukemia, the bone marrow makes abnormal blood cells that do not mature. The abnormal cells are usually white blood cells (leukocytes). And with leukemia, the bone marrow makes fewer healthy cells. Common symptoms of leukemia in children include feeling tired and weak, easy bruising or bleeding, and frequent or long-term infections. Leukemia is diagnosed with blood and bone marrow tests. Imaging may be done to look for signs of leukemia in different parts of the body. Chemotherapy is the main treatment for most leukemias in children. A child with leukemia may have complications from the leukemia and from the treatment. Ongoing follow-up care is needed during and after treatment. The earliest signs of leukemia can be hard to spot. They can also vary from child to child, as not all children with leukemia show the symptoms listed above. Many of the symptoms are common and can indicate a range of illnesses. The doctor will perform various tests and assessments before making a diagnosis. If a parent or caregiver notices any of the symptoms above, it is best to take the child to a doctor as soon as possible. A prompt diagnosis can ensure that the child receives the right treatment quickly. Children with leukemia have high white blood cell counts, but most of these cells are not functioning correctly. This is because abnormal cells replace healthy white blood cells. White blood cells help protect the body by fighting off infections. For this reason, recurrent or persistent infections can indicate that a child does not have enough healthy white blood cells.
Early Symptoms
If a child bruises easily and experiences severe nosebleeds or bleeds from the gums, this may point to leukaemia. A child with this type of cancer will have a lack of platelets that help prevent bleeding. In rare cases, leukemia leads to very severe weakness and exhaustion that can result in slurred speech. This occurs when leukemia cells collect in the blood, causing the blood to thicken. The blood may be so thick that circulation slows through small vessels in the brain. A child may not be able to describe their symptoms in detail, but they may appear to be generally ill. They may also experience frequent, unexplained headaches. When the cause of a child’s illness is unclear, make an appointment with a doctor. In a child with leukemia, swelling can affect various parts of the body, including: In the abdomen, when abnormal cells collect in the liver or spleen In the face and arms, when pressure on a vein called the superior vena cava causes blood to pool in the area The lymph nodes, causing small lumps to form on the sides of the neck, in the underarms, or around the collarbone, where lymph nodes reside Importantly, a child with swollen lymph nodes and no additional symptoms is more likely to have an infection than leukemia. Also, tumors from other types of cancers are more likely to put pressure on the superior vena cava and lead to facial swelling. The swelling would be worse when a child wakes up, and it will improve throughout the day. This is called Superior vena cava syndrome and rarely occurs in cases of leukemia. However, it can be life threatening and requires emergency care. When Leukemia cells cause swelling in the liver, kidneys, or spleen, these organs can press against the stomach. The result may be a feeling of fullness or discomfort, a lack of appetite, and subsequent weight loss. If a child seems to be in pain and complains that their bones or joints are sore or achy, this can indicate childhood leukemia. When leukemia develops, the abnormal cells can collect close to the surface of the bones or inside joints. Leukemia is a cancer of the blood. It starts in blood stem cells.
Mechanism
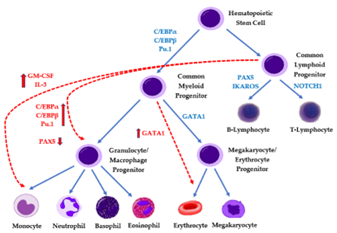
Stem cells are the earliest blood cells that develop into different types of specialized blood cells. As the stem cells of the blood develop, they produce immature blood cells (blasts). Blasts then develop into mature blood cells. Blood stem cells develop into either lymphoid stem cells or myeloid stem cells.
