Research Article
Black Swan: Atypical Left Ventricular Tamponade
- Iveta Tasheva *
- Irina Koleva
- Milena Miletieva
Clinic of Cardiology, Sofiamed University Hospital, Sofia, Bulgaria.
*Corresponding Author: Iveta Tasheva, Clinic of Cardiology, Sofiamed University Hospital, Sofia, Bulgaria.
Citation: Tasheva I, Koleva I, Miletieva M. (2023). Black Swan: Atypical Left Ventricular Tamponade. Journal of Clinical Cardiology and Cardiology Research, BRS Publishers. 1(1); DOI: 10.59657/2837-4673.brs.23.006
Copyright: © 2023 Iveta Tasheva, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 11, 2023 | Accepted: January 26, 2023 | Published: February 08, 2023
Abstract
Cardiac tamponade is a life-threatening condition that requires prompt recognition and treatment. The diagnosis is made clinically and echocardiographically. The right heart chambers are a system of low pressures and are the first to collapse in tamponade. However, there are cases where LV tamponade is also observed. It is rare and has two types: isolated LV pericardial effusion and circumferential pericardial effusion. This chapter aims to highlight the importance of recognizing atypical forms, which is often difficult due to the lack of classic signs of tamponade.
Keywords: LV tamponade, pathophysiology, treatment
Introduction
Pericardial diseases may be either isolated disease or part of a systemic disease. The main pericardial syndromes that are encountered in clinical practice include pericarditis (acute, subacute, chronic and recurrent), pericardial effusion, cardiac tamponade, constrictive pericarditis and pericardial masses. All medical therapies for pericardial diseases are off-label, since no drug has been registered until now for a specific pericardial indication.
Cardiac tamponade is a life-threatening condition that requires prompt recognition and treatment. It is an accumulation of fluid in the pericardium under pressure, compression of one or more cardiac chambers, leading to hemodynamic compromise. In tamponade the pericardial fluid distends the pericardium and raises the intrapericardial pressure. This high intrapericardial pressure compresses all heart chambers in diastole until the pressure inside them, equalizes with the intrapericardial pressure. The diagnosis is proven clinically and echo-cardiographically.
Clinically is observed the Beck's triad, which includes a collection of three clinical signs - hypotension; elevated systemic venous pressure, often with jugular venous distension; muffled heart sounds.
Echocardiographic findings- collapsing cardiac chambers, abnormal septal motion, inspiratory variations of transmitral and transtricuspid flow, IVC (inferior vena cava) dilatation with poor inspiratory collapse.
The right heart chambers are a system of low pressures and are the first to collapse in tamponade. However, there are cases where atypical LV (left ventricle) tamponade is also observed. It is rare and is divided into two types:
- Isolated LV pericardial effusion
- Circumferential pericardial effusion
Isolated LV pericardial effusion most often appears as a postoperative complication, localized around the free posterior or lateral wall of the LV [1]. The presence of adhesions in the pericardial space is thought to increase the regional intrapericardial pressure above the intracavitary diastolic pressure, leading to collapse of the ventricular wall. In isolated LV effusion, the absence of pericardial effusion along the anterior wall of the LV, leads to free expansion of the RV during inspiration, without interfering with the left ventricular filling, which is the reason for the absence of pulsus paradoxes and hypotension [2].
Circumferential pericardial effusion due to high pressures in the right-sided cardiac chambers that exceed the ones in the left, as in severe PAH (pulmonary arterial hypertension). This is usually seen in patients with severe right-sided HF (heart failure).
Prevalence
Prevalence data vary; however, some studies suggest that isolated LV pericardial effusion is common after cardiac surgery. It is observed after heart transplantation in about 21% of patients.
Pericardial effusion in the setting of pulmonary artery hypertension (PAH) is common (25–30%) and typically small in size, but rarely causes hemodynamic compromise.
