Review Article
Cancer Immunotherapy Beyond CAR T Cells: Exploring Frontiers Like Bispecific Antibodies, Oncolytic Viruses, Immune Check Point Inhibitors and Their Combinations
1Application scientist, Bio division, MSP Lab instruments Pvt. Ltd, Hyderabad, India.
2Technical sales specialist, Bio division, MSP Lab instruments Pvt Ltd, Hyderabad, India.
*Corresponding Author: Utkalendu Suvendusekhar Samantaray, Application scientist, Bio division, MSP Lab instruments Pvt. Ltd, Hyderabad, India.
Citation: Utkalendu S. Samantaray, Patil V. (2025). Cancer Immunotherapy Beyond CAR T Cells: Exploring Frontiers Like Bispecific Antibodies, Oncolytic Viruses, Immune Check Point Inhibitors and Their Combinations. Journal of BioMed Research and Reports, BioRes Scientia Publishers. 7(2):1-3. DOI: 10.59657/2837-4681.brs.25.130
Copyright: © 2025 Utkalendu Suvendusekhar Samantaray, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 13, 2025 | Accepted: January 27, 2025 | Published: February 03, 2025
Abstract
The development of CAR T-cell treatments has led to a revolution in oncology through cancer immunotherapy, substantially in blood cancers like Acute Lymphoblastic Leukemia (ALL), Diffuse Large B-Cell Lymphoma (DLBCL), Mantle Cell Lymphoma (MCL) etc. However, it always comes into topic when we discuss about the toxicity like cytokine release syndrome, patient specificity in case of allogenic treatment in and scaling up in general, high expense, and limited effectiveness of CAR T-cell treatment in solid tumours make it necessary to investigate complementary or alternative strategies. This study explores new cancer immunotherapy approaches, including immune checkpoint inhibitors, oncolytic viruses, and bispecific antibodies, as well as their combinations. We go over the mechanisms, preclinical and clinical developments, and potential future paths for these effective approaches.
Keywords: CAR T-cell; oncology; cancer immunotherapy; blood cancers; Acute Lymphoblastic Leukemia
Introduction
While CAR T-cell therapy has demonstrated remarkable success in hematological malignancies, its limitations highlight the need for diverse approaches, and bispecific antibodies, oncolytic viruses, immune checkpoint inhibitors, and their combinations are emerging as promising tools to address these challenges. This review provides an overview of these modalities, highlighting recent advancements and future perspectives. The goal of cancer immunotherapy is to use the immune system to identify and eliminate tumor cells.
The Mechanism of Action of Bispecific Antibodies
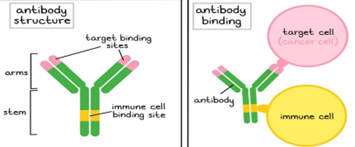
Engineered molecules known as bispecific antibodies (BsAbs) are made to bind two distinct antigens at the same time, usually one on tumor cells and the other on immune effector cells like T-cells or natural killer (NK) cells. Immune-mediated tumor cell death is facilitated by this dual targeting.
Although the body naturally produces antibodies, researchers have discovered methods to create and generate them in labs for use as medications to treat cancer. When researchers wish to use a certain antibody, they find the B cells that produce it, cultivate the B cells, and then extract the antibodies. Additionally, researchers can make modified antibodies by either changing the B cells' genetic coding to produce antibodies with better qualities or by modifying the antibodies themselves after they have been formed. Numerous beneficial antibodies are produced in mice (or other animals), but they must first be "humanized" to avoid the patient's immune system identifying them as "foreign." Humanized antibodies are created by modifying the genetic code of an animal-derived antibody and substituting the genetic code of a human antibody for everything save the target binding sites (the tips of the "Y" arms). Some antibody medications are also known as "chimeric" antibodies, which indicate that while they are almost humanized, a little portion of the original animal antibody remained preserved.
Advances in Preclinical and Clinical Research
Preclinical Research: In vitro and in vivo, BsAbs such as blinatumomab (CD19-CD3) have shown effectiveness in connecting T-cells to cancer cells, resulting in targeted cytotoxicity [1].
Clinical Trials: The FDA has approved blinatu-momab for B-cell acute lymphoblastic leukemia, and studies assessing BsAbs that target EGFR, HER2, and other antigens are still in progress [2].
Challenges and Opportunities
The efficacy of BsAbs in solid tumors remains limited due to the immunosuppressive tumor microenviron-ment. Novel designs, such as trispecific antibodies and extended half-life constructs, are under investigation.
Viruses that cause cancer (Oncolytic viruses)
Action Mechanism
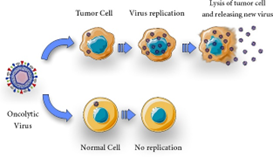
Tumor antigens are released, and systemic anti-tumor immunity is enhanced when oncolytic viruses (OVs) selectively infect and lyse tumor cells. Their immunostimulatory capacity and tumor selectivity are improved via genetic engineering.
