Case Report
Case of Post-Transplant Anemia Secondary to Parvovirus B19, Responsive to Cidofovir
- Pranjal Kashiv 1
- Sushrut Gupta 1
- Sunny Malde 2
- Shubham Dubey 1
- Twinkle Pawar 3
- Kapil N. Sejpal 1
- Prasad Gurjar 1
- Manish Balwani 4,1*
- Amit Pasari 5, 1
- Priyanka Tolani 6
- Amol Bhawane 7
- Charulata P. Bawankule 5
1Nephrology, Jawaharlal Nehru Medical College, Wardha, India.
2Department of Nephrology, Jawaharlal Nehru Medical College, Wardha, India.
3Department of Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Wardha, India.
4Nephrology, Saraswati Kidney care Center, Nagpur, India.
5Nephrology, Saraswati Kidney Care Center, Nagpur, India.
6Internal Medicine, Jawaharlal Nehru Medical College, Wardha, India.
7Department of Nephrology, All India Institute of Medical Sciences, Nagpur, Nagpur, India.
*Corresponding Author: Manish Balwani, Nephrology, Saraswati Kidney care Center, Nagpur, India.
Citation: Kashiv P., Gupta S., Malde S, Dubey S, Balawani M., et al. (2024). Case of Post-Transplant Anemia Secondary to Parvovirus B19, Responsive to Cidofovir. Clinical Case Reports and Studies, BioRes Scientia Publishers. 6(3):1-6. DOI: 10.59657/2837-2565.brs.24.140
Copyright: © 2024 Manish Balwani, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 28, 2024 | Accepted: June 19, 2024 | Published: July 25, 2024
Abstract
Persistent anemia is a common complication post-kidney transplantation. Parvovirus B19 (PVB19) infection, a rare but severe cause of anemia in this population, is characterized by direct cytotoxicity to erythroid progenitor cells. Diagnostic tools include molecular amplification techniques, serological estimation of antibodies, and complement levels. This report presents a unique case of persistent anemia due to PVB19 in a kidney transplant recipient from central India. Despite initial unresponsiveness to reduced immunosuppression and intravenous immunoglobulin (IVIG), the patient exhibited positive responses to antiviral treatment with Cidofovir. The case highlights the importance of vigilant management in addressing post-transplant complications, emphasizing the efficacy of Cidofovir in rare instances of persistent anemia due to PVB19 infection. The presented case contributes valuable insights into the limited literature on post-transplant PVB19 infection and underscores the significance of early detection and intervention to preserve graft function.
Keywords: immunosuppressive medications, cidofovir, parvovirus b19, post-transplant anemia, deceased donor kidney transplantation
Introduction
Anemia is a common complication post-kidney transplantation, affecting 25-50% of recipients, with lower hemoglobin levels independently predicting adverse outcomes such as hospitalization, mortality, and graft failure [1]. In the first month, anemia prevalence is 90%, gradually reducing to 35-45% by the end of the first year [1,2]. Neild et al. first documented human parvovirus B19 (PVB19) infection in kidney transplant recipients in 1986 [3]. Parvovirus b19 is a small, non-enveloped, single-stranded DNA virus belonging to the parvoviridae family and the erythrovirus genus, demonstrating a unique erythroid progenitor cell tropism. This virus exhibits direct cytotoxicity towards erythroid series units, significantly dropping hemoglobin levels [4]. Uncontrolled viral proliferation can lead to transient or prolonged aplastic crises, primarily manifesting as chronic red cell aplasia, causing persistent anemia [5].
Diagnostic tools include molecular amplification techniques for PVB19 DNA detection by RT-PCR, serological estimation of IgM and IgG antibodies, and complement levels [6,7]. While hemoglobin levels generally improve with a normally functioning allograft, persistent anemia may result from various factors, such as graft dysfunction, iron deficiency, viral infections, allograft rejection and the impact of immunosuppressive medications. Medications like angiotensin-converting enzyme inhibitors and opportunistic viral infections, such as cytomegalovirus, B.K.Polyomavirus, Epstein-Barr virus, and parvovirus B19 are associated with persistent anemia, posing a significant risk to kidney transplant recipients, particularly parvovirus B19, which may lead to life-threatening chronic pure red blood cell aplasia [8]. We report a case of persistent anemia due to PVB19 in a kidney transplant patient from central India. In a unique case where the majority exhibit improvement with reduced immunosuppressant dosage and IVIG, this patient, initially unresponsive, ultimately responded positively to treatment after the administration of the antiviral drug Cidofovir, underscoring the importance of careful management in such cases.
