Case Report
Bacteriophage-Based Interventions in The Therapy of Antimicrobial-Resistant Pathogenic Escherichia Coli
1Institute of Biotechnology, Addis Ababa University, Addis Ababa, Ethiopia.
2Department of Biotechnology, Woldia University, Amhara Region, North Wollo, Woldia, Ethiopia.
*Corresponding Author: Tamirat Salile Sada, Institute of Biotechnology, Addis Ababa University, Addis Ababa, Ethiopia.
Citation: Tamirat S. Sada, Tesfaye S. Tessema. (2024). Bacteriophage-Based Interventions in the Therapy of Antimicrobial-Resistant Pathogenic Escherichia Coli, Clinical Case Reports and Studies, BioRes Scientia Publishers. 5(2):1-24. DOI: 10.59657/2837-2565.brs.24.104
Copyright: © 2024 Tamirat Salile Sada, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 24, 2024 | Accepted: February 07, 2024 | Published: February 14, 2024
Abstract
Escherichia coli (E. coli) is a rod-shaped, facultatively anaerobic coliform bacteria that is typically found in the small intestine of warm-blooded species. E. coli species are not only an important member of the normal gut microbiota of humans and other animals but also contain many pathotypes that cause various diseases. These pathogenic E. coli diseases are usually treated with antibiotics. However, the antibiotic susceptibility of different E. coli strains varies greatly and they are resistant to many antibiotics. This multidrug resistance is a growing problem resulting from the overuse of antibiotics in humans and the use of antibiotics as growth promoters in animal feed. Bacteriophages or phages in short can be used as an alternative to antibiotics in the fight against infections caused by multidrug-resistant pathogens. Bacteriophages are the world's most widespread and ubiquitous organisms, which are viruses that kill bacteria. Both virulent and intermediate bacteriophages are present in the environment, but only virulent bacteriophages are used for a treatment known as phage therapy. Since their discovery, bacteriophages have been regarded as an indispensable weapon in the fight against bacterial diseases in humans and animals. Today, the advent of growing microbial resistance to antibiotics and attention to the use of bacteriophage in treatment has all but resurfaced. The purpose of this article is to highlight various studies of bacteriophage against pathogenic strains of E. coli from enteric and extraintestinal infections and effective phage therapies in humans and animals. In addition, the article discusses the benefits, challenges, and future prospects of phage therapy.
Keywords: antibiotics; bacteriophage; e. coli; resistance; therapy
Introduction
E. coli is a multispecies gram-negative bacterium that is generally regarded as innocuous and a natural component of the gut flora. However, certain strains have developed pathogenicity mechanisms, which means they can infect humans and animals with disease. These conditions can be extraintestinal (urinary tract infection (UTI), sepsis, pneumonia, and meningitis) or enteric (diarrhea) [1]. The six pathotypes of E. coli that cause intestinal diseases are enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), Shiga toxin-producing E. coli (STEC), and enterotoxigenic E. coli (ETEC). These classifications are based on the pathogenicity profiles (virulence factors, clinical disease, and phylogenetic profile). The adherent invasive coliform (AIEC), which is frequently linked to inflammatory bowel disease (IBD), and the Shiga-toxin enteroaggregative E. coli (STEAEC) are two additional pathotypes that have recently surfaced [2]. The classification of non-diarrheal E. coli is based on illness association; these groupings include uropathogenic E. coli (UPEC), E. coli linked to neonatal meningitis, and E. coli that causes sepsis [3].
The pathotypes of different E. coli strains are commonly replicate groups that share O and H antigens that define serogroups (O antigen only) or serotypes (O and H antigens) [4]. Pathogenic strains of E. coli employ a multistep model of pathogenesis similar to that of other mucosal pathogens, including the colonization of the mucosal site, evasion of host defenses, proliferation, and host damage. The majority of pathogenic E. coli is extracellular, but enteric strains are true intracellular pathogens that can both infect and replicate in macrophages and epithelial cells. Epithelial cells can internalize other strains of E. coli at low concentrations, but they don't seem to replicate intracellularly. Because these organisms are naturally found in high concentrations in human and animal feces, when the feces are disposed of and reach drainage systems, where already overused or misused antibiotics released from clinical aspects and agricultural run-offs predominate, coliforms are under pressure and resistant microbial strains emerge.
The emergence of drug resistance is a very serious problem in modern medicine, imposing significant social and economic burdens on society, including the loss of human life. The problem is further compounded by the fact that most antibiotics are broad-spectrum antibiotics. In practice, this means that antibiotic therapy not only targets pathogenic E. coli strains but can also cause significant collateral damage to the human microbiota by killing many other non-target and often beneficial bacteria [5]. Therefore, the phages that attack these bacteria may represent an alternative to antibiotics and have the potential therapeutic capacity to cure diseases. The use of lytic bacteriophage as a means of combating pathogenic E. coli contamination is an alternate technique.
Bacteriophages, also known as phages, are small viruses that infect and replicate within bacteria as well as in some cases archaea. They are ubiquitous in the environment and are currently being studied as potential therapeutic tools to eliminate bacterial pathogens. Lytic phages have potent bactericidal activity against host bacterial strains. Structurally, lytic bacteriophages are composed of DNA surrounded by proteins, making them non-toxic for human consumption [6]. During the lytic cycle, phage infects the cell using the cell's replication and translation machinery to replicate, then lyses the cell and releases new phage particles into the environment. In cases where overwhelming concentrations of phage are used, extrinsic lysis can also occur [7].
Phages are also very specific; they only attack their bacterial target hosts and cannot infect human cells or other eukaryotic cells. Even within bacterial taxa, in contrast to broad-spectrum antibiotics, phages typically lyse only strains or a subset of strains within a bacterial species, allowing for targeted bacterial therapy. In addition, the administration is easier because phages do not have to be administered repeatedly in quick succession over several days, as is the case with antibiotics since they can remain in the human body for a relatively long time. Compared to antibiotics, phages are said to have several other advantages. Phages are considered much safer and better tolerated because they can only replicate in the target bacterium but cannot infect mammalian cells. Using bacteriophages may be more cost-effective than using antibiotics that target multidrug-resistant pathogens and they easily eradicate the biofilms as well.