Numerous viruses seem to choose tumor cells as a reproductive site because of their susceptibility to apoptosis (22, 60). One of the many ways that viral infection destroys tumor cells is by direct cytolytic activity, which is believed to be the primary oncolytic mechanism. This activity is brought on by the OVs' ability to specifically infect, multiply, and destroy cancer cells (Figure 1). It is now well acknowledged that a variety of mechanisms, including as changes in the tumor's micro- and macroenvironment and complex immune regulation, contribute to virotherapy's effectiveness (40, 61, 62). Viral proliferation and host cell bursting cause OVs to be cytolytic (63), and the viral infection may cause the host cell to undergo apoptosis (20).
Advances in Preclinical and Clinical Research
• Preclinical Research: In models of melanoma, glioblastoma, and colorectal cancer, research on OVs based on the herpes simplex virus, adenovirus, and vaccinia virus has demonstrated strong anti-tumor effects [3].
• Clinical studies: FDA-approved for melanoma, talimogene laherparepvec (T-VEC) is an HSV-based OV that is now undergoing studies for several malignancies [4].
Opportunities and Difficulties
Limited distribution to tumor locations and immunological clearance restricts the effectiveness of OVs. To get beyond these obstacles, tactics including immune checkpoint blockage and nanoparticle distribution are being investigated.
Immune Checkpoint Inhibitors
Mechanism of Action
Immune checkpoint inhibitors (ICIs) work by blocking inhibitory pathways, including PD-1/PD-L1 and CTLA-4, to restore T-cell activation and promote anti-tumor responses. Preclinical and Clinical Advancements Preclinical studies have shown that checkpoint blockade improves survival and tumor regression in animal models, frequently in conjunction with other therapies ([5]); clinical trials: ICIs, such as pembrolizumab and nivolumab, have revolutionized the treatment of melanoma, non-small cell lung cancer, and other cancers [6]. Opportunities and Challenges Resistance to ICIs is still a major obstacle, and research into combining ICIs with other immunotherapies, radiation, or targeted therapies is ongoing. Study methodologies include preclinical models and in vitro studies, which use co-culture systems to evaluate the interactions between immune cells and tumor cells.
• Animal Models: Humanized and synthetic mice models used to assess toxicity and effectiveness.
Design of Clinical Trials
• Phase I–III Trials: Evaluate patient selection based on biomarkers, safety, and effectiveness.
• Outcomes: Immune response biomarkers, overall survival, and progression-free survival.
Prospects for the Future
Improved bispecific antibody designs with higher specificity and lower toxicity are anticipated during the next ten years.
• Oncolytic viruses that have been genetically modified to target malignancies.
• Combination regimen optimization by the use of multi-omics techniques.
Conclusion
Bispecific antibodies, oncolytic viruses, immune checkpoint inhibitors, and their combinations represent the next frontier in cancer immunotherapy. Addressing their respective limitations through innovative design and strategic combinations holds promise for improving patient outcomes across diverse cancers.
References
- Bargou, R., et al. (2008). Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science, 321(5891):974-977.
Publisher | Google Scholor - Kantarjian, H., et al. (2017). Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. New England Journal of Medicine, 376(9):836-847.
Publisher | Google Scholor - Russell, S. J., et al. (2012). Oncolytic virotherapy: Clinical success. Science, 334(6060):678-682.
Publisher | Google Scholor - Andtbacka, R. H., et al. (2015). Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. Journal of Clinical Oncology, 33(25):2780-2788.
Publisher | Google Scholor - Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4):252-264.
Publisher | Google Scholor - Topalian, S. L., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New England Journal of Medicine, 366(26):2443-2454.
Publisher | Google Scholor - Goebeler, M., et al. (2018). Bispecific T-cell engagers: A review. Annals of Oncology, 29(3):558-567.
Publisher | Google Scholor - Ribas, A., et al. (2017). Oncolytic virotherapy targeting melanoma. Nature Medicine, 23(1):111-119.
Publisher | Google Scholor - Kelly, E., & Russell, S. J. (2007). History of oncolytic viruses: Genesis to genetic engineering. Molecular Therapy, 15(4):651-659.
Publisher | Google Scholor - Garber, K. (2006). China approves world’s first oncolytic virus therapy for cancer treatment. Journal of the National Cancer Institute, 98(5):298-300.
Publisher | Google Scholor - Ricca, J. M., Oseledchyk, A., Walther, T., Liu, C., Mangarin, L., Merghoub, T., et al. (2018). Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Molecular Therapy, 26(4):1008-1019.
Publisher | Google Scholor - Ilkow, C. S., Marguerie, M., Batenchuk, C., Mayer, J., Ben Neriah, D., Cousineau, S., et al. (2015). Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nature Medicine, 21(5):530-536.
Publisher | Google Scholor - Desjardins, A., Vlahovic, G., & Friedman, H. S. (2016). Vaccine therapy, oncolytic viruses, and gliomas. Oncology (Williston Park, NY), 30(3):211-218.
Publisher | Google Scholor - Chiocca, E. A., & Rabkin, S. D. (2014). Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunology Research, 2(4):295-300.
Publisher | Google Scholor