Research Article
Characterization of a Unique Manganese-Containing Biosurfactant Produced from Pseudomonas Aeruginosa Strain S16, Isolated from Soil Found in A Mixed Farm, Located in Ibadan, Oyo State, Nigeria
- I. K. M. Okorie *
- A. A. Ogunjobi
Department of Microbiology, University of Ibadan, Ibadan, 200005, Nigeria.
*Corresponding Author: I. K. M. Okorie, Department of Microbiology, University of Ibadan, Ibadan, 200005, Nigeria.
Citation: Okorie I. K. M., Ogunjobi A. A. (2023). Characterisation of a Unique Manganese-Containing Biosurfactant Produced from Pseudomonas Aeruginosa Strain S16, Isolated from Soil Found in A Mixed Farm, Located in Ibadan, Oyo State, Nigeria. Clinical Case Reports and Studies, BioRes Scientia Publishers. 5(5):1-13. DOI: 10.59657/2837-2565.brs.24.124
Copyright: © 2024 I. K. M. Okorie, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 29, 2024 | Accepted: April 19, 2024 | Published: April 29, 2024
Abstract
The worldwide occurrence of antibiotic resistance, has triggered the quest for viable alternatives, to the failing antibiotics in common usage. Biosurfactants have been studied for their potential in this regard. The aim of this study, was to characterize a biosurfactant, produced from Pseudomonas aeruginosa strain S16 (accession number OQ734845), shown to possess effective biocontrol activity, against Staphylococcus aureus SO183 at 0.060 g/L. Pseudomonas aeruginosa strain S16 was isolated from soil in a mixed farm, at Ajibode area, Ibadan, Oyo State, Nigeria, with the use of the pour plate method, identified through biochemical tests, and confirmed through molecular studies. Scanning electron microscopy revealed that the biosurfactant possessed a surface composed of, globular and spindle-shaped projections, while energy dispersive x-ray spectroscopy revealed that its elemental composition was mainly oxygen (3.00%), carbon (36.10%), nitrogen (43.00%), phosphorus (3.00%) and sodium (6.60%); the least abundant was manganese (0.48%). High performance liquid chromatography and mass spectrometry, revealed that the monosaccharides, were mainly rhamnose, glucose, xylose and mannose, while the phenol-sulfuric acid method measured their concentration as 0.0047 g/L. Fourier transform infrared spectroscopy, revealed the presence of carboxyl, alcohol, amine and halo functional groups, amongst others. The biosurfactant produced from Pseudomonas aeruginosa strain S16 was a positively charged, manganese-containing, polymeric biosurfactant made up of carbohydrates, lipid and peptide molecules. It was likely a positively-charged, manganese-containing glycolipopeptide/ glycolipoprotein, polysaccharide-protein-fatty acid composite, or lipopolysaccharide-protein composite. The manganese probably conferred to it, the potential for antibacterial and diverse bioactivities. From available records, this is the first report of a manganese-containing biosurfactant.
Keywords: biosurfactant; characterization; Pseudomonas aeruginosa strain S16; antibiotic resistance; manganese-containing
Introduction
The worldwide occurrence of antibiotic resistance has triggered the quest for viable alternatives, to the present failing antibiotics in common usage. Antibiotic resistance is the descriptive term used, when bacteria are observed to possess the ability, to evade the harmful effects of drugs introduced either to kill them, or to suppress their growth. Antibiotic resistance is a major part of antimicrobial resistance (AMR) (WHO, 2021) that could develop on its own, but occurs mostly as a result of the inappropriate use of drugs, and the mismanagement of illnesses (Tanwar et al., 2014; Saha et al., 2021). The quest for alternative antibiotics has led, in part, to the use of biosurfactants. Biosurfactants possess antimicrobial properties (Pessione and Garcia-Contreras, 2022) amongst others. Biosurfactants are surface-acting compounds of biological origin. They are unique, secondary metabolites, synthesised in the absence of ribosomes, by some microbes (Ndlovu et al., 2017). The word “surfactant”, was created from the phrase “surface active agent” (Rosen and Kunjappu, 2012). Biosurfactants are amphiphilic chemical compounds (they have a hydrophobic tail group and a hydrophilic head group (Leong et al., 2021)), produced by microorganisms, with many advantages over conventional surfactants (Sandeep and Rajasree, 2017). Some of these advantages include easy biodegradability, minimal toxicity and unaltered activity in extreme conditions of salinity and pH (Costa et al., 2018).
Biosurfactants can broadly be grouped into two: biosurfactants with low molecular weights, and biosurfactants with high molecular weights (Adu et al., 2020). Biosurfactants with low molecular weights, have greater efficiency at surface tension and interfacial tension reduction, while those with high molecular weights (bioemulsifiers), demonstrate greater emulsification activity, than either surface, or interfacial tension reduction (Banat et al., 2010). Biosurfactants can also be classified, based on their source of production, their chemical properties, and their electrical charges. With reference to their source, biosurfactants could be, for example, bacterial or fungal. With regards to their chemical composition, the classification of biosurfactants, includes lipopeptide/lipoprotein biosurfactants, phospholipid biosurfactants, neutral lipid biosurfactants, glycolipid biosurfactants, glycopeptide biosurfactants, polymeric (polysaccharide-protein-fatty acid composites, lipopolysaccharide-protein composites, glycolipopeptide/ glycolipoprotein) biosurfactants and particulate biosurfactants (Gürkök and Özdal, 2021; Santos et al., 2016). Lipopeptide and lipoprotein biosurfactants, possess annular-shaped peptides, joined to chains of hydrophobic fatty acids (Sharma et al; 2021). An example is surfactin, produced by Bacillus subtilis species. Surfactin possesses a 7-amino acid cyclic shape, joined by a lactone to a fatty acid chain, and is a very powerful biosurfactant with activity against bacteria, viruses and fungi (Meena et al., 2017). Glycolipid biosurfactants are formed by the bonding of carbohydrates and lipids, and are situated in cell membranes (Hamid et al., 2015). The poster glycolipid biosurfactants, are the sophorolipids, rhamnolipids and trehalolipids (Chrzanowski et al., 2012). Neutral lipid biosurfactants, are ester-like in chemical composition, and waxy in texture, and are produced by marine-dwelling hydrocarbonoclastic bacteria (Gayathiri et al., 2022). Polymeric biosurfactants are surfactants that are huge in size, and are composed of a collocation of smaller, repeated sub-units; known examples include emulsan, alasan, liposan, and complexes of polysaccharide proteins (Vijayakumar and Saravanan, 2015). Other examples of polymeric biosurfactants include, glycolipopeptide biosurfactants and glycolipoprotein biosurfactants; these are made up of carbohydrate, lipids and peptide (or protein) chains. Lipopolysaccharide-protein composites are part of the external plasma membrane of Gram-negative bacteria (Solov'eva and IuS, 1983). Particulate biosurfactants, are biosurfactants that cause the disaggregation of microbial extracellular membrane vesicles; this disaggregation, results in the formation of tiny emulsions, that in turn, produce some force on alkane uptake into the cells (Vijayakumar and Saravanan, 2015). Examples of particulate biosurfactants, are the vesicles produced by Acinetobacter species, that consist of proteins, phospholipids, and lipopolysaccharides (Vijayakumar and Saravanan, 2015). Phospholipid biosurfactants are complexes of phosphorus and lipids, with surface tension-reducing properties, while fatty acid biosurfactants, are lipid subunits, with micelle-formation properties. Many microorganisms, produce fatty acid and lipids, that have very good biosurfactant characteristics; this fact, makes them much sought out for, by several industries (Gayathiri et al., 2022). Acinetobacter species also produce vesicles with huge quantities of the fatty acid biosurfactant, phosphatidyl ethanolamine (Santos et al., 2016). Surfactants can be classified based on the net electrical charges they possess, into anionic, cationic, amphoteric (zwitterionic) and non-ionic surfactants (Nakama, 2017); this classification can also be adapted for biosurfactants. The electrical charges emanate from the polar head of the biosurfactant (Santos et al., 2016). Biosurfactants are mostly either anionic or neutral; the positively charged biosurfactants are those that contain amines (Santos et al., 2016).