Phage cocktails or single phages are commonly used in therapeutic techniques to infect and kill certain microorganisms. Bacteriophages can infiltrate pathogenic bacteria without further harming the commensal microbiota because of their restricted host range specific to each species [8].
The rise of phage-resistant bacteria cannot be ignored, despite the undeniable potential of phage therapy [9]. Potential issues with phage-resistant "mutants" can be lessened by employing a strategy known as a "phage cocktail," which consists of several phage types with various host specificities [10]. Numerous mathematical models assess the mutation rates of phage-resistant bacteria and shed light on the maximum therapeutic efficacy of phage populations. Phage therapy, however, may be superior to antibiotic therapy since phages can mutate and subsequently efficiently destroy bacteria that have developed resistance [11].
E. coli phages are commonly isolated from marine environments including fresh and salt water, sewage, hospital waste, human and animal faces, various food sources (such as vegetables, fruits, dairy, and fish), soil, plants, and other environmental sources [12]. Phages are also common commensals in the human body and can be largely isolated from human skin, vagina, mouth, and the rest of the gastrointestinal tract, where their number is estimated to be around 1 × 10^15 [13]. Introduced into therapeutic practice in human medicine by D'Herelle at the end of World War I but neglected at the dawn of the antibiotic era, phage therapy is a tool to treat infections caused by multidrug-resistant strains of bacteria, including E. coli [14]. Moreover, phages are used in a variety of biotechnological applications, including vaccine carriers, antimicrobial enzymes, phage typing, and protein screening libraries [15, 16]. The use of bacteriophages as antimicrobial agents, on the other hand, necessitates a thorough understanding of phage biology to assess their potential as an alternative effective technique for the control of pathogenic bacteria [17].
Main Text
Pathogenic E. coli
E. coli is usually found within the lower gut of warm-blooded organisms (endotherms). According to Vogt & Dippold (2005), the majority of E. coli strains are not harmful, but some can lead to severe food poisoning, septic shock, meningitis, or urinary tract infections in humans [18]. The pathogenic strains of E. coli, in contrast to the normal flora, create toxins and other virulence factors that allow them to live in areas of the body where E. coli would not ordinarily reside and cause harm to host cells. Only pathogens have virulence genes that encode these harmful features [19].
Oral and fecal pathways are common routes for the spread of pathogenic E. coli. Unsanitary food preparation, agricultural contamination from manure fertilizer, crop irrigation using contaminated gray water or raw sewage, feral pigs on cropland, and direct ingestion of sewage-contaminated water are common modes of transmission [20]. The main sources of E. coli O157:H7 are dairy and beef cattle, which can harbor the infection asymptomatically and excrete it in their feces [21]. Cucumbers, raw ground beef, raw spinach or seed sprouts, raw milk, unpasteurized juice, unpasteurized cheese, and foods contaminated by infected food workers through the fecal–oral pathway are food products linked to E. coli outbreaks.
According to Eckburg et al. (2005), E. coli and similar bacteria make up approximately 0.1% of the gut flora [22]. The main route by which pathogenic strains of the bacterium cause illness is fecal-oral. Because they can only exist outside of the body for a brief period, cells are perfect indicator organisms to check environmental samples for the presence of feces [23]. The bacterium has been the subject of extensive research for more than 60 years and is also easily and affordably produced in a laboratory setting. Vibrant strains of E. coli can cause a variety of illnesses in both humans and domestic animals. Neonatal meningitis, urinary tract infections, and gastroenteritis are among these illnesses. Rarely, virulent strains can also cause extraintestinal diseases such as gram-negative pneumonia, peritonitis, mastitis, septicemia, and hemolytic-uremic syndrome [24].
Diarrheagenic gastroenteritis
Normally, E. coli stays within the intestinal lumen without causing any harm. However, in individuals who are immunocompromised or have weakened gastrointestinal barriers, even nonpathogenic strains of E. coli can lead to infections. Moreover, several highly adapted E. coli clones that have evolved to cause a wide range of diseases in humans and animals can infect even the healthiest people. Pathogenic E. coli infections can spread throughout the body or just affect mucosal surfaces. The three primary clinical syndromes resulting from infections produced by intrinsically harmful strains of E. coli include urinary tract infections, sepsis/meningitis, and enteric/diarrheal disorders.
In children and dairy calves, diarrheagenic E. coli (DEC) is a major cause of acute gastroenteritis. According to Liu et al. (2016), acute gastroenteritis ranks fourth in the world for children under the age of five years in terms of mortality and is a frequent cause of morbidity in both developing and developed nations during childhood [25]. E. coli pathotypes that are diarrheagenic (DEC) are distinguished from nonpathogenic and extraintestinal pathogenic (ExPEC) based on virulence factors found in their genomes and phenotypic traits. According to Kaper et al. (2004), the three types of ExPEC are neonatal meningitis-associated E. coli (NMEC), sepsis-inflicting E. coli (SEPEC), and uropathogenic E. coli (UPEC) [26].
The DEC group has been reexamined as seven distinct pathotypes by pathogenomics and phenotypic classification (Table 1). These pathotypes are defined by their essential virulence genes and differential features, which include enteropathogenic E. coli (EPEC), Shiga toxin-producing E. coli (Non-O157/STEC), enterohemorrhagic E. coli (EHEC/O157), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and E. coli that adheres diffusely (DAEC) [27].
i) Enteropathogenic E. coli (EPEC)
EPEC was the first pathotype of E. coli to be described. For many years, O:H serotyping was the only way to identify this pathotype and the mechanisms underlying EPEC-induced diarrhea remained a mystery. However, since 1979, numerous advances in understanding the pathogenesis of EPEC diarrhea have been made, such that EPEC is now among the best-understood pathogenic E. coli.
