Case Report
Benign Metastasizing Leiomyoma: A Case Report
- Ruyu Shao
- Faquan Shen
- Desheng Yao *
Department of Gynecologic Oncology, Guangxi Medical University Cancer Hospital, Nanning, People’s Republic of China.
*Corresponding Author: Desheng Yao,Department of Gynecologic Oncology, Guangxi Medical University Cancer Hospital, Nanning, People’s Republic of China.
Citation: Shao R, Shen F, D. D. (2024). Benign metastasizing leiomyoma: A case report. International Journal of Medical Case Reports and Reviews, BioRes Scientia Publishers. 3(5), 1-5. DOI: 10.59657/2837-8172.brs.24.053
Copyright: © 2024 Desheng Yao, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 22, 2024 | Accepted: May 06, 2024 | Published: June 22, 2024
Abstract
A patient with benign metastasizing leiomyoma (BML) was admitted to Department of Gynecology Oncology, Guangxi Medical University Cancer Hospital in December 2022. A 47‑year‑old patient with vaginal bleeding presented herself for a gynecological examination. Masses of uterine, lungs, and many other sites throughout the body were found by magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT). Complete excision of the removable tumors and total abdominal hysterectomy with bilateral ovariosalpingectomy was performed through laparotomy under general anesthesia. Observation during operation, frozen pathology and postoperative routine pathology of the tumors suggested that the mass of the left pelvis was an epithelioid angiomyolipoma and all the other masses were leiomyoma. The purpose of this study is to investigate the clinical and imaging characteristics of BML according to this case.
Keywords: benign metastasizing leiomyoma; diagnostic imaging; epithelioid angiomyolipoma
Introduction
Uterine leiomyoma is the most common gynecological tumor [1]. In addition to a few common types (Intramural uterine fibroids, submucosal uterine fibroids, subserous uterine fibroids), there are eight specific types of leiomyomata, and BML is one of them. Steiner first reported leiomyoma metastases from the uterus to the lungs in 1939, and the term "metastatic fibroleiomyoma" was proposed [2]. And it was renamed “Benign metastasizing leiomyoma (BML)”in 1977 [3]. BML is a very rare disease, which mainly occurs in premenopausal women, and it is characterized by the growth and formation of nodules or masses of benign smooth muscle cells in multiple sites of the body, generally including lung, pelvic cavity, muscle, lymph nodes, blood vessels and heart, among which pulmonary metastasis is the most common one, and it is called pulmonary benign metastasizing leiomyoma (PBML) [4]. On imaging, PBML presents multiple bilateral pulmonary nodules of different sizes, which is easily misdiagnosed as miliary pulmonary tuberculosis and malignant lung metastasis [5]. BML with metastasis of different sites is extremely rare. This kind of tumor is benign in terms of histopathology, but the biological behavior of the tumor is not benign, instead, it presents an invasive biological behavior, which can cause the disease to linger. The etiology of the disease is still controversial. The diagnosis of BML is based on the pathological findings of extra-uterine lesions with histological features of leiomyoma [6]. The difference between uterine leiomyoma and leiomyosarcoma is made by degree of cytological atypia, morphology, presence of tumor cell necrosis and mitotic index [7]. There is a lot of debate about this disease, one of the most important questions is whether these lesions are benign or malignant. Long-term survivors of BML often have a good prognosis. However, these lesions have been found to have a low degree of mitotic activity and slow but steady progression of growth. Therefore, many scholars regard this lesion as a metastatic, low-grade malignant sarcoma. At the same time, BML can also be confused with lymphangiomyomatosis (LAM). In ultrastructure, both cells have smooth muscle cell characteristics, and LAM and BML are both mainly found in female. However, the clinical, chest X-ray and pathological features of these two diseases are quite different. Now we present a case of BML with systemic metastasis with complete medical history data, laboratory examination, imaging and pathological findings, as well as the treatment to summarize the clinical manifestations, imaging features, pathological features and treatment of BML in order to improve the understanding of this disease and reduce misdiagnosis and missed diagnosis.
