Research Article
Breast Cancer Classification According to Immunohistochemical Markers: Clinicopathologic Features in Women Treated at Pietersburg Hospital, Limpopo, South Africa
1 Department of Radiation Medicine and Imaging, Faculty of Health Sciences, University of Limpopo, Sovenga, South Africa.
2 Department of Surgery, Faculty of Health Sciences, University of Limpopo, Sovenga, South Africa.
*Corresponding Author: Francis O. Ooko, Department of Radiation Medicine and Imaging, Faculty of Health Sciences, University of Limpopo, Sovenga, South Africa.
Citation: Francis O. Ooko, Joyce R. Mphahlele, Mirza M.U.Z Bhuiyan, V A Sebastian. (2023). Breast Cancer Classification According to Immunohistochemical Markers: Clinicopathologic Features in Women Treated at Pietersburg Hospital, Limpopo, South Africa. Journal of BioMed Research and Reports, BRS Publishers. 2(2); DOI: 10.59657/2837-4681.brs.23.012
Copyright: © 2023 Francis O. Ooko, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 14, 2023 | Accepted: February 09, 2023 | Published: February 15, 2023
Abstract
Background: Breast cancer molecular subtypes can provide critical prognostic information that is useful to individualize care in addition to avoiding unwarranted adjuvant chemotherapy in some patients. Micro-array-based genetic profiling used to type breast tumours is expensive and unavailable for most low- and middle-income countries. Molecular subtyping using immunohistochemical (IHC) markers has proved to be a viable alternative. We therefore used this immunohistochemical technique to classify the breast cancer subtypes in our patient population and evaluated its association with clinicopathologic characteristics in these patients.
Patients and Methods: We retrospectively evaluated immunohistochemical marker results (oestrogen, progesterone and Human Epidermal Growth Factor Receptor-2 (HER2) receptors) of 254 breast cancer patients treated at a tertiary referral hospital from 01 January 2010 to 31 December 2011 against their clinicopathological variables.
Results: Out of 329 patients with invasive breast cancer treated within the study period 254 who had complete IHC marker test results were evaluated. Of these 136(54%) were luminal A, 69(27%) triple negative breast cancer (TNBC), 29(11%) HER2- enriched, and 20(8%) luminal B. The mean age at diagnosis was 55.6, 54.4, 54.2, and 52.3 years for luminal A, TNBC, HER2, and luminal B, respectively. The rate of lymph node involvement was 80%, 66.7%, 66.2%, and 58.6% for luminal B, TNBC, luminal A, and HER2, respectively.
There was no association between molecular subtypes and clinical stage (p=0.578) or distant metastasis (p = 0.940). Grade 3 tumours were most prevalent in TNBC (59.4%) followed by luminal B (40%), HER2 (37.9%), and luminal A (37.5%). Five-year survival was 27.2%, 25%, 24.1% and 23.2% for luminal A, luminal B, HER2 and TNBC respectively (p=0.104).
Conclusion: A large proportion or our patients (22.8%, n=75) with missing IHC test results could not be classified into breast cancer molecular subtypes and may have missed an opportunity for an individualized care. There is need for IHC tests in every breast neoplasm biopsy specimen to include ER, PR, HER2, and Ki67 markers for classification of the lesion according to molecular subtypes.
Keywords: oestrogen receptor; progesterone receptor; HER2; molecular subtype
Introduction
Breast cancer is the most commonly diagnosed cancer in females worldwide with an estimated 2.3 million new cases annually [1]. In South Africa, breast cancer contributed to 23.22% of all histologically diagnosed neoplasms in females in 2019 [https://www.nicd.ac.za/centres/national-cancer-registry/cancer-statistics/] translating to 33.95 cases per 100 000 per year. The development of new therapies for breast cancer has resulted in significant increase in disease-free survival and reduction in cancer specific mortality [2].
However, selection and administration of therapies according to the patient and disease characteristics is critical in improving disease-free and overall survival, and also to prevent late treatment-related complications such as cardiac toxicities caused by anthracyclines, myelodysplastic syndrome, leukaemia and taxane-associated neuropathy [3].
Historically, confirmation of diagnosis and classification of invasive breast cancer was made according to the morphological appearance of malignant cells. The main histological types according to morphological classification are invasive ductal carcinoma not otherwise specified (IDC-NOS), and lobular carcinoma. However, breast cancer is now known to be a highly heterogeneous disease comprising distinct phenotypes with diverse clinical behaviour [4].