EPEC infections are linked to a distinct intestinal histopathology. Known as "attaching and effacing" (A/E) infections, these bacteria form close attachments to intestinal epithelial cells and induce dramatic cytoskeletal alterations, such as the accumulation of polymerized actin directly beneath the adherent bacteria. Microvilli effacement and close adherence between the bacteria and the epithelial cell membrane characterize this remarkable phenotype. A whole family of enteric pathogens that cause A/E lesions on epithelial cells has its origins in EPEC strains.
ii) E. coli that is enterohemorrhagic (EHEC/O157)
The E. coli strain EHEC is responsible for producing Shiga toxin. The intestinal wall lining is harmed by the toxin. EHEC was identified as the source of bloody diarrhea in 1982, which occurred when a person consumed raw or undercooked hamburger meat tainted with the bacteria. Since then, unpasteurized milk, unsalted apple juice or cider, salami, spinach, lettuce, sprouts, well water, and surface water areas that animals frequently visit have all been connected to EHEC outbreaks. According to Gomes et al. (2016), outbreaks have also been linked to animals at petting zoos and daycare facilities [28].
Hemorrhagic colitis, hemolytic-uremic syndrome (HUS), diarrhea, and hemorrhagic diarrhea are all caused by the enterohemorrhagic bacterial strain E. coli O157: H7, which is also a significant food source. Three to eight days following infection are when EHEC symptoms start to appear. They include diarrhea that can turn into bloody diarrhea (hemorrhagic colitis) and abdominal pain. Fever and vomiting are possible side effects. The primary means of transmission for EHEC pathogens is eating contaminated food. Raw or undercooked meat, raw (unpasteurized) dairy products, and occasionally raw vegetable products are the main food products affected, as the digestive tract of cattle serves as the primary natural reservoir of EHEC. Such animals may also become contaminated when they are milked or killed. Another possible source of contamination is ruminant feces in the ground, in manure, or in water (ponds and streams). Although uncommon, EHEC can also spread from person to person. Most often, it is seen in a community or family setting [29].
iii) E. coli that is enterotoxigenic (ETEC)
The bacterium enterotoxigenic E. coli (ETEC) is a pathogenic version or pathotype of E. coli that produces heat-labile (LT) and heat-stable (ST) enterotoxins that cause diarrhea. Nearly fifty years have passed since these bacteria were first linked to cholera-like watery diarrhea [30]. Despite this, the bacteria continue to pose a serious threat to global health, especially to young children living in low-resource areas of the world. Here, it is estimated that more than a billion cases of diarrheal illness occur in children under five each year, with hundreds of millions of episodes of diarrhea linked to ETEC alone [31]. Watery diarrhea is caused by ETEC and can vary in severity from a mild self-limiting illness to a severe purging illness. Symptoms of an ETEC infection can include headaches, cramping in the stomach, vomiting, and, in rare instances, a low-grade fever. According to some research, ETEC infection may have some side effects, including an increased risk of childhood stunting from malnourishment and immunological deficiencies, an increased risk of developing other infectious diseases, and even an impact on cognitive development [32]. Moreover, there is a connection between postinfectious irritable bowel syndrome and traveler's diarrhea. Food or water tainted with human or animal excrement is how it spreads. Hand washing with soap regularly and avoiding or properly preparing foods and beverages that may be contaminated with bacteria are two ways to prevent infection.
iv) Enter invasive E. coli (EIEC)
EIEC, which shares a close relationship with Shigella, is believed to induce watery diarrhea by invading the colon epithelial cells. They attach to and penetrate intestinal cells using adhesin proteins, making them extremely invasive. Although they do not produce any toxins, they mechanically destroy intestinal wall cells, causing severe damage. A few minor biochemical tests separate EIEC from Shigella, but these pathotypes share important factors that contribute to their virulence [33]. It is believed that EIEC infection is an example of inflammatory colitis, even though many patients appear to have small bowel syndrome with secretory symptoms. Abdominal cramps, malaise, tenesmus, and occasionally fever is among the symptoms. Dysentery or bloody diarrhea is an unusual consequence [34].
v) E. coli that is enteroaggregative
A pathotype of E. coli known as enteroaggregative E. coli (EAEC) causes both acute and chronic diarrhea in both developed and developing nations. Additionally, they might result in UTIs. According to Jensen et al. (2014), EAECs are identified by their "stacked-brick" pattern of adhesion to the HEp-2 human laryngeal epithelial cell line [35]. It is now accepted that EAEC is a newly discovered enteric pathogen. Specifically, EAEC is known to be a common cause of diarrhea in pediatric populations and the second most common cause of traveler's diarrhea, behind enterotoxigenic E. coli. Additionally, it has been linked to long-term infections in the latter group as well as in immunocompromised hosts, including those with HIV [36]. Intestinal infections are brought on by EAEC; these infections can cause fever, diarrhea, and stomach pain. The majority of severe cases may result in kidney failure, dehydration, or bloody diarrhea.
vi) Shiga toxin-generating E. coli (Non-O157 STEC)
The most well-known serotype of Shiga toxin-producing E. coli (STEC) is probably E. coli O157:H7 (EHEC), but non-O157 STEC refers to at least 150 other serotypes of STEC that can infect humans and animals. For a long time, E. coli O157:H7 was linked to the majority of STEC outbreaks that were known to occur. This was mostly due to the ease with which E. coli O157 could be found in stool cultures ordered by medical professionals and carried out in clinical laboratories. Although the virulence of non-O157 STEC is highly variable, some strains can undoubtedly be just as dangerous as O157, even having the capacity to cause hemolytic uremic syndrome (HUS) and even death. All non-O157 STEC pathogenic strains have the potential to result in bloody diarrhea and hospitalization. However, only strains carrying Stx2 (as opposed to only strains carrying Stx1) usually cause HUS. The sources and risk factors for non-O157 STEC outbreaks are often comparable to those of E. coli O157:H7. The main means of transmission are foodborne, although it can also spread through contact with animals, water, and other people.
Vii) E. coli that is diffusely adherent (DAEC)
One group of E. coli that has been linked to diarrhea is diffusely adherent E. coli (DEC). Their diffuse adherence pattern on HeLa or HEp-2 cultured epithelial cells is what distinguishes them [37]. This adherence phenotype is caused by adhesins from the Afa/Dr families, which are present in 75% of DAEC. Much attention has been given to DAEC strains possessing Afa/Dr adhesions, but only those that were positive for the daaC probe, which recognizes a conserved region from Afa/Dr adhesin operons, were found at a higher frequency in diarrheic patients than in asymptomatic controls [38].