Case Presentation
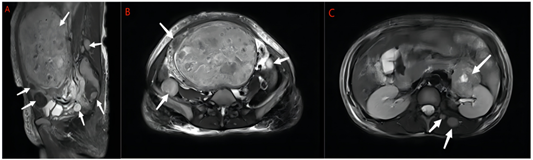
A 47‑year‑old patient with vaginal bleeding presented herself for a gynecological examination. 15 days ago, she suffered from vaginal bleeding without obvious inducement, the volume was more than the usual menstrual volume, bright red color, with blood clots, and she also suffered from lumbosacral distension, dizziness and fatigue, and no cough or hemoptysis. These symptoms caused severe anemia in her, and she had a blood transfusion at local hospital to ameliorate anemia. She underwent "radiofrequency ablation" for uterine leiomyoma in 2008, and found out a 13.9×8.9 cm uterine mass through transvaginal ultrasound in 2017. But she did not care about it 5 years ago. The patient had obstetrical history of one full‑term vaginal delivery. The rest of her medical history was nothing special. There were palpable masses in the neck and abdomen, and no significant abnormalities on other physical and gynecological examinations. Routine blood examination indicated that hemoglobin was 73g/L, and other laboratory examinations including tumor markers, coagulation function and so on were within the normal range. To rule out endometrial disease, we performed diagnostic curettage for her, and the results showed proliferative endometrium. Through pelvic MRI, multiple masses of different sizes were observed in the uterine cavity and the uterine muscle wall, with clear boundaries, the larger one was about 14.5×9.4×19.0cm, and similar multiple masses were also seen in the lower abdominal cavity and retroperitoneum, both sides of the lumbar back, both sides of the buttocks, and pelvic cavity, with clear boundaries, the larger one was about 3.2×3.1×3.1cm (Fig. 1).
Figure 1: A Sagittal T2-weighted MRI showing multiple masses of uterus and masses in pelvic cavity and muscle(arrows). B Axial T2-weighted MRI showing masses in pelvic cavity(arrows). C Axial T2-weighted MRI showing masses in retroperitoneum, lumbar back(arrows).
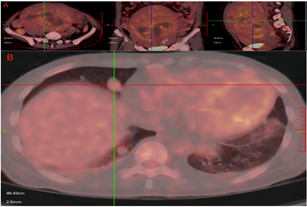
The radiologists considered them as specific types of uterine leiomyomas (BML?). PET/CT results: The uterus was significantly enlarged and a mixed density mass could be seen, with hemorrhage and liquefaction necrosis, and the maximum diameter of it was about 18.9cm, accompanied by uneven radioactive concentration, standardized uptake value (SUVmax): 6.2 (Fig. 2A).
Figure 2: A PET-CT showing masses in the uterus and pelvic cavity, with images presented in transverse, coronal, and sagittal planes from left to right, SUVmax: 6.2. B Bilateral lungs exhibit multiple scattered high-density solid nodules, with the largest located in the right middle lobe measuring approximately 1.4×2.1 cm, SUVmax: 2.2.
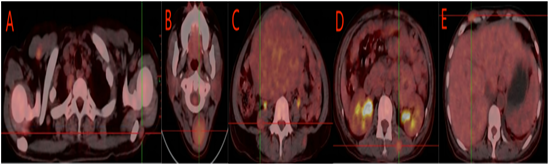
In both lungs, dense solid nodules were found, and the larger one was located in the middle lobe of the right lung, with a size of about 1.4×2.1cm, SUVmax: 2.2 (Fig. 3B).
Figure 3: Multiple nodules in both shoulders, with the larger one located on the left, SUVmax: 1.9. B Masses are observed in the posterior neck muscle space and subcutaneously, with the larger one measuring approximately 3.4×3.7 cm, SUVmax: 3.4. C A slightly low-density nodule in the right psoas major, SUVmax: 2.5. D Nodules in the muscle space of the back and buttocks, SUVmax: 4.1. E Nodules in the subcutaneous tissue and muscle space of the abdominal wall, SUVmax: 3.3.
Multiple isodense masses were observed in the right upper arm, posterior neck, bilateral shoulders, right chest wall, right psoas major, abdominal wall, back and hip muscle space and subcutaneous space, and the larger one was located in the posterior neck muscle space, with the size of about 3.4×3.7cm. SUVmax: 3.4 (Fig. 4).
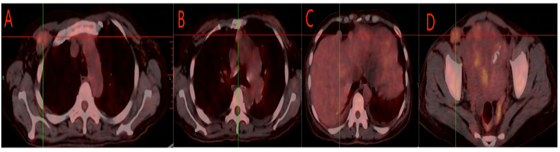
Figure 4: A A Nodule in the right infraclavicular fossa, SUVmax: 3.8. B Nodules in the 1st, 3rd, and 5th groups of the mediastinum, the larger one located in the 3rd group, SUVmax: 2.4. C Slightly high-density nodules in the prephrenic space and perihepatic region, with some showing unclear demarcation from the liver, SUVmax: 1.7. D A nodule in the left inguinal area, SUVmax: 3.8.