The different phenotypes present with diverse clinical history, morphological characteristics, therapeutic outcome, and prognosis [5]. The heterogeneousness of breast cancer was first reported twenty years ago with the discovery of intrinsic molecular subtypes that helped to explain the diversity of biological behavior and response to treatment [6, 7] which now demands that treatment for each patient be individualized.
Hierarchical cluster analysis of intrinsic genes associated with breast tumors reveal the existence of at least four molecular subtypes, namely: luminal A, luminal B, HER2-enriched, and basal-like or triple negative breast tumours [3]. Following the discovery of the molecular subtypes, several microarray-based gene expression profiling kits have been developed and are now used routinely to predict outcome for individual breast cancer patients with good prognosis who do not require adjuvant chemotherapy [8]. Examples of commercially available microarray-based gene testing kits include MammaPrint [MP], Oncotype DX, PAM-50 risk recurrence score, Breast Cancer Index, and Endo Predict [3].
Microarray-based tumour profiling using the 70-gene MammaPrint was introduced in South Africa in 2007 followed by the introduction of local referral criteria for payment by medical insurance companies [5, 9]. However, gene testing kits are expensive and not universally available to a majority of eligible patients in South Africa and other low- and middle-income countries [5, 10]. Additionally, most kits require fresh frozen tissue samples that make it necessary to repeat a biopsy to obtain fresh tissue sample for testing [10]. Initially in South Africa, analysis was performed on fresh tissue only, until 2012, when the use of formalin fixed paraffin embedded (FFPE) tissue became available and is now the only method in use [5]. This has made gene testing available to more patients than before thereby facilitating a limited degree of individualized patient care.
The process of individualizing therapy starts with the assessment of clinicopathologic characteristics such tumour size, grade, lymph node involvement, patient demographics, and several molecular markers found within the tumour. The most significant molecular markers commonly considered in treatment decision-making in a resource–constrained setting are oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and cytokeratin 5/6, usually identified by conducting immunohistochemistry (IHC) studies on biopsy specimen of a lesion [10].
IHC uses antibodies specific for each biomarker, with the estimated number of positively staining cells in the tumour correlating to positive or negative result. IHC is considered adequate for testing ER and PR. However, IHC alone for HER2 protein expression is not perfect and leads to approximately 10 percentage false negative rates [11] making it necessary to perform fluorescent or chromogenic in situ hybridization (FISH or CISH) to confirm HER2 gene amplification, or to establish its presence in inconclusive results. IHC and ISH show high concordance with microarray-based gene profiling and are often used as surrogates for gene expression analyses as they reflect the genetic subtypes in a nearly similar way to advanced genetic testing techniques for molecular markers. Thus, laboratory results of IHC and ISH can be used to identify histologic subtype or molecular phenotype in an accessible, affordable and easier way [12].
Even in the absence of microarray-based gene profiling, classification of breast cancers by immunohistochemistry into molecular subtypes is still crucial because the subtypes markedly influence the kind of treatment a patient is likely to respond to and the prognosis [10]. Therefore, identification of subtypes is critical in selecting the most effective therapy while minimizing unnecessary use of chemotherapy and in determining the prognosis for each patient. The aim of this study was to classify tumours in women with breast cancer treated at Pietersburg Hospital according to molecular subtypes based on immunohistochemistry markers, and to evaluate any association between molecular subtypes and clinicopathologic characteristics of these patients. The institutional ethics committee (Turfloop Research Ethics Committee) approved the study (Certificate number TREC/127/2021: PG). Permission to access patient records was provided by the Hospital administration.
Patients and Methods
We conducted a retrospective review of all breast cancer cases treated in Pietersburg Tertiary Teaching Hospital over a 2-year period from 01st January 2010 to 31st December 2011 who were subsequently followed up for up to 60 months. Cases were selected from the Medical and Radiation Oncology Clinic database.
Patients and Methods
We conducted a retrospective review of all breast cancer cases treated in Pietersburg Tertiary Teaching Hospital over a 2-year period from 01st January 2010 to 31st December 2011 who were subsequently followed up for up to 60 months. Cases were selected from the Medical and Radiation Oncology Clinic database.
Immunohistochemical staining
Immunohistochemical tests were performed on primary breast tumours obtained from the initial biopsy. Antibodies against ER, PR, and HER2 (Leica bond) were used according to the manufacturer’s specifications. IHC analyses were also run-on Leica bond III machine. External tissue controls were used to standardize and optimize the reagents. Automation of the process guaranteed the uniformity of results and avoided variations due to human error. We collected and analysed the information as found in each patient’s medical record without any alterations.