Case Presentation
A 57-year-old female underwent a deceased donor renal transplant with a donor-recipient HLA (human leukocyte antigen) mismatch of 2/6, and the pre-transplant DSA (donor specific antibodies) by single antigen bead was negative. She received induction with rabbit anti-thymocyte globulin (ATG) 125 mg, followed by standard triple immunosuppression of MMF, steroids, and tacrolimus. There was no significant blood loss intraoperatively, and the patient exhibited brisk urine output immediately after surgery. Hemoglobin (Hb) pre-transplant was 9.5 gm/dl, and she used to receive erythropoietin at a dose of 4000 IU subcutaneously twice per week. In the immediate postoperative period, the patient's Hb levels were satisfactory at 9 gm/dl. Over the subsequent days, Hb remained stable and static, around 7-8gm/dl, with an average platelet count. EPO was given at a dose of 4000 units subcutaneous. However, a gradual decline occurred, and by the postoperative day (POD) 12, Hb had decreased to 5.4 gm/dl. (Table 1).
Table 1: Laboratory values and treatment given during the course of treatment during post operative day 0 to 12.
| POD | 0 | 2 | 5 | 7 | 9 | 11 | 12 | Reference range |
| Hemoglobin (gm/dl) | 9 | 8.2 | 7.9 | 7.8 | 7.4 | 7 | 5.4 | 14- 18 |
| Platelet count (lakh/mm3) | 0.96 | 1.5 | 2.5 | 3.1 | 3.3 | 3.4 | 3.7 | 1.5-4.5 |
| Reticulocyte count (%) | - | - | - | - | - | 1.1 | 0.50-2.50 | |
| Creatinine (mg/dl) | 4.4 | 2.1 | 1.1 | 1 | 1 | 0.9 | 1 | 0.4-1.4 |
| Parvovirus B19 (RT-PCR quantitative) (IU/ml) | - | - | - | - | - | - | - | - |
| Tacrolimus level (ng/ml) | - | - | 12.4 | - | - | - | 11.1 | - |
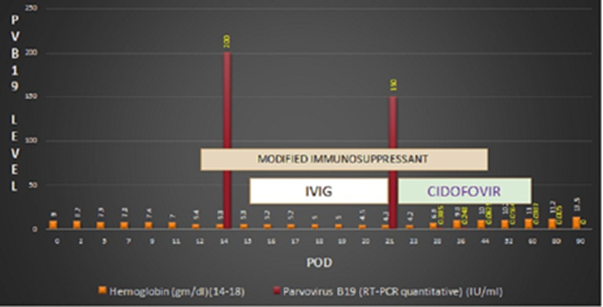
Evaluation for anemia revealed a normal iron study and a normal liver function test. Serial monitoring of kidney function tests (KFT) showed normal serum creatinine from POD 3. Adequate urine output was observed, ruling out common causes of persistent anemia in post-renal transplant patients. Her tacrolimus level was also normal. On pod 14, screening for viral infections, including adenovirus, cytomegalovirus, Epstein Barr virus, HSV 1 & 2, HHV 6 & 7, VZV, BKV, JCV, enterovirus, and parvovirus B19 were conducted, revealing a positive finding for parvovirus B19. Quantitative RT-PCR indicated a value exceeding >150 x 10^9 IU/ml. Management commenced by modifying the dosage of immunosuppressants, wherein MMF was reduced from 2 gm/day to 1 gm/day, and tacrolimus was adjusted from 3.5 mg/day to 3 mg/day, reaching 2.5 mg/day on the pod sixteen targeting a trough level of 8-12 ng/ml. Following these adjustments, the patient received intravenous immunoglobulin (IVIG) at a dose of 20 grams daily for five days. Unfortunately, despite these interventions, no improvement was observed in the patient's condition. Parvovirus quantitative PCR levels remained alarmingly high, exceeding >150 x 10^9 IU/ml on POD 21. The patient received one unit of nat-tested irradiated leukodepleted PRBCs to address the persistent anemia. On POD 23, antiviral therapy was initiated with Cidofovir at a dose of 100 mg, along with a tab. Probenecid. This intervention resulted in a notable reduction in parvovirus levels to 2.483 x 10^8 IU/ml then continued weekly treatment at a dose of 50 mg for four weeks. Subsequently, parvovirus quantitative PCR levels showed a progressive decline (3.85 x 10^8, 6.25 x 10^7, 5.64 x 10^7, 3.87 x 10^7), signifying the successful correction of the infection and resolution of anemia. (Table 2, 3) (Figure 1).