For a period of six months, 333 consecutive patients were examined serially after cardiac surgery, effusions were present in 178 patients (56%). Most of them appear on the second postoperative day, reach their maximum size by the 10th postoperative day, and usually resolve without sequelae in the first postoperative month. In 10 patients (5,3%), the pericardial effusion grew significantly, acquiring a negative hemodynamic effect, which necessitated evacuation by pericardial drainage [5].
Pericardial effusion is more common in patients who received aminocaproic acid during surgery. In a retrospective study of more than 4500 postoperative patients, only 48 had moderate or large effusion detected by echocardiography. Of these, 36 met the criteria for the diagnosis of tamponade. The symptoms and physical findings of significant postoperative pericardial effusions are often nonspecific, but echocardiographic detection and echo-cardio graphically guided pericardiocentesis when necessary, are safe and effective.
Cardiac LV tamponade after open heart surgery is more common in valvular surgery (73%) than in coronary artery bypass grafting (only 24%) and may be related to preoperative anticoagulant use. Prescribing indirect anticoagulants in patients with early postoperative pericardial effusion is risky, especially in those who have not undergone pericardiocentesis and effusion drainage [5].
Circumferential pericardial effusion cases are difficult to summarize in numbers, as there are only a few such cases described in the literature.
Pathophysiology
Pericardial effusion worsens the paradoxical interventricular septal motion characterized by displacement of the interventricular septum into the left ventricle during inspiration which impairs the preload of the left ventricle and leads to further decreases in cardiac output. When the increased intrapericardial pressure exceeds the left ventricular diastolic pressure, there is left atrial and/or ventricular diastolic collapse. The right ventricular diastolic collapse is absent due to the elevated right-sided pressures that are higher than the pericardial pressure [3]. In contrast, left atrial pressure is typically lower in PAH and therefore left atrial early diastolic collapse is more commonly seen. Exaggerated ventricular interdependence, such as a decrease in left ventricular filling with early inspiration, may also be present. Hypotension may be absent in some of these patients because of a compensatory increase in systemic vascular resistance. Pulsus paradoxus is absent because elevated right ventricular pressure and volume prevent the inspiratory drop in pleural pressure from further increasing right ventricular preload and stroke volume.
Pericardial effusion development in PAH appears to relate to right ventricular failure and a subsequent increase in right-sided filling pressures along with right atrial hypertension and increased pressure in the thebesian veins and coronary sinus. These processes result in increased filtration and lymphatic obstruction, resulting in pericardial effusion. Left ventricular tamponade is associated with the severity of PAH, rather than its etiology.
Thus, both aetiologies of LV tamponade result in absence or rarity of the classical signs of cardiac tamponade-hypotension, RV (right ventricle) collapse, and pulsus paradoxus [4].
Clinical Evaluation
Tamponade is a syndrome that is characterized by clinical manifestations consistent with low cardiac output and elevated central venous pressure - low mean arterial pressure, cold extremities, and signs of poor perfusion of the organs (eg, low urine output). It is associated with one of the following clinical findings: shortness of breath, orthopnea, cough, easy fatigue, nausea, anxiety.
During the physical examination, dilated jugular veins, oral cyanosis, tachypnea, decreased oxygen saturation, tachycardia, muffled heart sounds, ascites, edema of the lower extremities, increased jugular venous pressure, hepatomegaly can be observed.
Imaging studies
Electrocardiography has significant diagnostic value despite its relatively low sensitivity and specificity. Common ECG findings are sinus tachycardia, low ventricular complex voltage, and electrical alternation, which is defined as a change in the amplitude or electrical axis of the ventricular complex due to the floating motions of the heart.