Biosurfactants have antimicrobial activity, and this is mainly from the positive charges on their cationic head; the more hydrophobic a biosurfactant is, the more antimicrobial activity it exhibits (Pessione and Garcia-Contreras, 2022. Biosurfactants can therefore, be used as antibiotics, antivirals and antifungals (Sharma et al., 2021).
The metal manganese (Mn), is a chemical substance; it is the 25th element on the periodic table, and weighs 54.9 (Al-Fartusie. and Mohssan, 2017). Manganese is a blend of the colours, grey and white; and is splintery in composition (DOCCEEW, 2022). It is one of the ten principal elements, that exist in minute quantities (“trace elements”), in both greenfield and brownfield lands, and whose intake in gross amounts, is injurious to living things (Silva et al., 2019). In the soil, it is very reactive (Li et al., 2021). Manganese is also found in both air and water matrices (Gad, 2005). The major way through which manganese enters the human body, is the intake of foods rich in manganese, and the intake of water from wells (Harischandra et al., 2019). Manganese is also an important constituent of certain organocatalysts (Vincente et al. 2014); it takes part in the synthesis, and triggering of a large number of enzymes (Li and Yang, 2018). Manganese possesses many oxidation states, with a range from 3 – to 7 +; the most frequently encountered oxidation states in the natural world, are oxidation states 2 +, 3 +, and 4 + (Ghosh, 2020). Manganese does not exist on its own; it is always found in the bound form, in both carbon-containing, and non-carbon-containing composites (Avila et al., 2013). Some uses of non-carbon-containing manganese composites, include alloy-making, ceramic ware-production, pesticide-compounding and pigment-manufacturing, while the carbon-containing (organic) manganese composite, manganese carbonate, has been employed in the drug-manufacturing industry, amongst others (DOCCEEW, 2022). The maintenance of human health is a key factor in the determination of the quality of life and manganese contributes to this. Manganese aids in the maintenance of human health through its inclusion in metalloproteins (Horning et al., 2015). At the molecular level, metalloproteins that contain manganese, work as ligases, hydrolases and oxidoreductases, amongst other activities (Horning et al., 2015). In this manner, manganese performs the function of a coenzyme and many of the metabolic processes that occur in cells, require the presence of manganese (Erikson, 2005). The importance of manganese, in the cells of humans, is further appreciated in both its deficiency and its excess. Both a lack, and an excess, of manganese in man, could lead to severe health challenges, affecting organs like the sex organs, the lungs, the bones, and the brain (Keen et al., 2013; Harischandra et al., 2019; Avila et al., 2022), however, manganese deficiency in humans, is hardly seen (Chen et al., 2018). Low manganese in the human body (hypomanganesaemia), is reported to be associated with bone density loss, and abnormal cerebral neural discharges (Pennington and Schoen, 1996). Excessive manganese in the human body (hypermanganesaemia), mainly affects the brain, and could lead to “manganism”(Chen et al., 2018). Manganese deficiency in animals, causes diseases with overt manifestations, that include abnormal gait, skeletal malformations and hematological dyscrasias (Soetan et al., 2010). However, studies have revealed, that in animals that have been tamed by man, an excess of manganese, mainly causes a lack of iron, due to the disruptive effect of manganese on cellular iron intake; digestive, skeletal and cerebral functions can also be impaired (Keen and Zidenberg -Cherr, 2003) In plants, low manganese levels, affect photosystem II, and therefore, impairs the frequency of photosynthesis (Schmidt et al., 2016). The layering of cuticular waxes in foliage, is another plant metabolic activity, that is affected by manganese deficiency (Alejandro et al., 2020). Manganese toxicity also lowers the frequency of photosynthesis (Nable et al., 1988), and hinders the movement of important chemicals such as calcium from the surroundings into the plant (Blamey et al., 2015), amongst other metabolic activities. Overt manifestations of manganese deficiency in plants, are frequently absent, showing up much later on, as the deficiency persists or progresses (Alejandro et al., 2020). Manganese toxicity, on the other hand, frequently shows up as a yellowing of leaves, and the formation of foci of dead leaf tissue (Millaleo et al., 2010).
Fourier transform infrared (FTIR) spectroscopy, is a form of spectroscopic analysis, that exploits the infrared light- immersive capacity, of the majority of the chemically-bonded atomic particles, in a compound (Mishrah et al., 2018). It scrutinises the vacillatory motions of these chemically-bonded atomic particles (Berthomieu. and Hienerwadel, 2009). The FTIR spectrum that is finally recorded, is a product of the infrared radiation that leaves the compound being investigated (Sigma-Aldrich, 2023), and is measured between wavenumbers 4000 cm-1 and 400 cm-1 (Mishrah et al., 2018).
A knowledge of the surface form and layout, of a pure compound, provides important information, and a scanning electron microscope is well suited for this. Scanning Electron Microscopy (SEM) is a research tool that utilises a beam of excitable electrons, rather than that from light radiation, to create an elaborate and enlarged image, of a mapped-out section, of the top of a sample. Both secondary and back-scattered electrons, are produced from the collision of these excitable electrons, with varied parts of the sample (Nanakoudis, 2019). These electronic emissions are, translated to images, by the SEM (Nanakoudis, 2019). The elemental analysis, of a sample from a compound, is often achieved, through an inbuilt analyser, such as an energy dispersive X-ray spectroscope. Energy dispersive x-ray spectroscopy (EDX), is a scientific investigative procedure, that reveals the elemental constitution of a sample (Ismail et al., 2019). EDX determines the nature of an element or chemical, in a sample, and measures its quantity, in relation to the total amount of elements or chemicals, in the sample (Nasrollahzadeh et al., 2019). The spectral display of an EDX, is the result of the pooling, and the interpretation, of the x-ray radiation that emanates from the collision of the SEM electron beam with the compound (Raval et al., 2019). Nevertheless, the discerning of x-ray radiation, by any device, is affected by the combination of emitted energy between particles in a compound and not by the form of the particles of the compound; namely, if they are solid, liquid or gaseous (Scimeca et al., 2018).
SEM and EDX produce vital analyses of a compound, and the information garnered, can serve as baseline data, for future analyses. The pair of SEMS and EDX, has had several applications in many disciplines and industries, such as archaeology, for the dating of eras, by examining excavated pots (Zuluaga et al., 2011); forensic science, for studies on inflicted injuries (Gentile et al., 2020); and the aviation, petroleum and chemical manufacturing industries, to detect the causes of malfunctioning (Nasrazadani and Hassani, 2016).