Lymphoid stem cells develop into lymphoblasts and then lymphocytes. Lymphocytes are a type of white blood cell that help fight infection and destroy abnormal cells. The 3 main types of lymphocytes are B cells, T cells and natural killer (NK) cells. B cells make antibodies that help fight infection. T cells destroy damaged and infected cells in the body and tell B cells to make antibodies. NK cells attack cancer cells or cells that are infected with a virus. Myeloid stem cells develop into different blasts (myeloblasts, monoblasts, erythroblasts and megakaryoblasts), which then develop into granulocytes and monocytes red blood cells and platelets. Granulocytes and monocytes are white blood cells that destroy bacteria and help fight infection. Red blood cells carry oxygen to all tissues of the body. Platelets form clots in damaged blood vessels to stop bleeding. Leukemia causes an overproduction of blasts. These blasts develop abnormally and develop into mature blood cells. Over time the blasts crowd out normal blood cells so that they can’t do their jobs. leukemia are grouped based on the type of blood stem cell. Lymphoid leukemia develops from abnormal lymphoid cells. Myeloid leukemia develops from abnormal myeloid cells. Acute leukemia starts suddenly, developing within days or weeks. Chronic leukemia develops slowly over months or years. Acute lymphoblastic leukemia (ALL) is the most common type of leukemia diagnosed in young children. It occurs more often in boys than girls. Acute myelogenous leukemia (AML) is less common than ALL. Rare types of childhood leukemia and leukemia-like disorders can also develop. These include transient abnormal myelopoiesis (TAM) (also called transient leukemia), acute promyelocytic leukemia (APL), juvenile myelomonocytic leukemia (JMML), chronic myelogenous leukemia (CML) and myelodysplastic syndrome (MDS).
Figure 3
Conclusion
A child with leukemia needs ongoing care. Your child will be seen by oncologists and other healthcare providers to treat any late effects of treatment and to watch for signs or symptoms of the cancer returning. Your child will be checked with imaging tests and other tests. And your child may see other healthcare providers for problems from the cancer or from treatment.
References
- Reya T, Morrison SJ, Clarke MF, Weissman IL. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414:105-111.
Publisher | Google Scholor - Majeti R, Park CY, Weissman IL. (2007). Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell, 1:635-645.
Publisher | Google Scholor - Morrison SJ, Weissman IL. (1994) The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity, 1:661-673.
Publisher | Google Scholor - Osawa M, Hanada K, Hamada H, Nakauchi H. (1996). Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273:242-245.
Publisher | Google Scholor - Randall TD, Lund FE, Howard MC, Weissman IL. (1996). Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood 87:4057-4067.
Publisher | Google Scholor - Kondo M, Weissman IL, Akashi K. (1997). Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell, 91:661-672.
Publisher | Google Scholor - Akashi K, Traver D, Miyamoto T, Weissman IL. (2000). A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature, 404:193-197.
Publisher | Google Scholor - Rosenbauer F, Tenen DG. (2007). Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol,7:105-117.
Publisher | Google Scholor - Iwasaki H, Mizuno S, Arinobu Y, Ozawa H, Mori Y, Shigematsu H, et al. (2006).The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev, 20:3010-3021.
Publisher | Google Scholor - Nerlov C, Graf T. PU. (1998). Induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev, 12:2403-412.
Publisher | Google Scholor - Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. (2006). Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity, 25:731-744.
Publisher | Google Scholor - Xie H, Ye M, Feng R, Graf T. (2004). Stepwise reprogramming of B cells into macrophages. Cell, 117:663-676.
Publisher | Google Scholor - Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F. et al. (2008) PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA, 105:6057-6062.
Publisher | Google Scholor - Cirovic B, Schönheit J, Kowenz-Leutz E, Ivanovska J, Klement C. et al. (2017). C/EBP-induced transdifferentiation reveals granulocyte-macrophage precursor-like plasticity of B cells. Stem Cell Reports, 8:346-359.
Publisher | Google Scholor - Orkin SH, Shivdasani RA, Fujiwara Y, McDevitt MA. (1998). Transcription factor GATA-1 in megakaryocyte development. Stem Cells, 16(2):79-83.
Publisher | Google Scholor - Wang X, Crispino JD, Letting DL, Nakazawa M, Poncz M. (2002). Control of megakaryocyte-specific gene expression by GATA-1 and FOG-1: role of Ets transcription factors. EMBO J, 21:5225-5234.
