Case Report
Cardiac Cirrhosis and Right Heart Failure as a Consequence of Implantable Cardiac Device
- Mohamad Mubder 1*
- Issa Pour-Ghaz 2
- Omar Al-Taweel 3
- Buthainah Alhwarat 4
- Ahmad Abualhayjaa 5
- Ahsan H. Choudhury 3
- Neeraja Yedlapati 5
- Osama Mahmoud 6
- Deya Alkhatib 6
1Department of Internal Medicine, University of Nevada Las Vegas, Las Vegas, Nevada, United States.
2Department of Internal Medicine, University of Tennessee Health Science Center, Memphis, TN, United States.
3Department of Cardiology, Kirk Kerkorian School of Medicine at UNLV, Las Vegas, Nevada, United States.
4Hashemite University, Faculty of Medicine, Zarqa, Jordan.
5Department of Cardiology, University of Tennessee Health Science Center, Memphis, TN, United States.
6Cardiovascular Medicine, The University of Tennessee Health Science Center, Memphis, United States.
*Corresponding Author: Mohamad Mubder
Citation: M Mubder, I Pour-Ghaz, O Al-Tawee, B Alhwarat, A Abualhayjaa, et al. (2023). Cardiac Cirrhosis and Right Heart Failure as a Consequence of Implantable Cardiac Device. Journal of Clinical Cardiology and Cardiology Research, BioRes Scientia Publishers. 2(2):1-4. DOI: 10.59657/2837-4673.brs.23.011
Copyright: © 2023 Mohamad Mubder, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 20, 2023 | Accepted: September 04, 2023 | Published: September 18, 2023
Abstract
The utilization of cardiac implantable electronic devices has significantly enhanced the longevity and standard of living for many individuals with heart conditions. However, the utilization of these devices has also resulted in certain issues that need to be addressed to enhance patient outcomes. One of the complications that have received increased attention is tricuspid valve regurgitation (TR) secondary to devices’ leads. Gaining a more comprehensive understanding of these complications and underlying mechanisms can lead to better diagnosis, management strategies, and potentially improved device design.
Keywords: implantable cardioverter defibrillator; tricuspid valve; cardiac cirrhosis; heart failure; implantable cardiac device
Introduction
Prophylactic therapy with the implantable cardioverter defibrillator (ICD) was shown to prolong survival in patients with left ventricular dysfunction [1]. However, these devices can pose risks for the patients, including the possible development of infective endocarditis or device-mediated cardiomyopathy (CMP) [2]. Device leads can disturb the tricuspid valve resulting in mechanical tricuspid regurgitation (TR). The results from the Multi-center Automatic Defibrillator Implantation Trial (MADIT) II have demonstrated that the reduction in the mortality risk is associated with a significant 39% increase in the risk of subsequent heart failure hospitalization [1]. Here, we are presenting a case of torrential TR secondary to ICD lead presenting as cardiac cirrhosis.
Case Presentation
The patient was a 43-year-old man with a past medical history significant for heart failure with a non-ischemic cardiomyopathy with severely reduced left ventricular ejection fraction (LVEF) of 25 to 30% with New York heart classification class III symptoms with American College of Cardiology (ACC) stage D heart failure, history of ventricular tachycardia status post cardiac resynchronization therapy-defibrillator CRT-D approximately five years prior, chronic kidney disease who presented for further evaluation of his heart failure with right heart catheterization (RHC). He was noted to have ventricular waveforms in the right atrial pressure measurements consistent with severe TR. Due to ongoing severe heart failure symptoms, significant shortness of breath, and new worsening TR, the patient was brought into the hospital to evaluate for heart failure and the need for advanced cardiac care. On transthoracic echocardiogram (TTE), LVEF of 25 to 30% was noted, with findings of moderate-sized atrial septal defect versus patent foramen ovale (PFO). There was torrential TR. Trans-esophageal echocardiogram showed a moderate-sized PFO and intermittent right-to-left intra-cardiac shunting. There was torrential TR with restricted movement of the anterior and septal tricuspid leaflets. There was also partial thickening of the anterior and septal leaflets. Initially, there was a concern for carcinoid heart disease, which was ruled out by laboratory testing and ultrasound imaging of the liver. A repeated TTE was performed to evaluate the interaction between the CRT-D leads and the tricuspid valve leaflets. It was noted that the right ventricular lead was impinging the septal leaflet, significantly restricting its mobility and causing torrential TR. In addition, the coronary sinus lead looped across the tricuspid valve annulus and interfered with the anterior leaflet closure (refer to figure 1-2).