High performance liquid chromatography, also known as high pressure liquid chromatography (HPLC) is an invaluable analytical tool, that is broadly utilised for the determination of the type, and amount, of the molecules, that compose a drug or an organic compound (Malviya et al., 2010). In HPLC, a sample of the compound is mixed with a dissolving agent, called the mobile phase, and by utilizing high pressure, the mixture is brought into contact with a support material that is fixed in a column (stationary phase) (Petrova and Sauer, 2017). The separation into component parts that ensues, results from a difference in the masses and varied affinities of these parts, to the two phases in the HPLC; this is recorded as the retention times (Xiang et al., 2006) by the device detectors. HPLC could be described as normal-phase HPLC, reverse-phase HPLC (Deshmukh et al., 2019), ion-exchange HPLC, affinity HPLC, size-exclusion HPLC, adsorption HPLC, chiral-phase HPLC (Dong, 2006), gradient-separation HPLC and isocratic HPLC (Sadapha and Dhamak, 2022). The end-product of the HPLC analysis can be measured, using Mass Spectrometry (MS).
Mass Spectrometry is a very dynamic scientific procedure, that reveals the nature and quantity of a composite, as a result of the uniqueness of the mass-to-charge ratios of its dissociated ions (Rockwood et al., 2018). The ion source of the mass spectrometer, ionizes the compound, and the resulting ions are disaggregated, on the basis of their different mass-to-charge ratios (De Hoffmann and Stroobant., 2007). The separated ions that emerge from the second, in-built analyzer, are picked up by detectors, identified and measured, and the results are displayed (De Hoffmann and Stroobant., 2007). Mass spectrometry finds its application in many sectors, such as forensic science, geology and medicine (Baghel et al., 2017). The chemical analysis of a compound, performed via the phenol-sulfuric acid method, assesses the total sugar-concentration of the compound (expressed in milligrams /litre), through two reactions. The first reaction occurs between with the reagents, phenol and sulfuric acid, and the compound; while the second reaction occurs between the reagents and a stock solution of glucose (Chaplin, 1986). This analytical method utilizes a standard glucose curve, to interpret the test results (Chaplin, 1986). The aim of this study was to characterize a biosurfactant produced from Pseudomonas aeruginosa strain S16, that had been demonstrated to possess effective biocontrol activity, against Staphylococcus aureus SO183, at 0.060 g/L, in a biocontrol activity study, conducted as a Master’s project (Okorie, 2023). This was done, in response to the need for viable alternatives to the present failing antibiotics in common usage.
Materials and Methods
A biosurfactant was produced from Pseudomonas aeruginosa strain S16 (accession number OQ734845) and purified. An aliquot of the biosurfactant, was aseptically placed in a sterile bottle and taken for analytical procedures, in order to characterize it.
Fourier transform infrared (FTIR) spectroscopy
Fourier transform infrared spectroscopy (FTIR) was performed with a Perkin Elmer Spectrum 100 Series 3000 MX spectrometer. Potassium bromide plates were used to prepare the biosurfactant sample. An FTIR manual from Nanoscience Technology Centre (NTSC) (2008) was used as a guide for the procedure. The FTIR computerized display was switched on, and acetone was utilized to wipe clean its sample holder, taking care to avoid reagent spillage. The name of the sample, the range of the FTIR scan and the number of the FTIR scan, were fed into the computerized display, after which a combination of computer commands enabled the recording of the FTIR spectrum. The infrared spectra were analyzed with the aid of spectroscopic software Win-IR Pro Version 3.0. and the data was saved. The computerized display was logged off, and the device switched off, after which the sample holder was wiped clean once again. The infrared (IR) spectra were interpreted with the use of IR Spectra Table and Charts from Sigma-Aldrich Company (Sigma-Aldrich, 2023) and analyses of FTIR results from Nandiyanto et al. (2019), Smith (2017) and Aksnes and Aksnes (1963).
High performance liquid chromatography (HPLC) and Mass spectrometry (MS)
High performance liquid chromatography (HPLC) of the biosurfactant was performed to analyses the simple sugars present, using Agilent 1100 series HPLC System (Agilent Technologies, California, USA), equipped with a diode array detector (DAD) and a reverse phase C-18 Zorbax Eclipse extra-dense bundle (XDB-C18) column (250 × 4.6 mm, 5 µm particle-size, 300 Å pore size). The mass-to-charge ratio of the simple sugars, was measured with an Agilent 1100 Series LC/MSD benchtop mass spectrometer, that was coupled to the HPLC device.
HPLC procedures documented by Sharma et al. (2020), Anumula (1994) and Guzzetta (2001), were used, in addition to the Agilent 1100 Series High Value System User’s Guide, from Agilent Technologies (1999). In preparation for the HPLC, the solvent lines were degassed by HPLC-grade isopropanol purging. Solvent A (HPLC-grade water combined with 0.1% v/v formic acid) and Solvent B (HPLC-grade acetonitrile and 0.1% v/v trifluoroacetic acid), constituted the HPLC reverse phase solvent system, for elution (Guzzetta, 2001). Multiple injections of the same samples were done through an HPLC injector application set-up (Huber, 2010), to obtain the separation of the monosaccharide moieties. HPLC sorted out non-volatile compounds from the analyte to forestall matrix interference (Ho et al., 2003).
The incorporated Agilent 1100 Series LC/MSD (liquid chromatography/ mass selective detector) benchtop mass spectrometer, received the end-product of the HPLC. This device, initially ionized the end-product (sample) before it separated the ions of the sample in its electromagnetic field, based on variances in ionic mass/charge (m/z) ratios (Reusch, 2013). Finally, the combined ultraviolet and mass selective detectors of the mass spectrometer, quantified the disassociated ions, and displayed them as a spectrum (Reusch, 2013). Qualitative (composition) and quantitative (concentration) characterization were therefore, made possible through mass spectrometry (Ho et al., 2003).
The final result of both HPLC and MS was displayed as a blend of graphical displays called a total ion chromatogram.
Scanning electron microscopy (SEM) and Energy dispersive x-Ray analysis/spectroscopy (EDS/EDX)
The surface topography of the biosurfactant was revealed using a JEOL JSM-7600F Scanning electron microscope (SEM) (Tokyo, Japan) while the elements that composed the compound were deciphered, using the inbuilt energy dispersive x-ray spectroscope (EDX).
The protocol laid out by Core Facilities (2021) in preparing a SEM sample, was used as a reference. Formaldehyde and glutaraldehyde were utilized for primary fixation of a measured volume of the biosurfactant sample, while osmium tetroxide was utilized for secondary fixation. Ethanol was used for sample dehydration, before the sample was dried. Mounting of the sample, and firm fixation on a specimen stub, were performed next, prior to sample placement in the specimen slot. Sputter-coating of the sample, with platinum was performed, as a prelude to sample sectioning. The biosurfactant surface was observed by the SEM scanner.
An analysis of the chemical that compose the biosurfactant, was done by the in-built EDX. The supercharged electron beam from the SEM hit the biosurfactant sample and caused the biosurfactant surface atoms to release X-rays (Thambiratnam et al., 2020). The X-rays from this interaction, were pooled by the X-ray detector in the EDX, interpreted into data that was composed of the elemental-type and elemental-concentration; this data was finally displayed as an EDX spectrum (Thambiratnam et al., 2020).