To determine IHC subtype for each patient we combined the result of ER, PR, and HER2 receptor as follows: Luminal A (ER+ and/or PR+, HER2-), luminal B (ER+ and/or PR+, HER2+), HER2-enriched (ER-, PR-, HER2 +), and TNBC/basal type [ ER-, PR-, HER2). The TNBC and basal type tumours were considered together. Tumour grade was reported according to Elston-Ellis’s modification of Bloom-Richardson systems [13]. Clinical staging was determined according to the American Joint Committee on Cancer (AJCC) Staging Manual 8th edition [14].
Data collection
We collected demographic data of each patient such as age at diagnosis, menopausal status, histological type, ER, PR, HER2 results, tumour grade, tumour size (T stage), involvement of regional lymph nodes (N), and metastasis (M) status. We also recorded the time interval from diagnosis to the last recorded hospital visit or up to 60 months of follow-up. The last recorded date in the clinical record before 60 months was used as a surrogate for survival time in case of patients who did not complete 60-months follow-up.
Data analysis
Data were analysed on SPSS software version 27 [SPSS, Inc. Chicago IL, United States of America]. Descriptive statistics (mean, proportions and frequency) were used to analyse the variables. Analysis of variance (ANOVA) was used to assess the association between breast cancer subtypes and continuous variables such as age and menopausal status. The Chi-squared test was used to determine the association between clinicopathological features and breast cancer subtypes [discontinuous variables]. A p-value of 0.05 was considered statistically significant. Time interval from initial diagnosis until last recorded date, or 60 months follow-up was used to calculate overall survival by the Kaplan-Meier method. Data is presented as graphs and charts.
Results
Patient characteristics are shown in Table 1.
| Characteristic | Total (n=329) | TNBC n=69(21.0%) | HER2/neu n=29(8.8%) | Luminal A n=136(41.3%) | Luminal B n=20(6.1%) | Unknown n=75(22.8%) | P value |
| Age at diagnosis | |||||||
| Mean ± SD | 55.3 ±14.2 | 54.4 ±13.5 | 54.2 ±13.0 | 55.6 ±15.4 | 52.3 ±14.9 | 56.1±12.8 | 0.755 |
| Age by group | |||||||
| less then 40 | 42 (12.8%) | 7 (10.1%) | 5 (17.2%) | 19 (14.0%) | 5 (25.0%) | 6 (8.0%) | 0.991 |
| 40 - 49 | 81 (24.6%) | 21 (30.4%) | 4 (13.8%) | 33 (24.3%) | 4 (20.0%) | 19 (25.3%) | |
| 50 - 59 | 94 (28.6%) | 23 (33.3%) | 9 (31.0%) | 31 (22.8%) | 5 (25.0%) | 26 (34.7%) | |
| 60 - 69 | 52 (15.8%) | 5 (7.2%) | 7 (24.1%) | 26 (19.1%) | 4 (20.0%) | 10 (13.3%) | |
| ± 70 | 60 (18.2%) | 13 (18.8%) | 4 (13.8%) | 27 (19.9%) | 2 (10.0%) | 14 (18.7%) | |
| Age by group | 0.783 | ||||||
| less then 50 | 123(37.4%) | 28 (40.6%) | 9 (31.0%) | 52 (38.2%) | 9 (45.0%) | 25 (33.3%) | |
| 50 - 69 | 146(44.4%) | 28 (40.6%) | 16 (55.2%) | 57 (41.9%) | 9 (45.0%) | 36 (48.0%) | |
| ± 70 | 60 (18.2%) | 13 (18.8%) | 4 (13.8%) | 27 (19.9%) | 2 (10.0%) | 14 (18.7%) | |
| Menopausal Status | 0.578 | ||||||
| Premenopausal | 179(54.4%) | 40 (58.0%) | 16 (55.2%) | 74 (54.4%) | 12 (60.0%) | 37 (49.3%) | |
| Postmenopausal | 149(45.3%) | 29 (42.0%) | 13 (44.8%) | 62 (45.6%) | 8 (40.0%) | 37 (49.3%) | |
| Undetermined | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.3%) | |