Table 1: Major Pathotypes of E. coli that cause diarrhea [39].
| No | Strains | Diarrhea Pattern | Antecedent condition |
| 1 | Enteropathogenic E. coli (EPEC) | Watery | Can cause diarrhea outbreaks in newborn nurseries |
| 2 | Enterotoxigenic E. coli (ETEC) | Watery | Produces a toxin that acts on the intestinal lining, and is the most common cause of traveler’s diarrhea. |
| 3 | Enterohemorrhagic E. coli (O157/EHEC) | Bloody/non bloody | A type of EHEC on which Bloody diarrhea and hemolytic uremic syndrome (anemia and kidney failure) can be brought on by E. coli O157:H7. |
| 4 | Entero invasive E. coli (EIEC) | Bloody/non bloody | Invades (passes into) the intestinal wall to produce severe diarrhea. |
| 5 | Enteroaggregative E. coli (EAEC) | Watery | Can cause acute and chronic (long-lasting) diarrhea in children. |
| 6 | Shiga toxin producing (non-O157 STEC) | Bloody/non bloody | Causes of acute diarrhea, dysentery, and HUS. |
| 7 | Diffusely adherent E. coli (DAEC) | Watery diarrhea | Leads to diarrhea, stomach pain and cramps and low-grade fever |
Non-diarrheal pathogenic E. coli
Extraintestinal pathogenic E. coli encompasses several well-described pathogens, i.e., uropathogenic E. coli (UPEC), which causes sepsis and urinary tract infections, and neonatal meningitis E. coli (NMEC), which causes sepsis and brain infections. These subspecies are important pathogens and are implicated in the global spread of antibiotic resistance gene.
i) Uropathogenic E. coli (UPEC)
Approximately 90% of urinary tract infections (UTIs) in people with normal anatomy are caused by uropathogenic E. coli (UPEC) [24]. Fecal bacteria colonize the urethra and travel up the urinary tract to the bladder, kidneys (causing pyelonephritis), or, in the case of males, the prostate in ascending infections. Women are 14 times more likely than men to experience an ascending UTI due to their shorter urethras [40]. P fimbriae, or pyelonephritis-associated pili, are used by uropathogenic E. coli to bind urinary tract urothelial cells and colonize the bladder. This receptor is absent in approximately 1% of the human population, and its presence determines whether a person is susceptible to urinary tract infections caused by E. coli. Alpha- and beta-hemolysins are produced by uropathogenic E. coli that cause lysis of urinary tract cells [24].
The Dr family of adhesins, which is especially linked to cystitis and pregnancy-associated pyelonephritis, is another virulence factor frequently found in UPEC. The Dr blood group antigen (Dra), which is found on the decay accelerating factor (DAF) on erythrocytes and other cell types, is bound by Dr adhesins. According to Justice et al. (2006), the Dr adhesins cause the development of lengthy cellular extensions that encircle bacteria, activating multiple signal transduction cascades along the way, including PI-3 kinase [41]. By infiltrating superficial umbrella cells, UPEC can circumvent the body's innate immune defenses, such as the complement system, and create intracellular bacterial communities (IBCs). Additionally, they are capable of forming capsular polysaccharides, which aid in the formation of biofilms and the K antigen. Biofilm-generating E. coli is frequently the cause of persistent urinary tract infections because it is resistant to immune factors and antibiotic treatment. K antigen-synthesizing upper urinary tract infections caused by E. coli are frequent [42].
ii) Meningitis/sepsis-associated E. coli (MNEC)
Gram-negative neonatal meningitis is most frequently caused by this E. coli pathotype, which has a 15–40 percentage in many survivors1. While infections by gram-positive organisms seem to be declining, the incidence of infants with early onset sepsis caused by E. coli infections appears to be increasing. Meningitis-causing E. coli strains are primarily composed of K1 capsule strains, accounting for 80% of the strains, and represented by only a small number of O serogroups, similar to E. coli pathotypes with well-established genetic bases for virulence. An intriguing distinction between MNEC and E. coli strains that cause urinary tract or intestinal infections is that, while the latter strains are easily spread through urine or feces, infection of the central nervous system does not seem to provide a clear advantage for the selection and spread of highly pathogenic MNEC strains.
These strains, found in the mother's vagina, colonize the newborn's intestines and cause bacteremia, which eventually results in meningitis. Additionally, the lack of maternal IgM antibodies (which only transfer IgG across the placenta because FcRn only mediates the transfer of IgG) combined with the body's recognition of the K1 antigen as self-due to its similarity to cerebral glycopeptides causes severe meningitis in newborns. These strains, found in the mother's vagina, colonize the newborn's intestines and cause bacteremia, which eventually results in meningitis. Additionally, the lack of maternal IgM antibodies (which only transfer IgG across the placenta because FcRn only mediates the transfer of IgG) combined with the body's recognition of the K1 antigen as self-due to its similarity to cerebral glycopeptides causes severe meningitis in newborns [43].
E. coli infection diagnosis
Stool cultures are used to diagnose infectious pathogenic E. coli and identify antimicrobial resistance, with antibiotic sensitivity testing coming next. The bacteria may be cultured to confirm the diagnosis and identify specific toxins, such as those produced by E. coli O157:H7. To culture gastrointestinal pathogens, two days is the minimum and several weeks is the maximum. Although some human pathogens cannot be cultured, stool culture has varying rates of sensitivity (true positive) and specificity (true negative). Antimicrobial resistance testing takes an extra 12 to 24 hours to complete for culture-positive samples [44].
Currently, molecular diagnostic tests are much quicker at identifying E. coli and antimicrobial resistance in the strains that are identified than culture and sensitivity testing [45]. Microarray-based platforms with high sensitivity and specificity can identify particular pathogenic strains of E. coli and AMR genes unique to that strain in two hours or less; the size of the test panel, which includes all pathogens and antimicrobial resistance genes, is limited. Newer platforms for the identification and diagnostics of pathogenic E. coli based on PCR and sequencing are currently being developed to overcome the limitations of available molecular diagnostic technologies and culture [46].