Phenol-sulfuric acid method
The concentration of the total sugar content of the biosurfactant (expressed in milligrams /litre), was calculated employing glucose as the standard, (Chaplin, 1986). This was measured in the following manner. A quantity (0.1 ml), of the biosurfactant, was added to 2 mL of distilled water. Measured quantities of the reagents, 0.1 mL of 6% phenol and 5 mL of sulphuric acid 95% (v/v), were sequentially and swiftly added, to the dissolved biosurfactant, and the mixture was shaken. This mixture was kept aside and its optical density was read, at a wavelength of 490 nm. This procedure was performed in triplicate. Another measure of distilled water, 2 mL, was mixed with exactly the same volumes of reagent, 0.1 mL of 6% phenol, and 5 mL sulphuric acid 95% (v/v), and used as blank; the absorbance was also measured. The number of sugars in the biosurfactant was deduced using a constructed glucose standard curve, from a technique by Dubois et al., (1956) with some modifications. In 100 mL of distilled water, 0.1 g of glucose was dissolved, to produce the stock solution. Ten milliliters of this stock solution, was added to 90 mL, distilled water, to produce diluted stock solution measuring 100 mL. Five different sterile bottles, were neatly lined up, into which 0.1 mL, 0.2 mL 0.4 mL, 0.8 mL and 1.0 mL of the diluted stock solution were consecutively transferred. Each bottle was topped up to 3 mL, through the addition of the commensurate amount of distilled water. This procedure, created an array of varied, further dilutions, of the initially-diluted, glucose stock solution. The same measures of reagents, 0.1 mL of 6% phenol and 5 mL of 95% (v/v) sulphuric acid, were swiftly added to each bottle, and the mixtures were left to stand for about ten minutes. A measurement of the optical densities (OD) at 490 nm was performed, and a standard glucose curve was constructed, with the concentrations of chemically-treated glucose solutions (mg/L), on the x-axis, and the corresponding optical densities (at 490 nm), on the y-axis. A linear graph of the optical densities of the different concentrations of chemically-treated, biosurfactant (mg/L) against their corresponding optical densities (at 490 nm), was also constructed on the same graph, from its own best-fit line.
From the standard glucose curve, a mathematical equation was extrapolated:
y = mx + c
where: y = optical density/absorbance (at 490 nm); m = mass; x = biosurfactant monosaccharide-concentration; c = velocity (mathematical) constant (Jain et al., 2017).
Utilising precise data values, generated from the phenol-sulfuric acid method, the exact concentration of total biosurfactant sugars, was calculated from the formula: OD biosurfactant = 0.3492x + 1.2234
where the OD biosurfactant represents the average optical density of the total biosurfactant sugars
Results
Fourier transform infrared (FTIR) spectroscopy
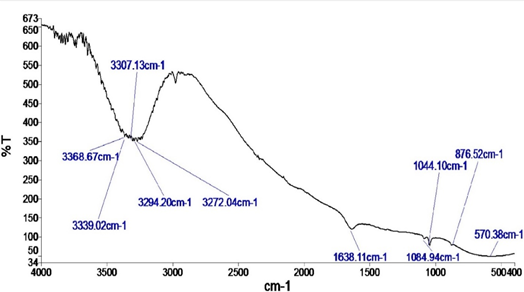
Figure 3.2 shows the FTIR spectrum of the biosurfactant from Pseudomonas aeruginosa strain S16 at 4000- 400 cm-1. The results showed a medium absorption peak at 3368.67 cm-1, generated by the N-H stretching of aliphatic primary amine groups, and a medium absorption peak at 3339.02 cm-1 generated by the N-H stretching of secondary amines. The strong broad peak at 3307.13 cm-1, was due to O-H stretching of an aromatic alcohol. The strong absorbance peak at 3294.20 cm-1, was due to O-H stretching, from a carboxylic acid group, or N-H stretching, in an amide salt. The strong absorption peak at 3272.04 cm-1 was generated by O-H stretching of a carboxylic acid/carboxylate (-COOH), or of a concentrated aromatic alcohol (-OH), present in the biosurfactant. The medium absorption peak at 1638.11 cm-1, corresponded to the C=C stretching of either an alkene group, or a conjugated alkene group. Also, this peak at 1638.11 cm-1, was within the usual range of 1900 to 1600 cm-1, known for carbonyl groups belonging to sugars. Peaks in the fingerprinting region (< 1500 S=O C=C>
High performance liquid chromatography (HPLC) and mass spectrometry (MS)
The total ion chromatogram, on Figure 3.4 shows the different masses of simple sugars in the biosurfactant that corresponded to different retention times, when compared to a standard for identification of each monosaccharide. A single simple sugar compound had several peaks, due to the different injection rates used in the HPLC, and the plausible presence of stereoisomers, in the biosurfactant. The total number of peaks for each monosaccharide, in the total ion chromatogram, was numbered. The biosurfactant from Pseudomonas aeruginosa strain S16 consisted of monosaccharide moieties mainly of rhamnose, glucose, xylose and mannose The other monosaccharides present, where D-ribose, galactose arabinose and inositol. There were fourteen peaks recorded, with glucose possessing three (peaks 1, 3 and 14) because of the different rates of injection used, and the possibility of the presence of D- and L- glucose stereoisomers. Galactose had two peaks (4 and 5) and is also a stereoisomer of glucose. Mannose had two peaks (3 and 4) and rhamnose also had two peaks (6 and 12).
Scanning electron microscopy (SEM) and energy dispersive x-ray analysis/spectroscopy (EDS/EDX)
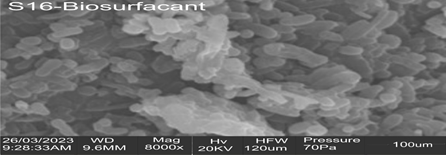
Plate 3.2 shows the SEM image for the biosurfactant from Pseudomonas aeruginosa strain S16. The image showed a high-resolution, three-dimensional, image of the scanned surface of a narrow stretch of the surface of the biosurfactant. The biosurfactant possessed a surface composed of globular and spindle-shaped projections. The x-ray radiation, emitted by the SEM electron beam was captured and read off by an in-built analyser- EDX. This recorded the elemental compositions of the biosurfactant as a percentage of its total weight with the following results: oxygen (3.00%); carbon (36.10%); nitrogen (43.00%); sulphur (1.28%); phosphorus (3.00%); sodium (6.60%); calcium (2.20%) potassium (0.88%) and manganese (0.46%). The relatively high nitrogen content correlated with the presence of peptide or protein moieties as is found in biosurfactants. Carbon and oxygen presence suggested carboxylic bonds. Phosphorus and oxygen were of the same quantity.
Phenol-Sulfuric Acid Method
The phenol-sulfuric acid method measured the concentration of the monosaccharides in the biosurfactant as 0.0047 g/L.
Plate 1: Scanning electron microscopy (SEM) micrograph of the biosurfactant from Pseudomonas aeruginosa strain S16.
Key: S-16 Biosurfactant: biosurfactant from Pseudomonas aeruginosa strain S16; WD: working distance; Mag: magnification; Hv: high voltage; HFW: horizontal field switch; MM: millimetres; KV: kilovolts; µm: micrometres; PA: Pascals
Figure 1: Energy dispersive x-ray analysis (EDX) spectrum for the biosurfactant from Pseudomonas aeruginosa strain S16.