| Tumor stage T | |||||||
| T1 | 27 (8.2%) | 6 (8.7%) | 3 (10.3%) | 9 (6.6%) | 0 (0%)) | 9 (12.0%) | 0.647 |
| T2 | 15 (4.6%) | 3 (4.3%) | 2 (6.9%) | 7 (5.1%) | 0 (0%) | 3 (4.0%) | |
| T3 | 55 (16.7%) | 13 (18.8%) | 3 (10.3%) | 24 (17.6%) | 6 (30.0%) | 9 (12.0%) | |
| T4 | 64 (19.5%) | 14 (20.3%) | 9 (31.0%) | 27 (19.9%) | 2 (10.0%) | 12 (16.0%) | |
| Tx | 168(51.1%) | 33 (47.8%) | 12 (14.4) | 69 (50.7%) | 12 (50%) | 42 (56.0%) | |
| Oestrogen receptor | |||||||
| Positive | 166(50.6%) | 0 (%) | 0 (0%) | 135 (99.3%) | 20 (100%) | 11 (14.9%) | 0.000 |
| Negative | 103(31.4%) | 69 (100%) | 29 (100%) | 1 (0.7%) | 0 (0%) | 4 (5.4%) | |
| Unknown | 59 (18.0) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 59 (79.7%) | |
| Progesterone receptor | |||||||
| Positive | 99 (30.1%) | 1 (1.4%) | 1 (3.4) | 78 (57.4%) | 12 (60%) | 7 (9.3%) | 0.000 |
| Negative | 166(50.5%) | 68 (98.6%) | 27 (93.1) | 58 (42.6%) | 8 (40%) | 5 (6.7%) | |
| Unknown | 64 (19.5%) | 0 (0%) | 1 (3.4) | 0 (0%) | 0 (0%) | 63 (84%) | |
| HER2 | |||||||
| Positive | 47 (14.3) | 0 (0%) | 28 (96%) | 0 (0%) | 19 (95%) | 0 (0%) | 0.000 |
| Negative | 205(62.3%) | 69 (100%) | 1 (3.4%) | 135 (99.3%) | 0 (0%) | 0 (0%) | |
| Unknown | 77 (23.4%) | 0 (0%) | 0 (0%) | 1 (0.7%) | 1 (5%) | 75 (100%) |
Table 1: Patient characteristics.
The median age was 55 years (±14.2 standard deviation (SD), range 26 to 96 years). The most common WHO histological type was IDC-NOS (81.8%, n=269) followed by mucinous (colloid) carcinoma (4.3%, n=14), medullary carcinoma (3%, n=10), papillary carcinoma (2.7%, n=9), and other histological types (3.9%, n=13). Histology was unknown in 14 cases (4.3%) [Table 2].
| WHO histological type | Number of patients (%) |
| Infiltrating ductal carcinoma (NOS) | 269 (81.8%) |
| Mucinous (colloid) carcinoma | 14 (4.3%) |
| Medullary carcinoma | 10 (3%) |
| Invasive papillary carcinoma | 9 (2.7%) |
| Others | 13 (3.9) |
| Unknown | 14 (4.3) |
Table 2: WHO histological types
Characteristics of 254 patients with complete information for subtype classification who were further analysed are shown in Table 3.
| Characteristic | Total (n=254) | Basal cell n=69(%) | HER2/neu n=29 (%) | Luminal A n=136 (%) | Luminal B n=20 (%) | P value |
| Lymph node status | ||||||
| Positive (N1-N3) | 169(66.5%) | 46 (66.7%) | 17 (58.6) | 90 (66.2%) | 16 (80.0%) | 0.886 |
| Negative (N0) | 63 (24.8%) | 16 (23.2%) | 10 (34.5%) | 33 (24.3%) | 4 (20.0%) | |
| Not determined | 22 (8.7%) | 7 (10.1%) | 2 (6.6%) | 13 (9.6%) | 0 (0%) | |
| Distant metastasis at diagnosis | ||||||
| No metastasis (M0) | 170(66.9%) | 47 (68.1%) | 18 (62.1%) | 92 (67.6%) | 13 (65%) | 0.940 |
| Metastasis (M1) | 84 (33.1%) | 22 (31.9%) | 11 (37.9%) | 44 (32.4%) | 7 (35%) | |
| AJCC stage | ||||||
| Stage I | 7 (2.8%) | 1 (1.4%) | 1 (3.4%) | 5 (3.7%) | 0 (0%) | 0.578 |
| Stage II | 38 (15.0%) | 13 (18.8%) | 4 (13.8%) | 18 (13.2%) | 3 (15%) | |
| Stage III | 119 (46.9) | 31 (44.9%) | 12 (41.8%) | 65 (47.8%) | 11 (55%) | |
| Stage IV | 85(33.5%) | 22 (31.9%) | 11 (37.9%) | 46 (33.8%) | 6 (30%) | |
| Unknown | 5 (2.0%) | 2 (2.9%) | 1 (3.4%) | 2 (1.5%) | 0 (0%) | |
| Histologic grade | ||||||
| Grade 1-2 | 109(42.9%) | 25 (36.2%) | 13 (44.8%) | 60 (44.1%) | 11 (55.0) | 0.587 |