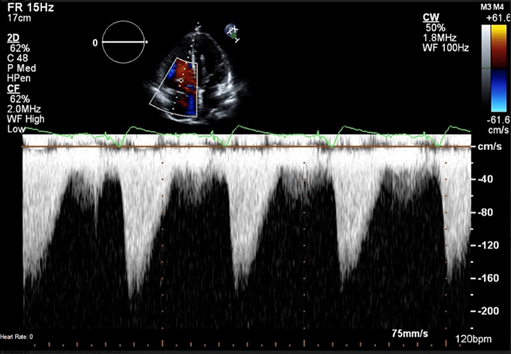
Figure 1: Continuous wave Doppler showing dense triangular TR jet suggestive of at least severe TR. TR: tricuspid regurgitation.
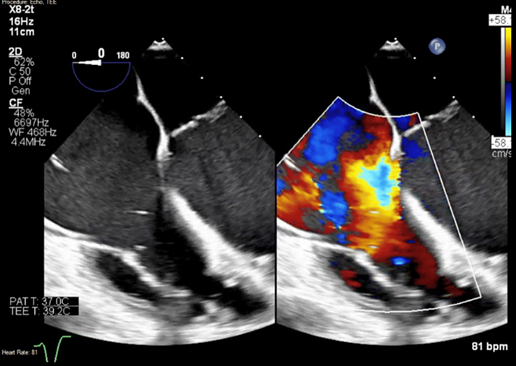
Figure 2: Color Doppler showing torrential TR jet. TR: tricuspid regurgitation.
Computer tomography (CT) of the chest, abdomen, and pelvis showed moderate-sized right pleural effusion with small ascites and mild mesenteric edema. Blood volume analysis showed significant plasma volume excess. A liver biopsy was performed to evaluate the cause of the cirrhosis's etiology, which showed cryptogenic cirrhosis. Laboratory testing was negative for hemochromatosis, genetic mutations, and carcinoid causes for cirrhosis, leaving the torrential TR through the years as the most likely culprit. Worsening respiratory status required intubation and ventilatory support. Multi-organ failure complicated by severe coagulopathy and sepsis resulted in rapid clinical deterioration. CT scan was significant for pneumatosis, and he was not a candidate for laparotomy given multi-organ failure and poor prognosis. Despite attempts to stabilize the patient for a possible multi-organ transplant, unfortunately, the patient deteriorated and passed away due to severe illness.
Discussion
Patients with severe left ventricular dysfunction can benefit from implantable cardioverter defibrillators (ICD), which have been shown to reduce mortality risk by 30-54% and improve survival [1, 3, 4]. The reduction in sudden cardiac death (SCD) is the primary reason for the lifesaving benefits of ICDs [3, 5]. The devices are equipped with anti-tachycardia pacing to terminate reentrant ventricular tachycardia (VT) and can deliver internal shocks to terminate VT or VF [3]. Additionally, the devices store records of all therapy administered, which can be routinely retrieved through device interrogation at regular intervals [3]. However, the implantation of CIED presents inherent risks including complications associated with the procedure, complications arising from leads and pulse generator usage, Valvular complications, the potential for infection, the development of arrhythmia, as well as complications stemming from medication usage and the psycho-social burden experienced by the patients [6]. The clinical syndrome known as right heart failure (RHF) is characterized by symptoms and signs resulting from the dysfunction of right heart structures, such as the right ventricle and tricuspid valve, leading to a decreased ability to supply blood to the lungs at normal central venous pressures. While most patients with RHF have a history of left-sided heart failure, pulmonary diseases such as COPD, or obstructive sleep apnea, some patients present with symptoms and signs of RHF without these underlying causes [2]. The use of an endocardial lead for pacing or defibrillation in the right ventricle has identified various adverse outcomes related to tricuspid valve (TV) structure and function [2,7]. The TV can be damaged by mechanical obstruction caused by the lead, fibrotic attachment between the lead and leaflet(s), lead entanglement with TV support structures, leaflet perforation, laceration, and avulsion [8, 9]. Procedural and technical factors can also affect the type and probability of valve damage during lead advancement and positioning (refer to table 1) [8].