Key- O: oxygen; C: carbon; N: nitrogen; S: sulphur; P: phosphorus; Na: sodium; Ca: calcium; K: potassium; Mn: manganese; KeV (x-axis): energy of the x-rays; y-axis: peak intensity (counts); Wt (%): percentage weight; S16: Pseudomonas aeruginosa strain S16.
Figure 2: FTIR spectrum of the biosurfactant from Pseudomonas aeruginosa strain S16.
Key: y-axis- absorbance (2-log%T); x-axis-wavelength measured in cm-1; S16: Pseudomonas aerugnosa strain S16
Discussion
Fourier transform infrared (FTIR) spectroscopy of the biosurfactant revealed the presence of certain functional groups that corresponded to specified peaks. The functional groups that were typical for a lipopeptide biosurfactant as reported by Umar et al. (2021), were seen. These were the carboxylic functional group that was represented as a strong broad peak at 3294.20 cm-1 from O-H stretching, and the aliphatic primary amine, and secondary amine groups that were represented by the broad peaks from N-H stretching, seen at 3339.02 cm-1 and 3339.02 cm-1, respectively. The presence of these amines, also indicated, that the biosurfactant from Pseudomonas aeruginosa strain S16 was positively charged (Santos et al., 2016). The absorbance peak at 3294.20 cm-1 that was strong and as a result of O-H stretching from a carboxylic acid group, or from N-H stretching in an amide salt, may have alluded to the formation of an amide, from its corresponding carboxylic acid (Moldoveaunu, 2019). Kumari et al. (2020), reported that the amide functioning unit, is a key factor in the constitution of many compounds that possess the ability to trigger a quantifiable action, on living cells and tissues. The two functional groups commonly present in carbohydrates (Roberts and Caserio, 1977), were also seen in the biosurfactant from Pseudomonas aeruginosa strain S16. These were the hydroxyl group, alluded to from the strong broad peak at 3307.13 that was from O-H stretching in an aromatic alcohol group, and the carbonyl group alluded to by the medium absorption peak at 1638.11 cm-1, that fell into the 1900-1600 cm-1 range that was usual for the group (Smith, 2017). The sulphoxide functional group (that generated a strong absorption at 1064.94 cm-1, and represented S=O stretching) present in the biosurfactant from Pseudomonas aeruginosa strain S16, may have contributed to the antibacterial activity of the biosurfactant, if the sulphoxide functional moiety, displayed chirality (Olbe et al., 2003). Interestingly, the alkene functional group (FTIR strong absorption at 876.52 cm-1), may also have proven important in the biosurfactant’s capacity to exert antibacterial; activity. This was because the report from research on a lipopeptide biosurfactant from Lactobacillus sp., conducted by Emmanuel etal. (2019), that demonstrated bioactivity against Escherichia coli, also contained an alkene functional group. It may be that the reactivity of the alkene functional group, due to the presence of the double bond between carbon atoms (Stauffer et al., 2007), could have contributed to the bioactivities of the biosurfactants in both studies. Although there was a strong absorbance at 570.36 cm-1 that was due to either C-I or C-Br stretching of a halo compound, this compound may have been volatile, as no halogen was detected on EDX analysis.
The total ion chromatogram from the high-performance liquid chromatographic, and mass spectrometric analyses, of the biosurfactant from Pseudomonas aeruginosa strain S16, revealed that the biosurfactant was composed of varied monosaccharides. These monosaccharides were predominantly rhamnose, glucose, xylose and mannose, and had a total sugar concentration of 0.0047 g/L when measured using the phenol-sulphuric acid method. This confirmed the heteropolysaccharide nature of the carbohydrate, present in the biosurfactant.
Scanning Electron Microscopy of the biosurfactant showed the surface was composed of globular and spindle-shaped projections. The surface morphology of a biosurfactant may directly influence its surface- and interfacial tension-reduction capacities probably in the same context as described for a bioflocculant in the report by Xiong et al., 2010) This report stated the great importance the configuration of the surface of a bioflocculant carries, towards its capacity to form flocs in a liquid medium. EDS of the biosurfactant revealed a relative abundance of carbon (36.10%) and nitrogen (43.00%) with a low amount of oxygen (3.00%). The amount of phosphorus (3.00%) was similar to that of oxygen while sodium (6.60%) was relatively more than either oxygen or phosphorus. Other detectable elements in the biosurfactant were calcium (2.20%), sulphur (1.26%), potassium (0.88%); the least abundant was manganese (0.46%). Nikolova and Gutierrez (2021) have stated, the chemical elemental composition of biosurfactants vary immensely between different species of biosurfactant-producers. Sabturani et al. (2016) reported in their research, the presence of carbon and oxygen and the absence of nitrogen in the biosurfactant they worked on. Tied with the detection of hexose sugars in the biosurfactant, they concluded that the biosurfactant was a glycolipid and not a lipopeptide biosurfactant or polymeric surfactant. However, the biosurfactant produced from Pseudomonas aeruginosa strain S16 was a polymeric biosurfactant made up of repeating aggregates of peptide, lipid and carbohydrate molecules. It was likely a manganese-containing phosphorylated glycolipopeptide/ glycolipoprotein, polysaccharide-protein-fatty acid composite or lipopolysaccharide-protein composite. Of particular interest, was the presence of the metal manganese in the biosurfactant. McCarron et al. (2018) have reported that synthesised compounds of Manganese (II) (Mn2+) with thiosemicarbazones, were active against Mycobacterium tuberculosis. The activities of certain man-made Manganese (III) compounds against the reactive peroxynitrite ion, have also been documented in the research by Carballal et al. (2018). In addition, the anticancer properties of one of the Manganese (II) artificially-made compounds by Zhang et al. (2012) have been documented in their research. These studies bring to light the diverse, bioactivities of manganese-containing compounds. The studies also create intrigues concerning, and drive speculation over, the actual ambit of bioactivities, a natural manganese-containing compound, like the biosurfactant from Pseudomonas aeruginosa strain S16, possesses. From available records, this is the first report of a manganese-containing biosurfactant.
Conclusion
The biosurfactant produced from Pseudomonas aeruginosa strain S16, was a positively charged, manganese-containing polymeric biosurfactant made up of carbohydrates, lipid and peptide molecules. It was more likely a positively-charged, manganese-containing, glycolipopeptide/glycolipoprotein; polysaccharide-protein-fatty acid composite or lipopolysaccharide-protein composite. From available records, this is the first report of a manganese-containing biosurfactant. The presence of manganese in the biosurfactant could open up applications particularly in medicine and the pharmaceutical industry, though the range of manganese applications is more diverse. Owing to toxicities related to manganese, however, clinical phase trials would be in keeping, before any intended use in the human body.
Declarations
Authors Contribution
I, Ikechukwu Kenneth M. Okorie (Corresponding author), carried out all the work in this study as my Masters (MSc.) research project. Professor Adeniyi A. Ogunjobi supervised my Masters research study.
Acknowledgements
The corresponding author would like to thank Professor Adeniyi A. Ogunjobi for supervising this study. Many thanks also go to Professor Tonye G. Okorie for editing and proofreading the article and to Dr. Augustine Akpoka for his useful suggestions. Mr. Gabriel O. Bamidele helped greatly in the laboratory work.
Funding
This study was self- funded by me, Ikechukwu. Kenneth. M. Okorie. No external fund was received.
Competing Interest
Both authors declare no competing financial interest.
References
- Adu, S.A., Naughton, P.J., Marchant, R. and Banat, I.M. (2020). Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations. Pharmaceutics 12(11):1099.