| Grade 3 | 111(43.7%) | 41 (59.4%) | 11 (37.9%) | 51 (37.5%) | 8 (40.0%) | |
| Unknown | 34 (13.4) | 3 (4.3%) | 5 (17.2%) | 25 (18.4%) | 1 (5.0%) | |
| Surgery | ||||||
| Yes | 143(56.3%) | 36 (52.2%) | 19 (65.5%) | 75 (55.1%) | 13 (65.5%) | 0.997 |
| No | 107(42.1%) | 32(46.40%) | 10 (34.5) | 58 (42.6%) | 7 (34.5%) | |
| Unknown | 4 (1.6%) | 1 (1.4%) | 0 (0%) | 3 (2.2%) | 0 (0%) | |
| Chemotherapy | ||||||
| Yes | 215(84.6%) | 57 (82.6%) | 25 (86.2%) | 114 (83.8%) | 19 (95.0%) | 0.870 |
| No | 39 (15.4%) | 12 (17.4%) | 4 (13.8%) | 22 (16.2%) | 1 (5%) | |
| Adjuvant hormone therapy | ||||||
| Yes | 124(48.8%) | 6 (8.7%) | 5 (17.2%) | 98 (72.1%) | 15 (75%) | 0.000 |
| No | 130(51.2%) | 63 (91.3%) | 24 (82.8%) | 38 (27.9%) | 5 (25.0%) | |
| Radiation therapy | ||||||
| Yes | 110(43.3%) | 26 (37.7%) | 13 (44.8%) | 63 (46.3%) | 8 (40.0%) | 0.183 |
| No | 139(54.7%) | 41 (59.4%) | 15 (51.7%) | 72 (52.9%) | 11 (55.0%) | |
| Unknown | 5 (2.0%) | 2 (2.9%) | 1 (3.4%) | 1 (0.7%) | 1 (5.0%) | |
| Survival | ||||||
| 0 – 12 months | 79(31.1%) | 27 (39.1%) | 7 (24.1%) | 39 (28.7%) | 6 (30.0%) | 0.104 |
| 13 – 24 months | 55 (21.7%) | 18 (26.1%) | 8 (27.6%) | 25 (18.4%) | 4 (20.0%) | |
| 25 - 36 months | 31 (12.2%) | 6 (8.7%) | 3 (10.3%) | 19 (14.0%) | 3 (15.0%) | |
| 37 – 48 months | 15 (5.9%) | 1 (1.4%) | 2 (6.9%) | 11 (8.1%) | 1 (5.0) | |
| 49 – 60 months | 9 (3.5%) | 1 (1.4%) | 2 (6.9 %) | 5 (3.7%) | 1 (5.0%) | |
| 61+ months | 65 (25.6%) | 16 (23.2%) | 7 (24.1) | 37 (27.2%) | 5 (25%) |
Table 3: Clinico-pathologic characteristics of the molecular subtypes.
Comparison of WHO histological types and molecular subtypes are shown in Table 4.
| Who Histological Type | IHC Sub-Type | |||
| Luminal A | Luminal B | HER2/neu | TNBC | |
| Infiltrating ductal carcinima (NOS) | 113 (83.1%) | 18 (90%) | 23 (79.3%) | 60 (87%) |
| Invasive papillary carcinoma (NOS) | 5 (3.7%) | 1 (5%) | 1 (3.4%) | 1 (1.4%) |
| Medullary carcinoma | 3 (2.2%) | - | - | 4 (5.8%) |
| Invasive lobular carcinoma (NOS) | 1 (0.7%) | - | 2 (6.9%) | - |
| Mixed ductal and lobular | - | - | 1 (3.4%) | - |
| Mucinous (colloid) carcinoma | 8 (5.9%) | - | - | 3 (4.4%) |
| Ductal carcinoma in situ (DCIS) | 1 (0.7%) | - | - | 1 (1.4%) |
| Others | 3 (2.2%) | - | 1 (3.4%) | - |
| Unknown | 2 (1.5%) | 1 (5%) | 1 (3.4%) | - |
| Total | 136 | 20 | 29 | 69 |
Table 4: Comparison of WHO histological types and molecular subtypes
Overall ER, PR and HER-2 positivity was 50.6%, 30%, and 14.3% (p=0.000) respectively. Luminal A (54%, n=136) was the most frequent molecular subtype followed by TNBC (27%, n=69), HER2-enriched (11%, 29), and luminal B (8%, n=20), respectively. Luminal A had a peak incidence in relatively younger patients (40-49 years old), whereas luminal B, TNBC, and HER2-enriched all had peak incidence at 50 – 59 years. The mean age at diagnosis for each of the molecular subtypes were 55.6±15.4 SD, 54.4±13.5 SD, 54.2±13.0 SD, and 52.3±14.9 SD for luminal A, TNBC, HER2-enriched, and luminal B, respectively (p = 0.755). The rate of lymph node involvement (N1 –N3) was 80%, 66.7%, 66.2%, and 58.6% for luminal B, TNBC, luminal A, and HER2-enriched subtypes respectively (p = 0.886). There was no association between the molecular subtype and clinical stage (p=0.578), and with distant metastasis (p = 0.940).