Antibiotic therapy and resistance
Bacterial infections are typically handled with antibiotics. However, there are significant differences in the antibiotic sensitivity of various E. coli strains. Since E. coli are gram-negative bacteria, they are immune to many antibiotics that work well against gram-positive bacteria. Amoxicillin and other semisynthetic penicillin, numerous cephalosporins, carbapenems, aztreonam, trimethoprim-sulfamethoxazole, ciprofloxacin, nitrofurantoin, and aminoglycosides are among the antibiotics that can be used to treat an E. coli infection. The issue of antibiotic resistance is becoming worse. A portion of this can be attributed to human antibiotic overuse, but a portion is most likely caused by the use of antibiotics in animal feed as growth promoters [47]. "On the order of 10−5 per genome per generation, which is 1,000 times as high as previous estimates," according to a study, is the rate of adaptive mutations in E. coli. This finding may be important for the management and study of bacterial antibiotic resistance ([48].
Moreover, E. coli may use a process known as horizontal gene transfer to transfer antibiotic resistance genes to other bacterial species, including Staphylococcus aureus. E. coli frequently carries several drug-resistant plasmids, which can easily spread to other species when under stress. Plasmids from and to other bacteria can be accepted and transferred by E. coli due to species mixing in the intestines. Consequently, E. coli and other enterobacteria are significant sources of antibiotic resistance that can be transferred [49]. Since the prevalence of bacterial strains that produce extended-spectrum beta-lactamases has increased in recent decades, resistance to beta-lactam antibiotics has become a particular issue. These beta-lactamases make many, if not all, of the penicillin and cephalosporins ineffective as therapies. Extended-spectrum beta–lactamase producing E. coli (ESBL E. coli) are highly resistant to an array of antibiotics, and infections by these strains are difficult to treat. In many instances, only two oral antibiotics and a very limited group of intravenous antibiotics remain effective [50].
Phage therapy
Fundamentals of phage biology
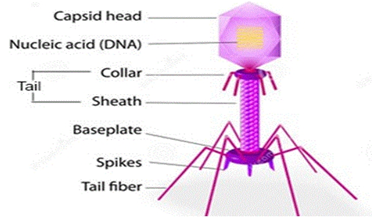
Phages are nonliving biological entities that are simple yet highly diverse. They are made of protein capsids containing either DNA or RNA (Figure 1). Phages are naturally occurring bacterial parasites that are dependent on their bacterial host for survival because they are unable to reproduce on their own and are therefore nonliving. Generally, phages attach themselves to particular receptors on the surface of the bacterial cell, inject their genetic material into the host cell, and either integrate this material into the bacterial genome (temperate phages reproduce vertically from mother to daughter cell) or use the bacterial replication machinery to produce the next generation of phage progeny (lytic phages), which lyse the destination cell. When the number of phage progeny reaches a critical mass, which varies based on the environment and can range from a few to over 1000 viral particles, the lytic proteins activate and hydrolyze the peptidoglycan cell wall, releasing new phage to restart the lytic cycle [51].
Figure 1: Typical bacteriophage structure [52].
The majority of phages exhibit infectious properties solely to bacteria harboring their corresponding receptor, thereby effectively defining the host range of lytic phages [53]. Phages differ in their host specificity; some are strain-specific, while others have shown the ability to infect a variety of bacterial strains and even genera [54]. Bacteria have developed a multitude of defense mechanisms against lytic phage infection, and phages possess an equally remarkable array of defense mechanisms against this resistance. The integration of phage DNA into the clustered regularly interspaced palindromic repeat/CRISPR-associated system and the alteration or loss of receptors in bacteria is examples of this [55]. For phages, this can include the recognition of new or altered receptors and anti-CRISPR genes. The two orders of lytic phages that are most frequently linked to human pathogens and the gut microbiota are microviridin, which are tailless single-stranded DNA viruses, and Cardioviral, also referred to as "tailed phages" because they have double-stranded DNA genomes [56].
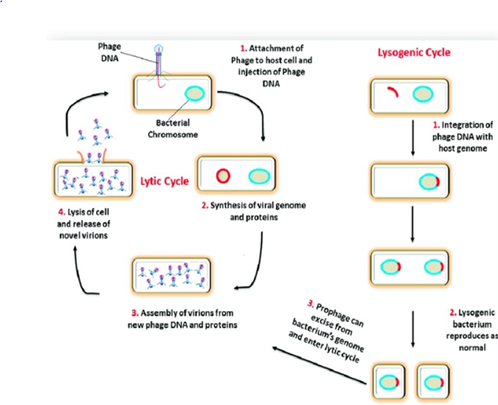
Lysogenic phages incorporate their genetic material into the bacterial chromosome as an endogenous prophage, as opposed to lytic phages (Figure 2). The bacterial lysogen then multiplies the prophage with each cell division. The lytic cycle and the release of phage progeny into the environment can be initiated by environmental stressors acting on the bacterial host, which can also induce the lysogenic phage from the latent prophage form. Prophage-encoded genes become accessible for transcription by the host upon integration of their genetic material into the bacterial genome [57]. Conventional phage therapy employs only lytic phages, which are inherently fatal to their bacterial host. Lithic phages are used in "phage cocktails," which are preparations made up of several phages that are effective in vitro against the pathogen of interest.
Figure 2: Bacteriophage lytic and lysogenic cycles [58].
Lytic bacteriophages undergo the lytic cycle, in which the host is lysed and offspring bacteriophages are released into the surroundings. Bacteriophages specifically attach to the bacterial host on a receptor present on the surface of the bacteria and inject their genetic material into the cell. The host cell supplies the necessary molecular building blocks and enzymes to replicate the bacteriophage's genetic material and produce offspring bacteriophages. During the release of new viruses, bacteriophage enzymes participate in disrupting the structures of host cell-cell lysis [59]. Bacteriophage-encoded proteins such as endolysin and holin lyse the host cell internally. Holins are small proteins that accumulate in the cytoplasmic membrane of the host and allow endolysin to degrade peptidoglycan, enabling offspring bacteriophages to escape. Subsequently, in the external environment, lytic bacteriophages can infect and destroy all nearby bacteria. The production of large numbers of offspring by lytic bacteriophages is an advantage when they are used in bacteriophage therapy.