Publisher | Google Scholor - Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. (1999). Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood, 93:2867-2875.
Publisher | Google Scholor - Welch JJ, Watts JA, Vakoc CR, Yao Y, Wang H. et al. Blood (2004). Global regulation of erythroid gene expression by transcription factor GATA-1., 104:3136-3147.
Publisher | Google Scholor - Rhodes J, Hagen A, Hsu K, Deng M, Liu TX, et al. (2005). Interplay of PU.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev Cel ,l 8:97-108.
Publisher | Google Scholor - Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. (1996). Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci U S A, 93:12355-12358.
Publisher | Google Scholor - Gutiérrez L, Tsukamoto S, Suzuki M, Yamamoto-Mukai H, Yamamoto M et al. (2008). Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood, 111:4375-4385.
Publisher | Google Scholor - Kulessa H, Frampton J, Graf T. (1995). GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev, 9:1250-1262.
Publisher | Google Scholor - Visvader JE, Elefanty AG, Strasser A, Adams JM. (1992). GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J, 11:4557-1164.
Publisher | Google Scholor - Nutt SL, Heavey B, Rolink AG, Busslinger M. (1999). Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature, 401:556-562.
Publisher | Google Scholor - Lindeman GJ, Adams JM, Cory S, Harris AW. (1994). B-lymphoid to granulocytic switch during hematopoiesis in a transgenic mouse strain. Immunity, 1:517-527.
Publisher | Google Scholor - Hu Y, Zhang Z, Kashiwagi M, Yoshida T, Joshi I. et al. (2016). Super enhancer reprogramming drives a B-cell-epithelial transition and high-risk leukemia. Genes Dev, 30:1971-1979
Publisher | Google Scholor - Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med (2016) 374:2209–21.
Publisher | Google Scholor - Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. (2013). Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. Cancer Genome Atlas Research Network. N Engl J Med, 368:2059-2074.
Publisher | Google Scholor - Rossi L, Lin KK, Boles NC, Yang L, King KY. et al. (2012). Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell 11:302-317.
Publisher | Google Scholor - Grammatico S, Vitale A, La Starza R, Gorello P, Angelosanto N. et al. (2013). Lineage switch from pro-B acute lymphoid leukemia to acute myeloid leukemia in a case with t(12;17) (p13;q11)/TAF15-ZNF384 rearrangement. Leuk Lymphoma, 54:1802-1805.
Publisher | Google Scholor - Imataki O, Ohnishi H, Yamaoka G, Arai T, Kitanaka A. et al. (2010). Lineage switch from precursor B cell acute lymphoblastic leukemia to acute monocytic leukemia at relapse. Int J Clin Oncol, 15:112-115.
Publisher | Google Scholor - Shivarov V, Stoimenov A, Galabova I, Balatzenko G, Guenova M. (2009). Very early onset of an acute myeloid leukemia in an adult patient with B-cell lymphoblastic leukemia. Int J Lab Hematol, 31:106-113.
Publisher | Google Scholor - van den Ancker W, Terwijn M, Regelink J, Westers TM, Ossenkoppele GJ, van de Loosdrecht AA, et al. (2009). Uncommon lineage switch warrants immunophenotyping even in relapsing leukemia. Leuk Res 33:e77-80.
Publisher | Google Scholor - Della Starza I, Ceglie G, Nunes V, Gianfelici V, Marinelli M, Fuligni F, et al. (2016). A case of lineage switch from B-cell acute lymphoblastic leukaemia to acute myeloid leukaemia. Role of subclonal/clonal gene mutations. Br J Haematol, 174:648-651.
Publisher | Google Scholor - Dorantes-Acosta E, Arreguin-Gonzalez F, Rodriguez-Osorio CA, Sadowinski S, Pelayo R, Medina-Sanson A. (2009). Acute myelogenous leukemia switch lineage upon relapse to acute lymphoblastic leukemia: a case report. Cases J, 2:154.
Publisher | Google Scholor - Krawczuk-Rybak M, Zak J, Jaworowska B. (2003). A lineage switch from AML to ALL with persistent translocation t(4;11) in congenital leukemia. Med Pediatr Oncol, 41:95-96.