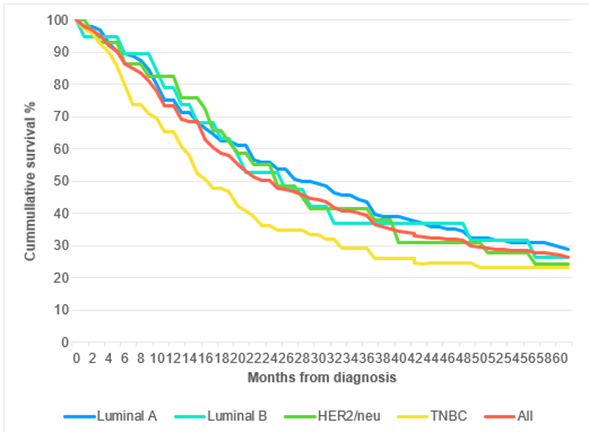
TNBC was associated with high-grade tumour with 41 out of 69 patients (59.4%) having grade 3 tumours, followed by luminal B (40%), HER2-enriched (37.9%), and luminal A (37.5%). Luminal A had the least percentage of grade 3 tumours (p=0.587). The five-year overall survival for the entire cohort was 25.5%. Five-year survival was 27.2%, 25%, 24.1% and 23.2% for luminal A, luminal B, HER2-enriched and TNBC, respectively (p=0.104) [Figure. 1].
Figure 1: Overall survival according to molecular subtypes.
Discussion
The mean age of women with breast cancer in South Africa at diagnosis is 54.4 years ±14.2 SD [15]. However, median age at diagnosis is variable across different African countries and among different population groups [16]. The predominant WHO histological type reported in African women was IDC-NOS [17, 18]. Hormone receptor positivity also shows a marked regional variation as reported in a systematic review of 80 African studies published between January 1980 and April 2014 [19]. In this review, variation in hormone receptor positivity rates was explained by the diverse methods of collection, preparation and handling of the specimen whereby mean ER positivity rate was 10% (range 4 –17%) lower for studies performed on archived tumour blocks compared to those performed on freshly collected specimen.
A study in South Africa reported breast cancer molecular subtype prevalence rates of 29.5%, 24.1%, 22.4%, 22.4%, 18.1% and 6%, for luminal A, luminal B HER2-negative, triple-negative, luminal B HER2-positive, and HER2/neu-positive, respectively [18]. The main difference between this study and ours is that the former factored in proliferation index (Ki-67) in determining molecular sub-types. However, our findings are consistent with another South African study which found 53.7%, 14.6%, 20.4%, and 11% of their patients with luminal A, luminal B, TNBC, and HER2/neu, respectively [17] and with another study from Peru [10] which used similar method to ours to characterize molecular subtypes. A study comparing the prevalence of molecular sub-types in Sudanese and German women found less occurrence of luminal A (36.9% versus 68.4%), nearly the same proportion of luminal B (13% versus 10.7%), twice the proportion of HER 2/neu (15.7% versus 6.8%), and twice the proportion of TNBC (34.5% versus 14.2%) in the Sudanese and German women [20]. The authors suggest that the disparity in the proportion of molecular subtypes between the two population groups could be a result of both environmental and inherent biologic factors such as exposure to agricultural pesticides and viral and parasitic infections, which could modulate immune and tumour microenvironment and possibly induce Sudanese women to develop a more aggressive disease [20].