Publisher | Google Scholor - Agilent Technologies. (1999). Agilent 1100 Series High Value System. User’s Guide. 11/99 Ed. Waldbronn: Agilent Technologies.
Publisher | Google Scholor - Aksnes, D. and Aksnes, G., 1963. Intensity of infrared absorption bands of phosphoryl groups in organic phosphorus compounds. Acta chemica scandinavica 17(5):1262.
Publisher | Google Scholor - Al-Fartusie, F. S. and Mohssan, S.N. (2017). Essential trace elements and their vital roles in human body. Indian Journal of Advances in Chemical Science 5(3):127-136.
Publisher | Google Scholor - Alejandro, S., Höller, S., Meier, B. and Peiter, E. (2020). Manganese in plants: from acquisition to subcellular allocation. Frontiers in plant science 11:517877.
Publisher | Google Scholor - Anumula, K. R. 1994. Quantitative determination of monosaccharides in glycoproteins by high-performance liquid chromatography with highly sensitive fluorescence detection. Analytical biochemistry, 220(2):275-283.
Publisher | Google Scholor - Australian Government Department of Climate Change, Energy, the Environment and Water (DOCCEEW). 2022, Manganese and Compounds. Environment: Environment protection: National Pollutant Inventory: Substances: Substance fact sheets.
Publisher | Google Scholor - Avila, D. S., Puntel, R. L. and Aschner, M. (2013). Manganese in health and disease. Interrelations between essential metal ions and human diseases 199-227.
Publisher | Google Scholor - Baghel, U.S., Singh, A., Singh, D. and Sinha, M. (2017). Application of mass spectroscopy in pharmaceutical and biomedical analysis. Spectroscopic Analyses-Developments and Applications 105-121.
Publisher | Google Scholor - Banat, I.M., Franzetti, A., Gandolfi, I., Bestetti, G., Martinotti, M.G., Fracchia, L., Smyth, T.J. and Marchant, R. 2010. Microbial biosurfactants production, applications and future potential. Applied microbiology and biotechnology 87:427-444.
Publisher | Google Scholor - Berthomieu, C. and Hienerwadel, R. (2009). Fourier transform infrared (FTIR) spectroscopy. Photosynthesis research 101:157-170.
Publisher | Google Scholor - Blamey, F. P. C., Hernandez-Soriano, M. C., Cheng, M., Tang, C., Paterson, D.J., Lombi, E., Wang, W. H., Scheckel, K. G. and Kopittke, P. M., (2015). Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiology 169(3):006-2020.
Publisher | Google Scholor - Carballal, S., Valez, V., Alvarez-Paggi, D., Tovmasyan, A., Batinic-Haberle, I., Ferrer-Sueta, G., Murgida, D.H. and Radi, R. (2018). Manganese porphyrin redox state in endothelial cells: Resonance Raman studies and implications for antioxidant protection towards peroxynitrite. Free Radical Biology and Medicine 126:379-392.
Publisher | Google Scholor - Chaplin, M. and Kennedy, J. J. M. S. (1986). Monosaccharides. Mass Spectrom, 1(7).
Publisher | Google Scholor - Chen, P., Bornhorst, J. and Aschner, M.A. (2018). Manganese metabolism in humans.
Publisher | Google Scholor - Chrzanowski, Ł., Dziadas, M., Ławniczak, Ł., Cyplik, P., Białas, W., et al. (2012). Biodegradation of rhamnolipids in liquid cultures: effect of biosurfactant dissipation on diesel fuel/B20 blend biodegradation efficiency and bacterial community composition. Bioresource technology 111.328-335.
Publisher | Google Scholor - Core Facilities. (2021). SEM sample preparation techniques. University of Gothenburg.
Publisher | Google Scholor - Costa, J. A.V., Treichel, H., Santos, L. O, Martins, V. G. (2018). Solid-State Fermentation for the Production of Biosurfactants and Their Applications. Current Developments in Biotechnology and Bioengineering. Elsevier. 16:357-372.
Publisher | Google Scholor - De Giani, A., Zampolli, J. and Di Gennaro, P. (2021). Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: Different perspectives on their properties, challenges, and potential applications. Frontiers in microbiology 12.655150.
Publisher | Google Scholor - De Hoffmann, E. and Stroobant, V., (2007). Mass spectrometry: principles and applications. John Wiley & Sons.
Publisher | Google Scholor - Deshmukh, S., Chavan, G., Vanjari, S. and Patil, R. (2019). A Review on Analytical Method Development and Validation by High Performance Liquid Chromatography Technique. Journal of Pharmaceutical sciences and research 11.1:.3599-3605.
Publisher | Google Scholor - Australian Government Department of Climate Change, Energy, the Environment and Water (DOCCEEW).
Publisher | Google Scholor - Dong, M.W., (2006). Modern HPLC for practicing scientists. New Jersey: John Wiley & Sons.
Publisher | Google Scholor - DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T. and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical chemistry 28.3:350-356.
Publisher | Google Scholor - Emmanuel, E.C., Priya, S.S. and George, S. (2019). Isolation of biosurfactant from Lactobacillus sp. and study of its inhibitory properties against E. coli biofilm. Journal of Pure and Applied Microbiology, 3:403-411.
Publisher | Google Scholor - Erikson, K. M., Syversen, T., Aschner, J. L. and Aschner, M. (2005). Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environmental toxicology and pharmacology.19.3:415-421.
Publisher | Google Scholor - Gad, S. C. (2005). Manganese. Encyclopedia of Toxicology. Cambridge: Academic Press, 11-13.
Publisher | Google Scholor - Gad, S. C. and Pham, T. (2014). Manganese. Encyclopedia of Toxicology. Cambridge: Academic Press, 150-152.
Publisher | Google Scholor - Gayathiri, E., Prakash, P., Karmegam, N., Varjani, S., Awasthi, M.K. (2022). Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—a review. Agronomy, 12.3:662.
Publisher | Google Scholor - Ghosh, S.K. (2020). Diversity in the family of manganese oxides at the nanoscale: from fundamentals to applications. ACS omega 5.40:25493-25504.
Publisher | Google Scholor - Gürkök, S. and Özdal, M. (2021). Microbial Biosurfactants: Properties, Types, and Production. Anatolian Journal of Biology 2.2:7-12.
Publisher | Google Scholor - Guzzetta, A. (2001). Reverse Phase HPLC Basics for LC/MS: An IonSource Tutorial. IonSource: Mass Spectrometry Educational Resource.
Publisher | Google Scholor - Hamid, N., Ma, Q., Boulom, S., Liu, T., Zheng, Z., Balbas, J. and Robertson, J., (2015). Seaweed minor constituents. Seaweed sustainability. Tiwari, B. K. Ed., Troy, D. J. Ed. Massachusetts: Academic Press, 193-242.
Publisher | Google Scholor - Harischandra, D.S., Ghaisas, S., Zenitsky, G., Jin, H., Kanthasamy, A. (2019). Manganese-induced neurotoxicity: new insights into the triad of protein misfolding, mitochondrial impairment, and neuroinflammation. Frontiers in Neuroscience 13: 654.
Publisher | Google Scholor - Ho, C. S., Lam, C. W. K., Chan, M. H., Cheung, R. C. K. et al. (2003). Electrospray ionisation mass spectrometry: principles and clinical applications. The Clinical Biochemist Reviews, 24(1):3.