Phage classifications
Bacteriophages are classified based on their morphological structure and genetic materials. The majority of phages are tailed phages with dsDNA and are members of the Caudovirales order. The DNA translocase molecular motors that pack the chromosomes of tailed phages into the procapsid are identical, but the DNA replication technique and the resulting genome end are different [60]. The type and unique features of the receptor on the surface of the host cell determine how the phages are absorbed. Phages are primarily divided into virulent and temperate phages based on their life cycles. The lytic life cycle is followed by virulent phages. However, under certain circumstances, temperate phages can occasionally switch from the lysogenic to the lytic cycle. The two main proteins employed by lytic phages to kill their host cells are holin and lysine. The International Committee on Taxonomy of Viruses (ICTV) categorizes phages based on their appearance and nucleic acids (Table 2). The Caudovirales order, which contains the Myoviridae family with a contractile tail, the Podoviridae family with a short tail, and the Siphoviridae family with a noncontractile long tail, makes up approximately 96% of the documented bacteriophages. The same order includes filamentous, cubic, and polymorphic phages, which are divided into 10 distinct families and account for approximately 3.6% of all known bacteriophages [61].
Table 2: Bacteriophage classification [62].
| Family | Morphology | Nucleic acid | Examples |
| Myoviridae | Contractile tail, non-enveloped | Linear dsDNA | T4 virus, P1, P2, FO1, Jilinvirus, Vequintavirus |
| Siphoviridae | Long non contractile tail, non-enveloped | Linear dsDNA | Lambda, T5, N15, Kagunavirus, Dhillonvirus |
| Podoviridae | Short non contractile tail, non-enveloped | Linear dsDNA | T7 virus, P22, T3, SP6 |
| Tectiviridae | Isometric, non-enveloped | Linear dsDNA | PRD1 |
| Corticoviridae | Isometric, non-enveloped | Circular dsDNA | PM2 |
| Lipothrixviridae | Rod-shaped, Enveloped | Linear dsDNA | Acidianus filamentous virus |
| Plasmaviridae | Pleomorphic, Enveloped | Circular dsDNA | Acholeplasma laidlawii virus L2 |
| Rudiviriade | Isometric, non-enveloped | Linear dsDNA | SIRV1 |
| Fuselloviridae | Lemon-shaped, non-enveloped | Circular dsDNA | SSV-1 |
| Inoviridae | Filamentous, non-enveloped | Circular ssDNA | M13 |
| Microviridae | Isometric, non-enveloped | Circular ssDNA | ΦX174 |
| Leviviridae | Isometric, non-enveloped | Linear ssDNA | |
| Cystoviridae | Spherical, Enveloped | Segmented dsDNA | Φ6 |
Therapeutic application of phages
i) History of phage therapy
Bacteriophages were separately discovered in 1915 by Frederick William Twort and in 1917 by Felix d'Hérelle. Twort reported on a possible "ultramicroscopic virus" that he recovered from vaccinia virus cultures using "white micrococcus" cultures. It appears that the lytic phages that were identified were bacteriophages that targeted Staphylococcus species that were present in a vaccinia virus culture.
On the other hand, Felix d'Hérelle isolated bacteriophage active against Shigella bacillus from the stools of patients recuperating from bacillary dysentery. According to Abedon et al. (2011), there were indications of bacteriophage presence even before their discovery, a time frame known as bacteriophage prehistory [63]. Felix d'Hérelle used bacteriophages in medicine for the first time in 1919 [64].
Worldwide, the use of bacteriophages to treat infectious disorders increased between the early 1920s and the late 1930s [65]. This phase of inflated expectations was succeeded by a period of waning excitement for phage therapy throughout much of the Western world, which was followed by antibiotics replacing its usage following World War II and a shift in emphasis toward the use of phages as model genetic systems. Phage therapy was difficult to administer since, at the time of its discovery, relatively little was understood about phages. In fact, until they were seen in the 1940s with the development of electron microscopy, their very existence was a matter of debate. Although phage research did not cease in the former USSR, with the establishment of the Eliava Institute in Tbilissi, Georgia, and other nations such as Poland (including its well-known Hirsfeld Institute in Wroclaw), phage therapy for animals was rediscovered in the English literature in the 1980s [66].
Phages have been used therapeutically for a very long time in Eastern Europe and the former Soviet Union [67]. It was proposed that bacteriophages could be used to prevent and/or treat bacterial infections before the discovery and widespread use of antibiotics [68]. After antibiotics were discovered, phage therapy was widely abandoned due to several logistical and technical challenges. The English literature rediscovered phage therapy in animals in the 1980s, although phage research was never abandoned in the former USSR. This was due to the establishment of the Eliava Institute in Tbilissi, Georgia, as well as other countries such as Poland, which included the well-known Hirsfeld Institute in Wroclaw.
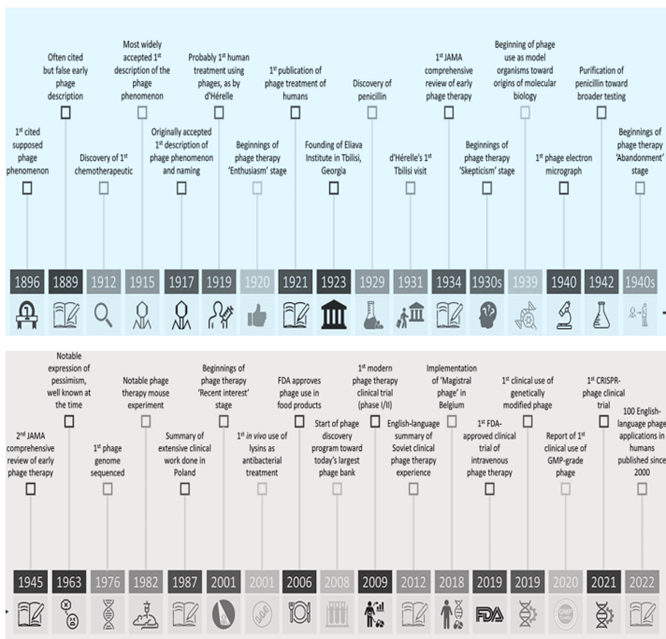
Eastern Europe and the former Soviet Union have long employed phages as medicinal agents [67]. It was proposed that bacteriophages had been used to prevent and/or treat bacterial infections before the discovery and widespread use of antibiotics [69]. Phage therapy was largely discontinued after antibiotics were discovered because of numerous logistical and technological difficulties. However, in the world before antibiotics, when the standard of care for treating bacterial illnesses was incredibly ineffective, phages, with their innate antibacterial qualities, might give much-needed hope. Poor use documentation and inconsistent results were major contributing factors to phage therapy [70]. However, there is still much data supporting their clinical application at present, and several historical innovations have been linked to significant phage therapy-influencing events (Figure 3).