Table 1: Mechanisms of mechanical TR in the setting of intra-cardiac leads. TLE: trans-venous lead extraction; TR: tricuspid regurgitation; TV: tricuspid valve.
| Procedural and technical factors |
| Valve obstruction due to lead placement between leaflets |
| Valve incomplete closure due to lead adherence with scar formation |
| Lead entrapment in the TV apparatus |
| Valve or leaflet perforation |
| Tricuspid annular dilation |
| TV apparatus damage after TLE |
Historically, two-dimensional echocardiography has been the mainstay of real-time cardiac imaging, but it is difficult to image the TV using this method. Multiple views are required, and no more than two leaflets are displayed in any one view [8-10]. Three-dimensional (3D) echocardiography, which provides a full-volume view of cardiac structures, should be the preferred imaging modality in patients with cardiac implantable electronic devices (CIEDs) [8, 11]. Other causes of tricuspid regurgitation (TR) in patients with CIEDs include intrinsic valvular disease/dysfunction or tricuspid annulus enlargement. When the pulmonary artery systolic pressure (PASP) exceeds 40 mm Hg, the incidence of TR approaches 90% due to chronic elevation of left heart filling pressures and subsequent development of pulmonary hypertension [12]. Most CIEDs require a right ventricular (RV) lead to be inserted and placed across the TV. Mechanical TR as a result of RV CIEDs leads has gained attention with more advanced echocardiographic techniques including 3D. The management of CIED-related severe TR depends on several factors, including right-sided heart failure symptoms, the severity of TR, the extent of lead-related valvular damage, the mechanism of CEIDs lead interfering with TV apparatus, the degree of RV dysfunction, and tricuspid annular dilation [13]. Medical therapy with diuresis is the primary treatment for patients with severe TR and symptomatic right heart failure. TLE with TV repair or replacement should be considered, with lead extraction alone being sufficient in some cases [8]. TLE is usually considered to be the first step. If TV function fails to significantly recover, then the next step will be TV repair or replacement. The need for CIEDs continues to grow, and studies are underway comparing trans-venous vs. subcutaneous ICDs, which are showing similar efficacy with fewer side effects related to the absence of trans-venous leads [2, 14].
Conclusion
Here we present a case of acute right-sided heart failure manifested as hepatic congestion and acute liver failure with pulmonary edema in a patient with recent implantation of CIED. TR is an increasingly recognizable cause of right ventricular heart failure. CIED leads have a major role in the development of TR in patients with post-device implantation right-sided heart failure. It is imperative to have high clinical suspicion and better understand the optimal diagnosis and risk factors for the development of TR post-CIED implantation and the best strategies to manage this problem.
References
- Pietrasik G, Goldenberg I, McNitt S, et al. (2009). Efficacy of Medical Therapy for the Reduction of Heart Failure Events in Patients with Implanted Cardioverter Defibrillators. Journal of Cardiovascular Electrophysiology, 20(4).
Publisher | Google Scholor - Cheema MA, Almas T, Ullah W, et al. (2021). RV lead placement - A forgotten cause of right heart failure. Ann Med Surg Lond. 7.
Publisher | Google Scholor - Moss AJ, Greenberg H, Case RB, et al. (2004). Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II) Research Group. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 21:3760-5.
Publisher | Google Scholor - Moss AJ, Hall WJ, Cannom DS, et al. (1996). Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 26:1933-40.
Publisher | Google Scholor - Greenberg H, Case RB, Moss AJ, et al. (2004). Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol. 21:1459-65.
Publisher | Google Scholor - Richardson CJ, Prempeh J, Gordon KS, et al. (2021). Surgical Techniques, Complications, and Long-Term Health Effects of Cardiac Implantable Electronic Devices. Cureus. 30:13001.
Publisher | Google Scholor - Paniagua D, Aldrich HR, Lieberman EH, et al. (1998). Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemakers leads. Am J Cardiol. 82:1130-2.
Publisher | Google Scholor - Ebrille E, Chang JD, Zimetbaum PJ. (2018). Tricuspid Valve Dysfunction Caused by Right Ventricular Leads. Card Electrophysiol Clin. 10:447-452.
Publisher | Google Scholor - Lin G, Nishimura RA, Connolly HM, et al. (2005). Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 17:1672-5.
Publisher | Google Scholor - Badano LP, Agricola E, Perez de Isla L, et al. (2009). Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography. Eur J Echocardiogr. 10:477-84.
Publisher | Google Scholor - Mediratta A, Addetia K, Yamat M, et al. (2014). 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging. 7:337-47.
Publisher | Google Scholor - Addetia K, Harb SC, Hahn RT, et al. (2019). Cardiac Implantable Electronic Device Lead-Induced Tricuspid Regurgitation. JACC Cardiovasc Imaging. 12:622-636.
Publisher | Google Scholor - Ren JF, Callans DJ, Marchlinski FE. (2014). Tricuspid regurgitation severity associated with positioning of RV lead or other etiology assessed by intracardiac echocardiography. JACC Cardiovasc Imaging. 7:1285-6.
Publisher | Google Scholor - Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. (2020). Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. 6:526-536.
Publisher | Google Scholor