And multiple isodense nodules were also observed in the right subclavian fossa, mediastinum, prephrenic, perihepatic, abdominal cavity and left inguinal area, and the larger one was about 2.7×3.4cm, SUVmax: 2.4 (Fig. 5). There were no enlarged lymph nodes or ascites.
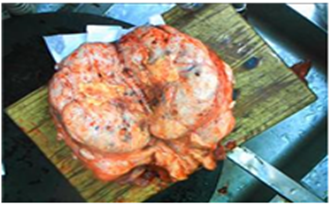
Figure 5: The largest mass on the uterus, with necrosis visible internally.
Treatment And Follow-Up
After obtaining informed consent from the patient, we perform a laparotomy for her at Guangxi Medical University Cancer Hospital on December 2022, under general anesthesia. We removed the whole uterus, bilateral adnexa, and masses of the left kidney, para-aortic, psoas major, abdominal wall, and chest wall. The largest one was on the uterus (21×18×10.5cm) (Fig. 5). Pathological results: The mass of the left pelvis was an epithelioid angiomyolipoma, and immunohistochemical results were HMB45 (+), MelanA (+), S100 (+), SMA (+), CD68 (+), CKpan (-), PAX-8 (-), Ki-67 (hot spot +, 10%). And all of masses of the other sites were leiomyoma, some were accompanied by venous thrombosis and infarct calcification. The tumor cell morphology is uniform, slight atypia, occasionally seen karyokinesis. Combined with clinical and pathological findings, BML was diagnosed. The patient exhibited a favorable recovery after surgery, and no notable abnormalities were detected during the one-year follow-up period.
Discussion
BML often occurs in women of reproductive age with a history of uterine leiomyoma surgery, but Few authors have reported that the estimated time from the primary surgery to BML diagnosis, which was conjectured to be approximately 10 to 15 years [8-10]. A review has reported that the mean age of primary surgery was 38.5 years in uterine leiomyoma patients, whereas the mean age for the diagnosis of BML was 47.3 years [11]. And the patient in our report was diagnosed with BML 14 years after "radiofrequency ablation of uterine leiomyoma", which is consistent with the above result. The patient's left pelvis mass was leiomyomata’s hamartomas. Fibroleiomyoma Tous hamartoma in lungs was thought to be lung metastases from a uterine leiomyoma (BML) [12]. But there has no study reported the relationship between leiomyomata’s hamartomas on pelvis and BML yet. Whether this kind of tumors come from metastases of uterine leiomyomas remains to be investigated. Because the patient refused to treat the lesions in the neck, lungs and muscles, there was no clear pathological diagnosis of these lesions, but according to the imaging findings, these lesions were more likely to be BML.
The pathogenesis of BML is not clear. BML is considered as slow growing “metastatic” tumors, which may metastasize via blood vessels from the trauma of myomectomy or hysterectomy [13-14]. And the viewpoint that BML is a very low-grade leiomyosarcoma which has the metastasis potential to other organs via lymphatic or hematological way was also accepted by studies [15]. And some researchers thought that the mechanisms of BML was similar with endometriosis [13]. In molecular pathology, A study described consistent chromosomal aberrations (19q and 22q terminal deletion) in five pulmonary BML cases, which could be found in approximately 3% of uterine leiomyoma, and could not be found in other types of tumors [16]. This suggested that BML might originated from a biologically distinctive subset of uterine myoma [16].
Diagnosis of BML mainly depends on history of uterine fibroids, clinical manifestations, imaging examination and pathology. The clinical presentation of BML depends on the site involved, but sometimes there is no symptom. Radiographically, BML is often multiple masses with clear boundaries and no enhancement on CT. In addition, BML has no significant metabolic activity on PET-CT scans [15]. Pathologically, BML is characterized by extra-uterine smooth muscle cell proliferation, but there is no evidence of nuclear pleomorphism or necrosis, and its histological appearance is similar to that of primary benign uterine leiomyomas. Nevertheless, BML is still easily confused with malignant tumors, and immunohistochemistry helps in the differential diagnosis. Primary or secondary malignancies, especially lung cancer and leiomyosarcoma, must be excluded before diagnosis of BML. Because the incidence of BML is extremely low, there is no standard treatment at present. Currently, the existing treatment regimens include surgery, traditional Chinese medicine therapy, chemoradiotherapy and anti-estrogen therapy, etc. When ER is positive, anti-estrogen therapy can lead to tumor degeneration and good prognosis of patients [17]. A study reported that most patients with BML had positive ER and PR, so surgery combined with hormone therapy may be a good option [18].