Lymph node involvement indicates tumour infiltration beyond the primary disease and determines treatment outcome. Our study found no association between lymph node involvement and any molecular subtype. This is consistent with other studies that found no association between molecular subtype and axillary lymph node involvement [18, 21]. Similarly, a number of studies found no difference in the prevalence of distant metastasis amongst the four molecular subtypes [18, 21, 22]. However, one study found highly significant association between molecular subtypes and axillary node status (p=0.001), and a greater prevalence of distant metastasis in HER2/neu tumours (p=0.014) compared to the other subtypes [10].
Two studies in the literature found significant association between molecular subtype and T-stage [10, 21]. In one study, mean tumour diameter at diagnosis differed significantly among the sub-types (P less than 0.0001) with TNBC and HER 2/neu presenting with larger tumour diameter compared to either luminal A or luminal B [21]. Similarly, a higher percentage of T3 (54.4%) in HER2/neu, and T4 (38.6%) tumours in TNBC has been reported [10]. However, in our study there was no association between AJCC stage and molecular subtype as most patients had locally advanced or metastatic tumours.
Histological grade and molecular subtypes are both independent markers for outcome. The association between tumour grade and subtype was reported in the systematic review mentioned earlier [19]. TNBC is often associated with high grade tumours reflecting loss of oestrogen expression in more advanced forms of the disease. Other studies have also consistently revealed significant association between molecular subtypes and histological grade [10, 18, 21]. Spitale et al. (21) in a European study observed significant differences among molecular subtypes in which TNBC and HER2/neu cases show the highest prevalence of poorly differentiated phenotype (75.9% and 66.7%, respectively), whereas luminal A tumours are more frequently well to moderately differentiated (84.6%).
Vallejos et al. [10] found a significant association (p<.0001) between molecular subtype and histologic grade, with well to moderately differentiated tumours (grade 1 and 2) appearing most frequently in the luminal A (76.6%), whereas a greater percentage (70.3%) of poorly differentiated tumours (grade 3) occur in TNBC. In South Africa, Kakudji et al. [18] also found statistically significant association (p less than 0.001) where both luminal and non-luminal molecular subtypes are associated with grade 2 and 3 tumours.
Predictors of overall survival among breast cancer patients reported in a large study were age, menopausal status, AJCC stage, distant metastasis at diagnosis, histologic grade, Ki-67 proliferation index, tumour size and molecular subtype [21]. Among molecular subtypes TNBC and HER2/neu showed reduced survival probability at 2 years after diagnosis (89.4% and 91.7% respectively) compared to luminal A and B cases (96.5% and 96.7% respectively). Histologic type (ductal versus lobular carcinomas) did not convey significant differences in overall survival [21]. In another study with a 5-year overall survival of 73.5%, clinicopathological characteristics predicting survival were age, clinical stage, distant metastasis at diagnosis, histologic grade, axillary lymph node involvement, and tumour size [10]. Menopausal status and AJCC tumour stage did not influence survival. However, significant differences in overall survival according to molecular subtypes was observed with the highest probability of 5-year survival seen in luminal A tumours (81.9%), followed by luminal B (72.8%), and TNBC (67.1%). HER2/neu had the worst probability of survival at 62.4% [10].
Limitations
This study has some limitations mainly resulting from missing immunohistochemical data in some records which made molecular typing difficult in 23% (n=75) of our patients. Secondly, patients who were lost to follow-up affected accurate estimation of overall survival thus possibly contributing to the survival gap in this hospital-based study. The significance of survival gap in many African breast cancer studies has been noted and the need for population-based cancer survival estimates underscored to not only reduce loss to follow-up but also to quantify heterogeneity by clinical and epidemiological factors [16].
Conclusion
This study has revealed the need for minimum IHC testing for all suspected breast neoplasm biopsy specimen to include ER, PR, HER2 (with FISH or CISH if equivocal), and Ki67 markers to facilitate classification of the lesion according to molecular subtypes. Physicians concerned with primary treatment of breast cancer patients, in resource-constrained LMICs especially surgeons should employ protocols whereby basic IHC tests are requested for each biopsy specimen submitted to the laboratory while the histopathology laboratory should also ensure that basic IHC tests are conducted on every specimen.
Declarations
Acknowledgement: Not applicable
Authors’ contributions
F.O and R.M designed the study and collected the data and performed data analysis
M.B provided quality control of the data and reviewed the design and quality of the study
F.O wrote the manuscript
S.A provided advice on the study design and proof-read the manuscript
All the authors read and approved the final manuscript
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article and/or its related files. Any other information that supports the findings of this study are available from the corresponding author, [F.O], upon reasonable request.