Publisher | Google Scholor - Horning, K.J., Caito, S.W., Tipps, K.G., Bowman, A.B. and Aschner, M. (2015). Manganese is essential for neuronal health. Annual review of nutrition 35: 71-108.
Publisher | Google Scholor - Huber, U. (2010). Performing multiple injections in an isocratic purification experiment using the Agilent 1100 Series purification system: Application Note. GiMiTEC.com/file.
Publisher | Google Scholor - Ismail, A. F., Khulbe, K. C., and Matsuura. T. (2019). RO Membrane Characterization. Reverse Osmosis. Amsterdam: Elsevier. Chapter 3. 57-90.
Publisher | Google Scholor - Jain, V. M., Karibasappa, G. N., Dodamani, A. S. and Mali, G. V. (2017). Estimating the carbohydrate content of various forms of tobacco by phenol-sulfuric acid method. Journal of education and health promotion 6.
Publisher | Google Scholor - Keen, C. L. and Zidenberg-Cherr, S. (2003). Manganese. Encyclopedia of Food Sciences and Nutrition. Cmbridge: Academic Press. 3686-3691.
Publisher | Google Scholor - Keen, C. L., Ensunsa, J. L., Lönnerdal, B. A. and Zidenberg-Cherr, S. (2013). Manganese. Encyclopedia of Human Nutrition. Cambridge: Academic Press.
Publisher | Google Scholor - Kumari, S., Carmona, A.V., Tiwari, A.K. and Trippier, P.C. (2020). Amide bond bioisosteres: Strategies, synthesis, and successes. Journal of medicinal chemistry 63(21):12290-12358.
Publisher | Google Scholor - Lee, S., Chintalapudi, K. and Badu-Tawiah, A.K. (2021). Clinical chemistry for developing countries: mass spectrometry. Annual Review of Analytical Chemistry 14:437-465.
Publisher | Google Scholor - Leong, B.F., Chuah, W.C. and Chye, F.Y. (2021). Recent advances and emerging trends in the utilization of dairy by-products/wastes. Valorization of Agri-Food Wastes and By-Products, 371-389.
Publisher | Google Scholor - Li, H., Santos, F., Butler, K. and Herndon, E. (2021). A critical review on the multiple roles of manganese in stabilizing and destabilizing soil organic matter. Environmental science & technology 55(18):12136-12152.
Publisher | Google Scholor - Li, L. and Yang, X. (2018). The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxidative medicine and cellular longevity (2018).
Publisher | Google Scholor - Malviya, R., Bansal, V., Pal, O.P. and Sharma, P.K. (2010). High performance liquid chromatography: a short review. Journal of global pharma technology 2(5):22-26.
Publisher | Google Scholor - McCarron, P., McCann, M., Devereux, M., Kavanagh, K., Skerry, C. (2018). Unprecedented in vitro antitubercular activitiy of manganese (II) complexes containing 1, 10-phenanthroline and dicarboxylate ligands: increased activity, superior selectivity, and lower toxicity in comparison to their copper (II) analogs. Frontiers in Microbiology 9:1432.
Publisher | Google Scholor - Meena, K.R., Sharma, A. and Kanwar, S.S., (2017). Microbial lipopeptides and their medical applications. Annals of Pharmacology and Pharmaceutics, 2(24):1126.
Publisher | Google Scholor - Millaleo, R., Reyes-Díaz, M., Ivanov, A.G., Mora, M.L. and Alberdi, M. (2010). Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. Journal of soil science and plant nutrition 10(4):470-481.
Publisher | Google Scholor - Moldoveaunu, S. C. (2019). Pyrolysis of Organic Molecules: Applications to Health and Environmental Issues. 2nd ed. Amsterdam: Elsevier Science.
Publisher | Google Scholor - Nable, R. O., Houtz, R. L. and Cheniae, G. M. (1988). Early inhibition of photosynthesis during development of Mn toxicity in tobacco. Plant Physiology, 86(4):1136-1142.
Publisher | Google Scholor - Nanakoudis, A. (2019). SEM: Types of Electrons and the Information They Provide. ThermoFisher Scientific: Advancing Materials: Electron Microscopy 101.
Publisher | Google Scholor - Nandiyanto, A. B. D., Oktiani, R. and Ragadhita, R. (2019). How to read and interpret FTIR spectroscope of organic material. Indonesian Journal of Science and Technology 4(1):97-118.
Publisher | Google Scholor - NanoScience Technology Centre (NTSC) (2008). FTIR Procedure and instructions: uploads: sites. University of Central Florida.
Publisher | Google Scholor - Nasrazadani, S. and Hassani, S. (2016). Modern analytical techniques in failure analysis of aerospace, chemical, and oil and gas industries. Handbook of Materials Failure Analysis with Case Studies from the Oil and Gas Industry Oxford: Butterworth-Heinemann, Chapter 2:39-54.
Publisher | Google Scholor - Nasrollahzadeh, M. Ed., Sajjadi, M. Ed., Sajadi, S.M. Ed. and Issaabadi, Z. Ed. (2019). An Introduction to Green nanotechnology. Interface science and technology 28., Atarod, M. Ed. Amsterdam: Elsevier. Chapter 1:1-27.
Publisher | Google Scholor - National Centre for Biotechnology Information (NCBI) GenBank.
Publisher | Google Scholor - Nakama, Y. (2017). Surfactants. Cosmetic Science and Technology: Theoretical Principles and Applications. Sakamoto, K., Lochhead, R. Y. Maibach, H. I., Yamashita Y. Eds. Amsterdam: Elsevier. Chapter:15:231-244.
Publisher | Google Scholor - Ndlovu, T., Rautenbach, M., Vosloo, J.A., Khan, S. and Khan, W. (2017). Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 7(1):1-19.
Publisher | Google Scholor - Nikolova, C. and Gutierrez, T. (2021). Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives. Frontiers in Bioengineering and Biotechnology 9:626639.
Publisher | Google Scholor - Okorie, I. K. M. (2023). Biocontrol of selected bacteria using biosurfactant, bioflocculant and exopolysaccharide, produced from Pseudomonas species. MSc. Project. Dept of Microbiology.
Publisher | Google Scholor - Olbe, L., Carlsson, E. and Lindberg, P. (2003). A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nature reviews drug discovery 2(2):132-139.
Publisher | Google Scholor - O’Neal, S.L. and Zheng, W. (2015). Manganese toxicity upon overexposure: a decade in review. Current environmental health reports 2:315-328.
Publisher | Google Scholor - Pennington, J.A. and Schoen, S.A. 1996. Contributions of food groups to estimated intakes of nutritional elements: results from the FDA total diet studies, 1982-1991. International Journal for Vitamin and Nutrition research. Internationale Zeitschrift fur Vitamin-und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition, 66(4):342-349.
Publisher | Google Scholor - Petrova, O.E. and Sauer, K., (2017). High-performance liquid chromatography (HPLC)-based detection and quantitation of cellular c-di-GMP. c-di-GMP Signaling: Methods and Protocols 33-43.
Publisher | Google Scholor - Pessione, E. and Garcia-Contreras, R. (2022). Non-Conventional Antimicrobial Agents. Encyclopedia of Infection and Immunity. Amsterdam: Elsevier.