| POD | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | Reference range |
| Hemoglobin (gm/dl) | 5.3 | 5.3 | 5.2 | 5.2 | 5 | 5 | 4.5 | 4.3 | 14- 18 |
| Platelet count (lakh/mm3) | 3.5 | 3.5 | 3.4 | 3.5 | 3.5 | 3.6 | 3.3 | 3.4 | 1.5-4.5 |
| Reticulocyte count (%) | 2 | 2 | 2 | 2.2 | 2.4 | 2.5 | 2.3 | 2.3 | 0.50-2.50 |
| Creatinine (mg/dl) | 1 | 1 | 1.1 | 0.9 | 1 | 1.1 | 1 | 1 | 0.4-1.4 |
| Parvovirus B19 (RT-PCR quantitative) (IU/ml) | >150x10^9 (DETECTED) | - | - | - | - | - | - | >150x10^9 | - |
| Tacrolimus level (ng/ml) | - | - | - | - | - | - | - | 10.4 | - |
Table 2: Laboratory values and treatment given during the course of treatment during post operative day 14 to 21.
| POD | 23 | 28 | 36 | 44 | 52 | 60 | 80 | 90 | Reference range |
| Hemoglobin (gm/dl) | 4.3 | 6.9 | 9.8 | 10 | 10.2 | 11 | 11.2 | 13.5 | 14-18 |
| Platelet count (lakh/mm3) | 2.5 | 3.9 | 3.9 | 4.1 | 4.1 | 4.2 | 4.1 | 3.66 | 1.5-4.5 |
| Reticulocyte count (%) | 2.1 | 2.2 | 2 | 1.8 | 1.5 | - | - | - | 0.50-2.50 |
| Creatinine (mg/dl) | 1 | 0.9 | 1 | 1.1 | 0.8 | 0.9 | 1.1 | 1 | 0.4-1.4 |
| Parvovirus B19 (RT-PCR quantitative) (IU/ml) | - | 3.85x 10^8 | 2.483x 10^8 | 6.25x 10^7 | 5.64x 10^7 | 3.87x 10^7 | 5.32x 10^4 | Not detected | - |
| Tacrolimus level (ng/ml) | - | - | - | 10.5 | - | - | - | 10.4 | - |
Table 3: Laboratory values and treatment given during the course of treatment during post operative day 23 to 90.
Figure 1: Course of treatment POD: Post operative day
The practical management approach allowed for the necessary escalation of immunosuppressant dosages, which is crucial for preventing graft failure. Subsequently, the patient transitioned to normal immunosuppressant doses. Currently, she is under regular follow-up; her quantitative PCR level for parvovirus is 5.32 x 10^4 IU/ml. Concurrently, her hemoglobin level has stabilized at 11 gm/dl, reflecting the resolution of prior anemia. Importantly, graft function remains normal, evidenced by adequate urine output and normal kidney function test results. This case underscores the rarity of persistent anemia due to parvovirus b19 in kidney transplant recipients and highlights the efficacy of Cidofovir in treating this challenging post-transplant complication.
Discussion
Kidney transplant recipients, especially within the first post-transplant year, are more susceptible to infections due to immunosuppressive drugs like anti-thymocyte globulin, used to prevent rejection. PVB19 is a notable opportunistic infection within the initial year, following closely behind CMV and EBV. PVB19 infection can lead to primary syndromes such as anemia, thrombocytopenia, and damage to the transplanted kidney [9]. Parvovirus B19 (PVB19) targets human erythroid progenitor cells (EPCs), disrupting erythropoiesis by inducing red blood cell aplasia through the activation of a nonstructural protein (NS1)- mediated caspase pathway [5,8]. During the viremic phase, this selective affinity temporarily disrupts erythropoiesis, yet no significant clinical manifestations are observed. PVB19 encodes two structural proteins (VP1 and VP2) alongside NS1, interacting with the blood group P-antigen on erythroblasts to trigger apoptosis. The virus's direct cytotoxicity induces normocytic normochromic anemia in both aplastic crisis and pure red-cell aplasia. Understanding these pathways is crucial for comprehending PVB19 transmission in kidney transplant recipients [10]. Capenko et al. found that active PVB19 infection significantly contributes to severe post-transplantation anemia [11]. In immunosuppressed patients, PVB19 infection presents with fever, rash, arthralgia, normochromic normocytic anemia, and erythropoietin unresponsiveness. Baek et al. identified correlations between deceased donor kidney grafts, tacrolimus treatment, and low hemoglobin levels with PVB19 infection in the initial post-renal transplantation year [12].