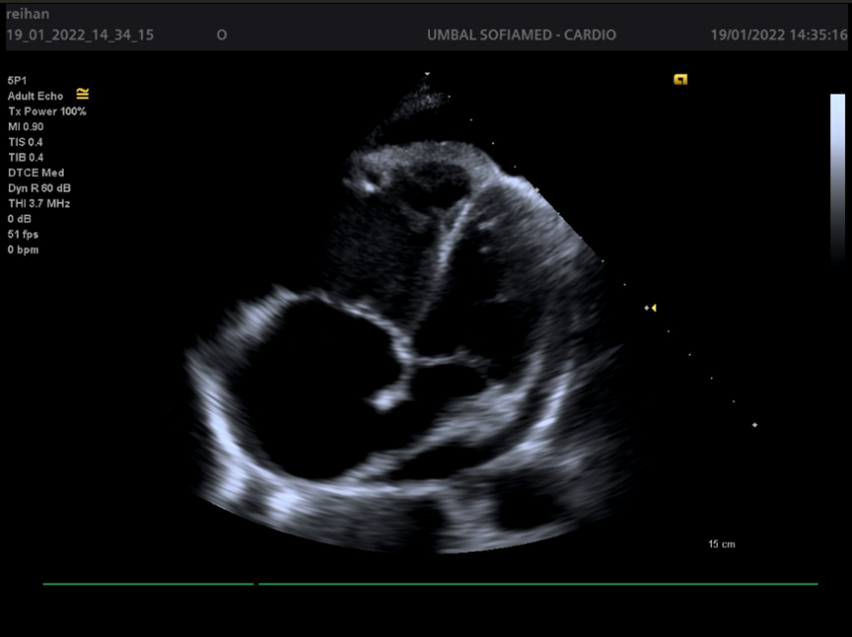
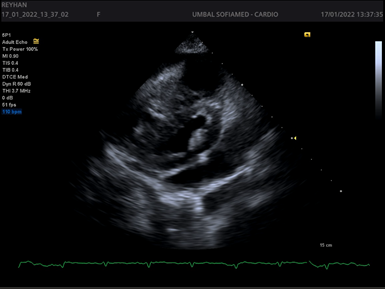
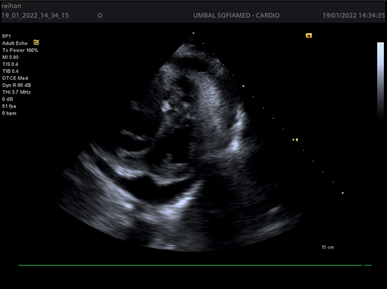
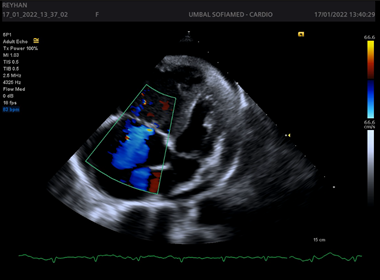
Echocardiography has leading diagnostic value. It has a high sensitivity and specificity regarding the recognition of cardiac tamponade and its cause. However, after cardiac surgery the TTE imaging windows may be limited by the presence of drains, surgical dressings and pacing wires; under this circumstance, TOE may be the better modality. Echocardiography is used to determine the size, location and hemodynamic effects of the pericardial effusion. It is important to evaluate from several imaging windows, as the apparent size of the effusion will vary depending on patient position and fluid location. Thin fibrinous strands, loculations or blood clots may also be seen within the fluid collection. Echocardiography findings consistent with left-sided chamber collapse, inferior vena cava dilatation, increased respiratory-driven valvular flow variation, reduced hepatic venous flow and abnormal motion of the interventricular septum during spontaneous inspiration with LV septal bounce.
Conventional radiography has low sensitivity and specificity.
CT and MRI are rarely considered, especially when echocardiography is available, although they have relatively good diagnostic capabilities for early recognition of cardiac tamponade.
Management
Pathophysiology of left ventricular tamponade determines the therapeutic approach.
The treatment of pulmonary hypertension requires a complex strategy that includes: - assessment the severity of the disease and change of functional class (I to IV), are necessary to establish appropriate treatment and for applying general and supportive therapies (diuretics, oxygen, anticoagulants, digoxin, anemia and iron status) [6].