Publisher | Google Scholor - Lounici A, Cony-Makhoul P, Dubus P, Lacombe F, Merlio JP, Reiffers J. (2000). Lineage switch from acute myeloid leukemia to acute lymphoblastic leukemia: report of an adult case and review of the literature. Am J Hematol, 65:319-321.
Publisher | Google Scholor - Weinberg OK, Arber DA. (2010). Mixed-phenotype acute leukemia: historical overview and a new definition. Leukemia, 24:1844-1851.
Publisher | Google Scholor - Wolach O, Stone RM. (2015). How I treat mixed-phenotype acute leukemia. Blood, 125:2477-2485.
Publisher | Google Scholor - Matutes E, Pickl WF, Van’t Veer M, Morilla R, Swansbury J. et al. (2011). Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood, 117:3163-171.
Publisher | Google Scholor - Mejstrikova E, Volejnikova J, Fronkova E, Zdrahalova K, Kalina T. et al. (2010). Prognosis of children with mixed phenotype acute leukemia treated on the basis of consistent immunophenotypic criteria. Haematologica, 95:928-935.
Publisher | Google Scholor - Fujisaki H, Hara J, Takai K, Nakanishi K, Matsuda Y, Ohta H. et al. (1999). Lineage switch in childhood leukemia with monosomy 7 and reverse of lineage switch in severe combined immunodeficient mice. Exp Hematol, 27:826-233.
Publisher | Google Scholor - Slamova L, Starkova J, Fronkova E, Zaliova M, Reznickova L. et al. (2014).CD2-positive B-cell precursor acute lymphoblastic leukemia with an early switch to the monocytic lineage. Leukemia, 28:609-620.
Publisher | Google Scholor - Rossi JG, Bernasconi AR, Alonso CN, Rubio PL, Gallego MS. et al. (2012). Lineage switch in childhood acute leukemia: an unusual event with poor outcome. Am J Hematol, 87:890-897.
Publisher | Google Scholor - Cumano A, Paige CJ, Iscove NN, Brady G. (1992). Bipotential precursors of B cells and macrophages in murine fetal liver. Nature, 356:612-615.
Publisher | Google Scholor - Montecino-Rodriguez E, Leathers H, Dorshkind K. (2001). Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol, 2:83-88.
Publisher | Google Scholor - Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC. et al. (2012). Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature, 481:506-510.
Publisher | Google Scholor - Podgornik H, Debeljak M, Zontar D, Cernelc P, Prestor VV. et al. (2007). RUNX1 amplification in lineage conversion of childhood B-cell acute lymphoblastic leukemia to acute myelogenous leukemia. Cancer Genet Cytogenet, 178:77-81.
Publisher | Google Scholor - Mantadakis E, Danilatou V, Stiakaki E, Paterakis G, Papadhimitriou S. et al. (2007). T-cell acute lymphoblastic leukemia relapsing as acute myelogenous leukemia. Pediatr Blood Cancer, 48:354-357.
Publisher | Google Scholor - Muntean AG, Hess JL. (2012). The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol, 7:283-301.
Publisher | Google Scholor - Sanjuan-Pla A, Bueno C, Prieto C, Acha P, Stam RW, Marschalek R, et al. (2015). Revisiting the biology of infant t(4;11)/MLL-AF4+ B-cell acute lymphoblastic leukemia. Blood, 126:2676-2685.
Publisher | Google Scholor - Lin S, Luo RT, Ptasinska A, Kerry J, Assi SA. et al. (2016). Instructive role of MLL-fusion proteins revealed by a model of t(4;11) pro-B acute lymphoblastic leukemia. Cancer Cell, 30:737-749.
Publisher | Google Scholor - Balducci E, Nivaggioni V, Boudjarane J, Bouriche L, Rahal I. et al. (2017). Lineage switch from B acute lymphoblastic leukemia to acute monocytic leukemia with persistent t(4;11)(q21;q23) and cytogenetic evolution under CD19-targeted therapy. Ann Hematol, 96(9):1579-1581.