Figure 3: Breakthroughs in the fields of phage therapy and science: JAMA, Journal of the American Medical Association; IV, intravenous; GMP, good manufacturing practice [71].
ii) Phage therapy principles
The key to antibacterial therapy success is building a library of different bacteriophages. According to Cui et al. (2017), bacteriophages exhibit stringent specificity and can range in host range from very narrow to broad [72]. A range of virulent bacteriophages that can kill the same strain as well as different lytic bacteriophages that can kill different species should be kept in the library. The establishment of stringent enrollment criteria for bacteriophages intended for clinical use is crucial.
For bacteriophage therapy to be used in clinical settings, standard operating procedures for bacteriophage preparations, storage, and transportation must be developed. Several crucial steps for their application were included in the documented clinical trials of bacteriophage therapy: bacteriophage isolation, characterization, susceptibility testing, endotoxin removal, and production of relevant products. Monitoring of bacteria resistant to bacteriophages and assessment of bacteriophage pharmacokinetics during therapy were also aspects of a documented successful case of bacteriophage therapy. Nevertheless, bacteriophage pharmacokinetics, endotoxin removal, and monitoring of bacteriophage-resistant bacteria were not part of a double-blind phase 1/2 trial. In the event of a bacterial infection in the gastrointestinal tract, it is important to prevent the phage from being neutralized by stomach acid when administering it orally. Additionally, other methods of treatment that have the potential to deactivate phages, such as antiseptic agents, should not be utilized in conjunction with phage preparations [73]. Before lytic phages can be used therapeutically in the West, more study is needed to gather reliable pharmacological data about them, including thorough toxicological studies. Therapeutic phages are believed to kill their target bacteria by multiplying inside and lysing the host cell through a lytic cycle as part of their bactericidal function. However, later research showed that lytic and lysogenic phages have significantly different replication cycles and that not all phages replicate in the same way (Figure 2).
Lytic phage lysis of host bacteria is a complex process involving a cascade of events involving several structural and regulatory genes, as demonstrated by the recent delineation of the full sequence of the T4 phage and years of elegant studies of the mechanism of T4 phage replication. Since the T4 phage is a typical lytic phage, it is conceivable that many therapeutic phages work like this; however, it is also conceivable that some therapeutic phages possess particular, as yet undiscovered genes or mechanisms, that enable them to successfully lyse their target bacteria [70].
Phages have been given to humans orally, in tablet or liquid formulations (105 to 1011 PFU/dose); rectally; locally (skin, eye, ear, nasal mucosa, etc.), in tampons, rinses, and creams; compared to the first four methods, there have been almost no reports of serious complications related to the use of aerosols or intrapleural injections, and intravenously [74].
Two different phage therapy methods were created at the time of the early 2000s phage therapy renaissance [74]. These are one-size-fits-all strategies and personalized phage therapy approaches. Broad-spectrum-defined phage cocktails, which were intended to target the majority of bacteria thought to be responsible for several infectious disorders, were used in what might be considered the one-size-fits-all method [76]. These predetermined broad-spectrum phage mixtures were created, manufactured, and evaluated using the pharmacoeconomic models that are now in use and were created to support "static" medications such as antibiotics [77]. However, true broad-spectrum phage cocktails that were effective against the majority of gram-positive and/or gram-negative bacteria frequently found in infectious disorders needed a significant number of phages and proved to be extremely challenging to create. It was possible to create phage cocktails with a narrower spectrum that were only effective against one or a small number of bacterial species, to be utilized in specific situations and with the knowledge of the bacterial species that would be infected beforehand. Phages with very extensive host ranges have been isolated and characterized for various bacterial species, including E. coli [78].
In the case of personalized phage medicine, one or more phages were chosen for phage therapy concepts from phage banks or the environment, and they may have been modified (in vitro selection of phage mutants exhibiting increased infectivity) to more effectively infect the bacteria isolated from the patient's infection site. Large therapeutic phage banks were set up and maintained by several phage therapy facilities. These banks were frequently updated with new phages, expanding and adapting the bank's host range to the constantly shifting bacterial populations. As only the infecting bacterium is targeted, there is less selection pressure toward the development of bacterial phage resistance, making personalized phage therapy approaches potentially more sustainable [43]. They shipped bacterial strains and corresponding phages all over the world, which made them more intricate and logistically challenging than one-size-fits-all methods.
Phage therapy practice against intestinal pathogenic E. coli
i) Animal studies
A mouse study by Chibani-Chennoufi et al. (2004) showed that broad host range T4-like coliphages for diarrhea-associated E. coli serotypes were isolated from stool samples from patients with diarrhea infants and ambient water samples [79]. All of these isolated phages showed very efficient passage through the digestive tract of adult mice when added to drinking water. Viable phages were recovered from the feces in a dose-dependent manner. Just 103 PFU of phage per milliliter of drinking water was the lowest oral dose required for sustained fecal recovery. In conventional mice, orally administered phage remained confined to the gut lumen and, as expected for non-invasive phage, no histopathological changes were observed in the gut mucosa of phage-exposed animals. E. coli strains recently introduced into the gut of conventional mice and monitored for ampicillin-resistant colonies were successfully lysed in vivo by phage added to the drinking water. Similarly, an in vitro phage-sensitive E. coli strain freshly inoculated into axenic mice was lysed in vivo by orally administered phage, while E. coli was resistant invitro E. coli was not lysed. In contrast, the normal intestinal E. coli flora of conventional mice was only minimally affected by oral phage administration, although most intestinal E. coli colonies were sensitive to the given phage cocktail in vitro. A resident of E. coli is physically or physiologically protected from phage infection.