The radical solution is to evacuate the fluid from the pericardium. Up to this point, the main goal of treatment is maintaining adequate hemodynamics (temporary measure with low efficiency). Mechanical ventilation is avoided due to the risk of worsening hemodynamics. For hypotension, some agents can be administered, although their effectiveness is limited. Infusion of fluids - helps to maintain intravascular volume and higher filling pressure.
Disadvantageously, it also increases intrapericardial pressure. Administration of inotropic agents is used to maintain adequate arterial and perfusion pressure.
In patients with LV tamponade caused by severe PAH, pericardiocentesis has a temporary effect. Therefore, the first-choice approach is to start conservative treatment.
The three main classes of drugs–endothelin receptor antagonists, prostacyclin analogs and phosphodiesterase-5 inhibitors affect the known pathogenetic mechanisms of PAH [6]. Endothelin antagonists are the most commonly used, phosphodiesterase inhibitors are an integral part of combined therapy, and the group of prostacyclin's is the most diverse. An improvement in physical capacity, hemodynamic parameters and quality of life could be observed in patients with PAH. Vasoreactivity tests are mandatory in patients with PAH to identify individuals who will respond to long-term high-dose calcium channel blocker therapy. They are performed in highly specialized referral centers and include inhaled nitric oxide (NO), intravenous epoprostenol or inhaled iloprost.
The use of calcium blockers is determined by the importance of hypertrophy, hyperplasia and proliferation of smooth muscle cells in the pathogenesis of PAH. Only a minority of patients are known to have a favorable response to calcium antagonist therapy. The need to administer them in high doses explains why they are not well tolerated by patients
If there is no response to vasoreactivity tests, or if there is vasoreactivity but no response or intolerance to high-dose calcium antagonists, we have to switch to PAH-specific treatment.
Phosphodiesterase type 5 inhibitors
Inhibition of cyclic guanosine monophosphate (cGMP) degrading enzyme phosphodiesterase 5 leads to vasodilation by activation of the NO/cGMP-pathway. Phosphodiesterase inhibitors also have an antiproliferative effect and cause strong vasodilation of the pulmonary vessels. Approved for the treatment of PAN are Sildenafil, Riociguat and Tadalafil [6].
Prostacyclin analogues and prostacyclin receptor agonists
Prostacyclin is produced primarily by endothelial cells and results in potent vasodilation. It is one of the strongest inhibitors of platelet aggregation and has cytoprotective and antiproliferative properties. Prostacyclin has an important role in the pathogenesis of PAH. Its synthetic analogues are characterized by different pharmacokinetic properties, but possess similar pharmacodynamic effects. Тhe medicaments approved for the treatment of PAH so far are Epoprostenol (intravenous), Iloprost (inhaled), Treprostinil (subcutaneous, intravenous and inhaled - only in the USA), Selexipag and Beraprost (only approved in Japan and South Korea) [6].
Combined Therapy
After initiation of therapy, the next step is an assessment of clinical response, which is usually done after 3-6 months of treatment. Clinical response was assessed based on several different parameters, including functional capacity (WHO), 6-minute walk test, cardiac index, right atrial pressure, NT-proBNP levels, as well as echocardiographic parameters. In the absence of an adequate clinical response, combined therapy is used.
When the patient is hemodynamically unstable, pericardiocentesis can be performed urgently, aimed as faster relief of symptoms. It has high efficiency, speed of implementation, low frequency of complications and low cost. Percutaneous catheter pericardiocentesis under echocardiographic or X-ray guidance is usually performed urgent, it can be performed with a blind needle in a subxiphoid approach. Relatively contraindications are poorly controlled anticoagulant therapy, thrombocytopenia below 50,000/mm2, coagulopathy.