Competing interest
The authors declare that there is no conflict of interest
Consent for publication
Not applicable
Ethics approval and consent to participate
This study was approved by the institutional review board of University of Limpopo. Permission to access and use patient records was granted by the Pietersburg Hospital Administration
Funding: None
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 71(3):209-249.
Publisher | Google Scholor - Florescu DR, Nistor DE. (2019). Therapy-induced cardiotoxicity in breast cancer patients: a well-known yet unresolved problem. Discoveries. 7(1).
Publisher | Google Scholor - Güler EN. (2017). Gene expression profiling in breast cancer and its effect on therapy selection in early-stage breast cancer. European journal of breast health. 13(4):168.
Publisher | Google Scholor - Tang Y, Wang Y, Kiani MF, Wang B. (2016). Classification, treatment strategy, and associated drug resistance in breast cancer. Clinical breast cancer. 16(5):335-343.
Publisher | Google Scholor - Grant KA, Myburgh EJ, Murray E, Pienaar FM, Kidd M, Wright CA, et al. (2019). Reclassification of early stage breast cancer into treatment groups by combining the use of immunohistochemistry and microarray analysis. South African Journal of Science. 115(3-4):1-6.
Publisher | Google Scholor - Perou CM, Sørlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA, et al. (2000). Molecular portraits of human breast tumours. Nature. 406(6797):747-752.
Publisher | Google Scholor - Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. (2003). Repeated observation of breast tumor subtypes in independent gene expression data sets. Proceedings of the national academy of sciences. 100(14):8418-823.
Publisher | Google Scholor - Weigelt B, Baehner FL, Reis‐Filho JS. (2010). The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 220(2):263-80.
Publisher | Google Scholor - Grant KA, Apffelstaedt JP, Wright C, Myburgh E, Pienaar R, De Klerk M, et al. (2013). MammaPrint Pre-screen Algorithm (MPA) reduces chemotherapy in patients with early-stage breast cancer. South African Medical Journal..103(8):522-6.
Publisher | Google Scholor - Vallejos CS, Gómez HL, Cruz WR, Pinto JA, Dyer RR, Velarde R, et al. (2010). Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a peruvian hospital database. Clinical breast cancer. 10(4):294-300.
Publisher | Google Scholor - de Ronde JJ, Hannemann J, Halfwerk H, Mulder L, Straver ME, Vrancken Peeters M-JT, et al. (2010). Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast cancer research and treatment. 119(1):119-126.
Publisher | Google Scholor - Engstrøm MJ, Valla M, Bofin AM. (2017). Basal markers and prognosis in luminal breast cancer. Breast cancer research and treatment. 163(2):207-217.
Publisher | Google Scholor - Elston CW, Ellis IO. (1991). Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology. 19(5):403-410.
Publisher | Google Scholor - Edge S, Byrd D. (2017). AJCC cancer staging manual. AJCC cancer staging manual.
Publisher | Google Scholor - Cubasch H, Dickens C, Joffe M, Duarte R, Murugan N, Chih MT, et al. (2018). Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009 to 11. Cancer epidemiology. 52:120-127.
Publisher | Google Scholor - McCormack V, McKenzie F, Foerster M, Zietsman A, Galukande M, Adisa C, et al. (2020). Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. The Lancet Global health. 8(9):e1203-e12.
Publisher | Google Scholor - McCormack VA, Joffe M, van den Berg E, Broeze N, dos Santos Silva I, Romieu I, et al. (2013). Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Research. 15(5):1-13.
Publisher | Google Scholor - Kakudji BK, Mwila PK, Burger JR, du Plessis JM, Naidu K. (2021). Breast cancer molecular subtypes and receptor status among women at Potchefstroom Hospital: a cross-sectional study. The Pan African Medical Journal. 38.
Publisher | Google Scholor - Eng A, McCormack V, dos-Santos-Silva I. (2014). Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS medicine.11(9):e1001720.
Publisher | Google Scholor - Sengal AT, Haj Mukhtar NS, Vetter M, Elhaj AM, Bedri S, Hauptmann S, et al. (2017). Comparison of receptor-defined breast cancer subtypes between german and sudanese women: a facility-based cohort study. Journal of global oncology. 4:1-12.
Publisher | Google Scholor - Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. (2009). Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Annals of oncology. 20(4):628-635.
Publisher | Google Scholor - Ekpe E, Shaikh AJ, Shah J, Jacobson JS, Sayed S. (2019). Metastatic breast cancer in Kenya: presentation, pathologic characteristics, and patterns—findings from a tertiary cancer center. Journal of Global Oncology. 5:1-11.
Publisher | Google Scholor