Publisher | Google Scholor - Rayes A, McMasters RL, O’Brien MM. (2016). Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer 63:1113-1115.
Publisher | Google Scholor - Gardner R, Wu D, Cherian S, Fang M, Hanafi LA. et al. (2016). Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood, 127:2406-2410.
Publisher | Google Scholor - Jacoby E, Nguyen SM, Fountaine TJ, Welp K, Gryder B, Qin H, et al. (2016). CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun, 7:12320.
Publisher | Google Scholor - Burda P, Laslo P, Stopka T. (2010). The role of PU.1 and GATA-1 transcription factors during normal and leukemogenic hematopoiesis. Leukemia 24:1249-1257.
Publisher | Google Scholor - Aikawa Y, Yamagata K, Katsumoto T, Shima Y, Shino M. et al. (2015). Essential role of PU.1 in maintenance of mixed lineage leukemia-associated leukemic stem cells. Cancer Sci, 106:227-236.
Publisher | Google Scholor - Aikawa Y, Katsumoto T, Zhang P, Shima H, Shino M. et al. (2010). PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2. Nat Med, 16:580-585.
Publisher | Google Scholor - Yeh JR, Munson KM, Chao YL, Peterson QP, Macrae CA. (2008). AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression. Development,135:401-410.
Publisher | Google Scholor - Monma F, Nishii K, Ezuki S, Miyazaki T, Yamamori S. et al. (2006). Molecular and phenotypic analysis of Philadelphia chromosome-positive bilineage leukemia: possibility of a lineage switch from T-lymphoid leukemic progenitor to myeloid cells. Cancer Genet Cytogenet, 164:118-121.
Publisher | Google Scholor - Morrison SJ, Scadden DT. (2014). The bone marrow niche for haematopoietic stem cells. Nature, 505:327-334.
Publisher | Google Scholor - Schepers K, Campbell TB, Passegue E. (2015). Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell ,16:254-267.
Publisher | Google Scholor - Jiang Y, Nakada D. (2016) Cell intrinsic and extrinsic regulation of leukemia cell metabolism. Int J Hematol,103:607-616.
Publisher | Google Scholor - Dong L, Yu WM, Zheng H, Loh ML, Bunting ST, Pauly M, et al. (2016) Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature, 539:304-308.
Publisher | Google Scholor - Zhang W, Trachootham D, Liu J, Chen G, Pelicano H. et al. (2012) Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol, 14:276-286.
Publisher | Google Scholor - Barabe F, Kennedy JA, Hope KJ, Dick JE. (2007). Modeling the initiation and progression of human acute leukemia in mice. Science, 316:600-604.
Publisher | Google Scholor - Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. (2008) Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell 13:483-495.
Publisher | Google Scholor - McClellan JS, Dove C, Gentles AJ, Ryan CE, Majeti R. (2015) Reprogramming of primary human Philadelphia chromosome-positive B cell acute lymphoblastic leukemia cells into nonleukemic macrophages. Proc Natl Acad Sci U S A 112:4074-4079.
Publisher | Google Scholor - Maeda K, Baba Y, Nagai Y, Miyazaki K, Malykhin A. et al. (2005). IL-6 blocks a discrete early step in lymphopoiesis. Blood, 106:879-885.
Publisher | Google Scholor - Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M. et al. (2011). IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell, 20:661-673.
Publisher | Google Scholor - Schurch CM, Riether C, Ochsenbein AF. (2014). Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell, 14:460-472.
Publisher | Google Scholor - de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. (2012). IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood, 119:1543-1554.
Publisher | Google Scholor - Stass S, Mirro J, Melvin S, Pui CH, Murphy SB, Williams D. (1984). Lineage switch in acute leukemia. Blood, 64:701-706.
Publisher | Google Scholor - Zoghbi A, Zur Stadt U, Winkler B, Müller I, Escherich G. (2017). Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr Blood Cancer, 64:e26594.
Publisher | Google Scholor