Must be taken in consideration that as the effusion in such a circumstance is circumferential, the drainage of the fluid anterior to RV itself will decrease the fluid within the whole pericardium and result in resolution of LV collapse.
Оn the other hand the Symptomatic treatment of isolated LV pericardial effusion is the same as for acute pericarditis (NSAIDs or colchicine for several weeks or months, even after the effusion disappears). The therapeutic choice for resistant forms is: prolonged (3-6 months) oral corticosteroids or preferably pericardiocentesis and intrapericardial placement of triamcinolone 300 mg/m2. Reoperation and pericardectomy are required very rarely. Primary prevention of post-pericardiotomy syndrome with short-term perioperative administration of corticosteroids or colchicines is currently under investigation.
In patients with LV tamponade due to loculated effusion, the subxiphoid approach may not be useful as the pigtail catheter may not reach the effusion due to the loculations and rather be dangerous carrying the risk of RV perforation, thus mandating an axillary approach by echocardiography and fluoroscopy guidance.
The most serious complications are perforation of the myocardium and coronary vessels, pneumothorax, embolism, arrhythmia.
Figures 1-4.
Conclusion
LV tamponade is a syndrome that we can define as a "Black Swan" - an event that initially looks strange and difficult to predict, but in the end, it becomes more logical, based on the developing processes. Thus, patients with cor pulmonale and circumferential pericardial effusion develop an atypical form of cardiac tamponade with isolated LV compression. Tamponade may be suspected in patients with symptoms and signs of right-sided heart failure that suddenly worsen. When pericardial pressure begins to rise in patients with pulmonic heart, the increased pressures in the right heart chambers prevent compression of the right atrium and ventricle.
Continuing to increase, the pericardial pressure exceeds the pressure in the left cavities, which leads initially to the collapse of the LA in diastole and later of the LV. Signs of impaired LV filling lead to a reduction in stroke volume. The most likely mechanism of pericardial effusion recruitment in patients with PAH is increased transudation and impaired reabsorption of pericardial fluid due to increased venous pressure.
This aims to highlight the importance of recognizing atypical forms, which is often difficult due to the lack of classic signs of tamponade. Understanding the physiology of the LV tamponade, leads to early and accurate diagnosis and can prevent hemodynamic collapse, shock and death.
At this stage, there is limited evidence-based medical data to guide us in the treatment of left ventricle pericardial tamponade. In summary, pericardiocentesis as a stand-alone treatment can at least temporarily reduce symptoms, although it does not correct the underlying cause. The clinical prognosis after pericardiocentesis depends on the causes of the pericardial disease and the general condition of the patient.
Ongoing and future randomized clinical trials, registries, and guidelines are needed to improve the effectiveness of LV pericardial disease treatment.
References
- Chuttani K, Tischler MD, Pandian NG, Lee RT, Mohanty PK. Diagnosis of cardiac tamponade after cardiac surgery: relative value of clinical, echocardiographic and hemodynamic signs.
Publisher | Google Scholor - Kuvin JТ, Khabbaz K, Pandian NG. Left ventricular apical diastolic collapse: an unusual echocardiographic marker of postoperative cardiac tamponade.
Publisher | Google Scholor - Frey MJ, Berko B, Palevsky H, Hirshfield JW Jr, Herrmann HS. Recognition of cardiac tamponade in the presence of severe pulmonary hypertension.
Publisher | Google Scholor - Barun Kumar, Ashwin Kodliwadmath, Anupam Singh, Amar Upadhyay, Anshuman Darbari, Bhanu Duggal, Left ventricular tamponade- pathophysiology determines the therapeutic approach: a case series, European Heart Journal - Case Reports, 5(2).
Publisher | Google Scholor - Aleksieva E, Tsenev A, Vasilev A. Frequency and evolution of pericardial effusion at patients after cardiac surgery in the early period of rehabilitation.
Publisher | Google Scholor - ESC Guidelines for the diagnosis and treatment of pulmonary hypertension.
Publisher | Google Scholor