Conclusion
In conclusion, this case report aims to present an example of a benign lesion associated with a malignant biological behavior. Clinically, if one has a history of uterine fibroids and has multiple body masses found on imaging, we should be alert to whether it is BML. BML can be confirmed when pathological findings suggest leiomyoma. However, the pathogenesis and treatment of BML still need further study.
Declarations
Contributors
The article was researched and written by Dr Ruyu Shao and Dr Faquan Shen, with the supervision of Dr Desheng Yao, who assisted with edits and guidance.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
None declared.
Patient consent for publication
Consent obtained directly from patient(s).
References
- Lewis TD, Malik M, Britten J, San Pablo AM, Catherino WH. (2018). A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. Biomed Res Int, 2414609.
Publisher | Google Scholor - Steiner PE. (1939). Metastasizing fibroleiomyoma of the uterus: Report of a case and review of the literature. Am J Pathol. 15(1): 89-110.7.
Publisher | Google Scholor - Horstmann JP, Pietra GG, Harman JA, Cole NG, Grinspan S. (1977). Spontaneous regression of pulmonary leiomyomas during pregnancy. Cancer. 1977. 39(1):314-321.
Publisher | Google Scholor - Jiang H, Ma L, Qi XW, et al. (2021). Pulmonary benign metastasizing leiomyoma: a case report and literature review. Ann Palliat Med, 10(5):5831-5838.
Publisher | Google Scholor - Fan R, Feng F, Yang H, et al. (2020). Pulmonary benign metastasizing leiomyomas: a case series of 23 patients at a single facility. BMC Pulm Med, 20(1): 292.
Publisher | Google Scholor - André D, Gouveia F, Luís H, et al. (2021). Benign metastasizing leiomyoma - a case of benign metastasis. JRSM Open, 12(12):20542704211064482.
Publisher | Google Scholor - Aoki K, Yamamoto T, Terauchi R, Mori T, Shirai T, Kitawaki J. (2021). Benign metastasizing leiomyoma in femur and thigh with a history of uterine leiomyoma: A case report and literature review. J Obstet Gynaecol Res, 47(2):812-817.
Publisher | Google Scholor - Taftaf R, Starnes S, Wang J, et al. (2014). Benign metastasizing leiomyoma: a rare type of lung metastases-two cases reports and review of the literature. Case Rep Oncol Med, 842801.
Publisher | Google Scholor - Wei WT, Chen PC. (2015). Benign metastasizing leiomyoma of the lung: A case report and literature review. Oncol Lett, 10(1): 307-312.
Publisher | Google Scholor - Chen S, Zhang Y, Zhang J, et al. (2013). Pulmonary benign metastasizing leiomyoma from uterine leiomyoma. World J Surg Oncol, 11:163.
Publisher | Google Scholor - Barnaś E, Książek M, Raś R, Skręt A, Skręt-Magierło J, Dmoch-Gajzlerska E. (2017). Benign metastasizing leiomyoma: A review of current literature in respect to the time and type of previous gynecological surgery. PLoS One, 12(4): e0175875.
Publisher | Google Scholor - Cheng D, Zhang F, Hu K. (2019). An unusual case report of multiple pulmonary leiomyomatous hamartomata. Medicine (Baltimore), 98(30): e16496.
Publisher | Google Scholor - Awonuga AO, Shavell VI, Imudia AN, Rotas M, Diamond MP, Puscheck EE. (2010). Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv, 65(3):189-195.
Publisher | Google Scholor - Yuan X, Sun Y, Jin Y, et al. (2019). Multiple organ benign metastasizing leiomyoma: A case report and literature review. J Obstet Gynaecol Res, 45(10): 2132-2136.
Publisher | Google Scholor - Pacheco-Rodriguez G, Taveira-DaSilva AM, Moss J. (2016). Benign Metastasizing Leiomyoma. Clin Chest Med, 37(3): 589-95.
Publisher | Google Scholor - Nucci MR, Drapkin R, Dal Cin P, Fletcher CD, Fletcher JA. (2007). Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am J Surg Pathol. 31(5):737-43.
Publisher | Google Scholor - Lewis EI, Chason RJ, DeCherney AH, Armstrong A, Elkas J, Venkatesan AM. (2013). Novel hormone treatment of benign metastasizing leiomyoma: an analysis of five cases and literature review. Fertil Steril, 99(7):2017-2024.
Publisher | Google Scholor - Chan JW, Law WL, Cheung SO, et al. (2005). Benign metastasising leiomyoma: a rare but possible cause of bilateral pulmonary nodules in Chinese patients. Hong Kong Med J, 11(4):303-306.
Publisher | Google Scholor