Publisher | Google Scholor - Raman, K. (2016). HPLC: Biochemical Analysis. A Step-By-Step Method Guide. SciGine: Scientific Methods for -Biology and Biochemistry- Analytic Techniques.
Publisher | Google Scholor - Raval, N., Maheshwari, R., Kalyane, D., Youngren-Ortiz, S. R., Chougule, et al. (2019). Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. Basic Fundamentals of Drug Delivery: Advances in Pharmaceutical Product Development and Research. Cambridge: Academic Press. 10:369-400.
Publisher | Google Scholor - Reusch, W. (2013). Virtual Text of Organic Chemistry. 1 st Ed. East Lansing: Michigan State University.
Publisher | Google Scholor - Roberts, J.D. and Caserio, M.C. (1977). Basic Principles of Organic Chemistry. 2nd ed. California: WA Benjamin, Incorporated.
Publisher | Google Scholor - Rockwood, A.L., Kushnir, M.M. and Clarke, N.J. (2018). Mass spectrometry. Principles and applications of clinical mass spectrometry Rifai, N., Horvath, A.R., Wittwer, C.T., Hoofnagle, A. Eds. Amsterdam: Elsevier. Chapter 2:33-65.
Publisher | Google Scholor - Rosen, M.J. and Kunjappu, J.T. (2012). Surfactants and interfacial phenomena. 4th ed. New Jersey: John Wiley & Sons.
Publisher | Google Scholor - Sabturani, N., Latif, J., Radiman, S. and Hamzah, A. (2016). Spectroscopic analysis of rhamnolipid produced by produced by Pseudomonas aeruginosa UKMP14T. Malays. J. Anal. Sci, 20:31-43.
Publisher | Google Scholor - Sadapha, P. and Dhamak, K. (2022). Review Article on High-Performance Liquid Chromatography (HPLC) Method Development and Validation. International Journal of Pharmaceutical Sciences Review and Research 74.2:23-29.
Publisher | Google Scholor - Sandeep, L. and Rajasree, S. (2017). Biosurfactant: pharmaceutical perspective. Journal of Analytical and Pharmaceutical Research 4(3):11-12.
Publisher | Google Scholor - Saha, M. and Sarkar, A. (2021). Review on multiple facets of drug resistance: a rising challenge in the 21st century. Journal of xenobiotics 11(4):197-214.
Publisher | Google Scholor - Santos, D. K. F., Rufino, R.D., Luna, J.M., Santos, V.A. and Sarubbo, L. A. (2016). Biosurfactants: multifunctional biomolecules of the 21st century. International journal of molecular sciences 17(3):401.
Publisher | Google Scholor - Schmidt, S.B., Jensen, P.E. and Husted, S. (2016). Manganese deficiency in plants: the impact on photosystem II. Trends in Plant Science 21.7: 622-632.
Publisher | Google Scholor - Scimeca, M., Bischetti, S., Lamsira, H. K., Bonfiglio, R. and Bonanno, E. (2018). Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. European journal of histochemistry: EJH, 62(1).
Publisher | Google Scholor - Sharma, J., Sundar, D. and Srivastava, P. (2021). Biosurfactants: potential agents for controlling cellular communication, motility, and antagonism. Frontiers in Molecular Biosciences 893.
Publisher | Google Scholor - Sharma, K., Sharma, N., Handa, S. and Pathania, S. (2020). Purification and characterization of novel exopolysaccharides produced from Lactobacillus paraplantarum KM1 isolated from human milk and its cytotoxicity. Journal of Genetic Engineering and Biotechnology 18:1-10.
Publisher | Google Scholor - Soetan, K. O., Olaiya, C. O. and Oyewole, O. E. (2010). The importance of mineral elements for humans, domestic animals and plants: A review. African journal of food science, 4(5):200-222.
Publisher | Google Scholor - Solov'eva, T.F. and IuS, O. (1983). Lipopolysaccharide-protein complexes of the outer membrane of gram-negative bacteria. Bioorganicheskaia Khimiia, 9.6:725-733.
Publisher | Google Scholor - Sigma-Aldrich. (2023). IR Spectrum Table & Chart. Photometry and Reflectometry. Merck.
Publisher | Google Scholor - Silva, C. S., Moutinho, C., Ferreira da Vinha, A. and Matos, C. (2019). Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. International Journal of Science and Research Methodology 13(3):57-80.
Publisher | Google Scholor - Smith, B.C. (2017). The carbonyl group, part I: introduction. Spectroscopy, 32(9):31-36.
Publisher | Google Scholor - Stauffer, E., Dolan, J. A. and Newman, R. (2007). Fire Debris Analysis, 1st ed. Cambridge: Academic Press.
Publisher | Google Scholor - Tanwar, J., Das, S., Fatima, Z. and Hameed, S. (2014). Multidrug resistance: an emerging crisis. Interdisciplinary perspectives on infectious diseases.
Publisher | Google Scholor - Thambiratnam, K., Reduan, S. A., Tiu, Z. C. and Ahmad, H. (2020). Application of two-dimensional materials in fiber laser systems. Nano-Optics: Fundamentals, Experimental Methods, and Applications: A volume in Micro and Nano Technologies. Thomas, S., Grohens, Y., Vignaud, G., Kalarikkal, N., James, J. Eds. Amsterdam: Elsevier.Chapter 6:227-264.
Publisher | Google Scholor - Umar, A., Zafar, A., Wali, H., Siddique, M.P., Qazi, M.A., et al. (2021). Low-cost production and application of lipopeptide for bioremediation and plant growth by Bacillus subtilis SNW3. AMB Express 11(1):1-21.
Publisher | Google Scholor - Vincente, A. R., Manganaris, G. A., Ortiz, C. M., Sozzi, G. O. et al. (2014). Nutritional Quality of Fruits and Vegetables 2014.
Publisher | Google Scholor - Vijayakumar, S. and Saravanan, V. (2015). In vitro cytotoxicity and antimicrobial activity of biosurfactant produced by Pseudomonas aeruginosa strain PB3A. Asian Journal of Scientific Research 8(4):510.
Publisher | Google Scholor - World Health Organisation. (2021). Antimicrobial Resistance. Newsroom: Fact sheets.
Publisher | Google Scholor - Xiang, Y., Liu, Y. and Lee, M. L. (2006). Ultrahigh pressure liquid chromatography using elevated temperature. Journal of Chromatography A, 1104(1-2):198-202.
Publisher | Google Scholor - Xiong, Y., Wang, Y., Yu, Y., Li, Q., Wang, H., Chen, R. and He, N. (2010). Production and characterization of a novel bioflocculant from Bacillus licheniformis. Applied and environmental microbiology 76(9):2778-278.
Publisher | Google Scholor - Zhang, F., Lin, Q.Y., Zheng, X. L., Zhang, L.L., Yang, Q. and Gu, J. Y. (2012). Crystal structures, interactions with biomacromolecules and anticancer activities of Mn (II), Ni (II), Cu (II) complexes of demethylcantharate and 2-aminopyridine. Journal of fluorescence 22:1395-1406.
Publisher | Google Scholor - Zuluaga, M.C., Alonso-Olazabal, A., Murelaga, X. and Ortega, L.A. (2011). A comparison of scanning electron microscopy energy dispersive X-ray (SEM/EDX) and inductively coupled plasma optical emission spectrometry (ICP-OES) for provenance inferences of grog-tempered Bronze Age pottery. Microchemical Journal 99(2):443-448.
Publisher | Google Scholor