Possible transmission routes of PVB19 in kidney transplant recipients include viral reactivation, respiratory infections, blood transfusions and exposure through transplanted organs. RT-PCR, a direct detection method, demonstrates heightened sensitivity compared to antibody assays in immunocompromised patients, as elucidated in Eid et al.'s exhaustive review of 98 cases [13]. In instances where RT-PCR yields negative results despite clinical suspicion, diagnostic confirmation is achieved through a bone marrow biopsy, revealing giant pronormoblasts with inclusion bodies [14]. Viral infections typically manifest in the second stage (1-6 months) post-surgery, yet studies show PVB19 can occur within two weeks, leading to severe anemia. Capenko et al. found a significant association between PVB19 infection and anemia, with onset at seven weeks post-transplantation [11]. Baek et al. Demonstrated that nearly 90% of positive cases were reported within the first-year post-transplant [12]. Active PVB19 infection in kidney transplant recipients is associated with allograft dysfunction, acute and chronic aplastic anemia and thrombotic renal allogeneic microangiopathy. Furthermore, the persistent existence of PVB19 DNA in the kidney is associated with the onset of chronic allograft injury [15].
The standard immunosuppressive regimen following kidney transplantation greatly diminishes PVB19 clearance rates, resulting in prolonged viral infections. While PVB19 reinfection or reactivation is unlikely, individuals with immunodeficiency are susceptible to secondary infections due to insufficient protective antibodies. The primary strategy for managing parvoviremia in kidney transplant recipients involves adjusting the immunosuppressive regimen. In cases where changing the immunosuppressive regimen proves insufficient, the secondary approach involves passive immunization through intravenous immunoglobulin (IVIG) [13,15]. Our patient's persistent unexplained anemia post-kidney transplantation, attributed to PVB19 infection, was confirmed by RT-PCR, as a bone marrow biopsy was unfeasible due to financial constrain. Despite modifying the immunosuppressive regimen and administering IVIG therapy for five days, the patient continued to experience persistent anemia. Resolution occurred only after the administration of inj. Cidofovir (100 mg) led to an improvement in her hemoglobin levels.
Despite not being specific for PVB19, Cidofovir showed significant improvement in our case. Wong et al. Reported the ineffectiveness of multiple IVIG therapy courses for pure red cell aplasia PRCA linked to PVB19 infection in a tacrolimus-treated kidney transplant recipient [16]. Nair et al. Highlighted Cidofovir's curative potential in treating parvovirus b19 infection [17 ]. Cidofovir, a monophosphate nucleotide analogue, exhibits in vitro activity against various DNA viruses, including herpesviruses, adenovirus, polyomavirus, papillomavirus, and poxvirus, and has demonstrated in vitro anti-PVB19 activity [18]. Early diagnosis and prompt intervention played pivotal roles in resolving the anemia and safeguarding graft function, making this case a valuable contribution to the limited literature on post-transplant parvovirus b19 infection and the utility of Cidofovir in such scenarios. Physicians must be vigilant about PVB19 infection as an underestimated cause of PRCA in kidney transplant recipients following kidney transplant, irrespective of immunosuppression protocols or donor types. Although PVB19 infection typically resolves on its own, its potential to cause harm to immunocompromised transplant patients should not be underestimated.
Conclusion
Parvovirus B19 (PVB19) infection is a rare but clinically significant cause of persistent anaemia post-renal transplantation. A comprehensive understanding of the factors contributing to persistent anaemia includes considerations of viral infections, immunosuppressive medications, and graft-related issues. Diagnostic modalities include molecular amplification techniques, serological estimation of antibodies and bone marrow biopsy, with molecular amplification technique being an investigation of choice for achieving an early and accurate diagnosis. Successful treatment approaches encompass clinical strategies, including reducing immunosuppressant dosage, repeated intravenous immunoglobulin (IVIG) administration, and employing the antiviral drug Cidofovir. The efficacy of Cidofovir underscores the importance of timely interventions. This case underscores the importance of early detection and intervention in addressing anemia and preserving graft function during post-transplant parvovirus B19 infection, highlighting the utility of Cidofovir
Additional Information
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
Dr. Pranjal drafted the manuscript, designed it, and did data analysis dr sunny, dr sushrut, dr shubham and dr twinkle helped in designing of the manuscript. Critical review of the manuscript for important intellectual content was given by dr kapil, dr prasad, dr charulata, dr amol, dr priyanka. Dr manish balwani, Dr amit pasari, Dr kapil reviewed the final version and supervised the work.
References
- Yaghoubi F, Dalil D, Tavakoli F, et al. (2023). Relapsing anemia associated with parvovirus B19 infection in a kidney transplant recipient: a case report and review of the literature. Clin Case Rep., 7:905-906.
Publisher | Google Scholor - Singh V, Dogra PM, Singh P, et al. (2023). Parvovirus B19 infection after kidney transplantation: a single centre experience. Med J Armed Forces India, 79:665-671.
Publisher | Google Scholor - Neild G, Anderson M, Hawes S, Colvin BT. (1986) Parvovirus infection after renal transplant. Lancet, 22:1226-1227
Publisher | Google Scholor - Simunov B, Mrzljak A, Jurekovic Z, et al. (2022). Parvovirus B19 status in liver, kidney and pancreas transplant candidates: a single center experience. World J Transplant, 18:378-387.
Publisher | Google Scholor - Annavarajula, Sashi Kiran, Momin, et al. (2003). Pure red cell aplasia caused by parvovirus b19 infection in an early kidney transplant recipient. Indian Journal of Transplantation, 17:381-382.
Publisher | Google Scholor - Egbuna O, Zand MS, Arbini A, Menegus M, Taylor J. (2006). A cluster of parvoviruses B19 infections in renal transplant recipients: a prospective case series and review of the literature. Am J Transplant, 6:225-231.
Publisher | Google Scholor - Krishnan P, Ramadas P, Rajendran PP, et al. (2015). Effects of parvovirus b19 infection in renal transplant recipients: a retrospective review of three cases. Int J Angiol., 24:87-92.
Publisher | Google Scholor - Thongprayoon C, Khoury NJ, Bathini T, et al. (2020). Epidemiology of parvovirus B19 and anemia among kidney transplant recipients: a meta-analysis. Urol Ann., 12:241-247.
Publisher | Google Scholor - Porignaux R, Vuiblet V, Barbe C, et al. (2013). Frequent occurrence of parvovirus B19 DNAemia in the first year after kidney transplantation. J Med Virol., 85:1115-1121.
Publisher | Google Scholor - Luo Y, Lou S, Deng X, Liu Z, Li Y, Kleiboeker S, Qiu J. (2012). Parvovirus B19 infection of human primary erythroid progenitor cells triggers ATR-Chk1 signaling, which promotes B19 virus replication. J Virol., 85:46-55.
Publisher | Google Scholor - Čapenko S, Kozireva S, Folkmane I, Bernarde K, Rozentāls R, Murovska M. (2012). Anemia as a complication of parvovirus b19 infection in renal transplant recipients. Medicina (Kaunas, Lithuania), 48:299-304.
Publisher | Google Scholor - Baek CH, Kim H, Yang WS, Han DJ, Park SK. (2017). Risk factors and long-term outcomes of parvovirus B19 infection in kidney transplant patients. Transplant infectious disease : an official journal of the transplantation society, 19:73-74.
Publisher | Google Scholor - Eid AJ, Brown RA, Patel R, Razonable RR. (2006). Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis, 1:40-48.
Publisher | Google Scholor - Ahsan N, Holman MJ, Gocke CD, Groff JA, Yang HC. (1997). Pure red cell aplasia due to parvovirus B19 infection in solid organ transplantation. Clin Transplant, 11:265-270.
Publisher | Google Scholor - Sharma N., Bajwa R. (2020). Parvovirus infection-related anemia after kidney transplantation. Case Reports in Transplantation, 12:3-4.
Publisher | Google Scholor - Wong TY, Chan PK, Leung CB, Szeto CC, Tam JS, Li PK. (1999). Parvovirus B19 infection causing red cell aplasia in renal transplantation on tacrolimus. Am J Kidney Dis., 34:1132-1136.
Publisher | Google Scholor - Nair V, Jandovitz N, Jhaveri KD, et al. (2020). Treatment of parvovirus B19 viremia to facilitate kidney transplantation in a patient with collapsing glomerulopathy. Clin Nephrol Case Stud, 29:41-45.
Publisher | Google Scholor - De Clercq E. (1996). Therapeutic potential of cidofovir (hpmpc, vistide) for the treatment of dna virus (i.e. herpes-, papova-, pox- and adenovirus) infections. Verhandelingen - Koninklijke Academie voor Geneeskunde van Belgie. 58:19-47.
Publisher | Google Scholor