Dissanayake et al. (2019) reported that phage treatment was effective in reducing viable E. coli O157:H7 in infected mice with similar efficacy to ampicillin therapy [80]. However, the bacteriophage preparation had a lesser effect on the gut microflora than ampicillin. Prophylactic or therapeutic uses of lytic bacteriophage preparations can be useful in the prevention or treatment of bacterial infections of the gastrointestinal tract, including those caused by the consumption of food contaminated with important foodborne bacterial pathogens such as L. monocytogenes, Salmonella spp., and enterohemorrhagic E. coli (eg., E. coli O157:H7) – with no adverse effects on the normal - and often beneficial - gut flora.
Bacteriophages may also be a valuable adjunct tool for combating drug-resistant bacterial infections and/or for fine-tuning the mammalian microbiome (targeting problem bacteria without damaging commensal microflora) to provide various health benefits [81].
Enteropathogenic E. coli (EPEC) is a drug-resistant E. coli that causes severe diarrhea. EPEC resistance to commonly used antibiotics has increased in recent years. The report by Vahedi et al. (2018) comparing the therapeutic effects of phage and ciprofloxacin in an EPEC-infected mouse model, found that phage therapy could not only reduce EPEC levels in vivo but also ensure normal growth of mice [82].
The study by Zhao et al. (2017) also showed that phage therapy has proven to be an attractive option to prevent and control multidrug resistance in rabbits [83]. Atypical EPIC causes intestinal changes characterized by attachment and atrophy, and intimin encoded by the attachment and withdrawal gene is thought to contribute to attachment and destruction of microvilli [84]. Orally infected rabbits with pathogenic EPIC can be cured with a single specific therapy with phage. The phage eliminated almost all host cells in vivo and had little effect on other bacteria. Compared to those who received antibiotic treatment; The phage-treated rabbits had a slightly higher residual load in the cecal contents at the end of the 3-day test. More research is needed over a longer period to see if phage therapy is as effective as antibiotic therapy.
ii) Human Studies
The reports from Dalmasso et al. (2016) demonstrate that three human intestinal phages showed promise as potential phage therapy [85]. According to them, the three-phage cocktail completely inhibited the growth of E. coli. The phage cocktail also reduced biofilm formation and prevented the emergence of phage-resistant mutants that appeared in a single phage. Phage combined with ciprofloxacin alone or in cocktails inhibited the growth of E. coli and interrupted the emergence of resistant mutants. These new phage isolates are promising agents for the biological control of E. coli infections. The human gut is a natural reservoir of many phages with promising antibacterial properties [86].
Bruttin and Brussow. (2005), used E. coli T4 phage to treat acute infectious bacterial diarrhea in adults and children. Fifteen healthy adult volunteers received a lower dose of E. coli T4 phage (103 PFU/ml), a higher dose of phage (105 PFU/ml), and a placebo via the drinking water. Fecal coliphage was detected in a dose-dependent manner in volunteers who were orally exposed to the phage. All volunteers receiving the highest dose of phage showed fecal phage 1 day after the challenge; this rate was only± 50% in subjects receiving the lowest dose of phage. One week after a 2-day oral phage application, no fecal phage was detectable. Oral administration of the phage did not cause a reduction in the total number of E. coli in the stool. In addition, in the commensal population of E. coli. No side effects associated with the use of phage have been reported. They found that while the E. coli T4 phage is safe, its therapeutic efficacy is still controversial [87].
One of the pathogenic Shiga toxin-producing E. coli is enterohemorrhagic E. coli (EHEC), which causes diarrhea in children. The most popular EHEC, E. coli O157:H7, commonly acquired from the community through contaminated food or water, is known to be a causative agent of diarrhea and urinary problems (hemolytic uremic syndrome) in humans. It is clear that treatment of phage O157:H7 is not without challenges, with only a modest 10-fold reduction in O157:H7 reported even when phage was used for a large number of infections [88].
In a separate study, Sarker et al., (2012) found that oral administration of phage to hospitalized children with acute diarrhea did not improve diarrhea scores, possibly due to phage's insufficient ability to fight a broad spectrum of diarrhea or genetic variability of E. coli [89]. In addition, it was not clear whether E. coli was responsible for diarrhea since the fecal samples were largely dominated by streptococci. Reduced efficiency of the phage titers after passing through the gastric acid was identified as another possible reason for the failure of the assay.
In addition, possible differences between the fecal and intestinal physiological status of E. coli and a low titer of the fecal pathogen could have prevented E. coli phage replication. These results confirm that much more knowledge of phage-bacteria interactions in vivo is required if we are to develop effective phage therapy assays. On a positive note, the coliphages administered during the study passed the gut safely, which helped demonstrate the safety aspects of phage therapy [90].
Phages against extraintestinal pathogenic E. coli
i) Urinary tract infections
Urinary tract infections (UTIs) are a major concern for medical experts and patients, as they are associated with almost 40% of nosocomial infections in ICUs [91]. Specifically, these illnesses are thought to be among the most prevalent bacterial infections, impacting an estimated 150 million individuals annually globally. They fall into two categories clinically: simple and complex. The simple form describes conditions when patients were healthy before to infection, were not pregnant, did not require catheterization, and did not have any structural abnormalities. Conversely, patients with immunocompromised status or those with risk factors such as pregnancy and urinary retention are thought to have complicated UTIs [92, 93]. Infection can occasionally be made more difficult by nosocomial polyresistant strains of E. coli (UPEC), which are common infections. One of the main characteristics of uropathogenic bacteria, which makes them more resistant to the host immune system and to chemical antibiotics, is their ability to form a monospecific or mixed biofilm. This structure is deposited on both abiotic and biological surfaces [94].
The usage of antibiotics has been severely limited in recent years due to the increased frequency of MDR bacteria. Furthermore, patients receiving long-term antibiotic therapy frequently experience toxicity, antibiotic resistance, and disruption of the normal microbiota. As a result, interest in alternative therapies like phage therapy is rising. Phages cannot infect eukaryotic cells in this situation because they are self-replicating, and their resistance mechanisms are distinct from those of antibiotics resistance [95]. The effectiveness of topical and oral administration of a multispecies bacteriophage cocktail in treating patients with acute and chronic urogenital inflammation was reported by Perepanova et al. (1994) [96]. However, there is insufficient documentation of this trial to be used as a model for human phage therapy. Three phages were tested for their capacity to remove UPEC biofilms, and Sybesma et al. (2016) documented the lytic activity of store-bought bacteriophage cocktails against strains of E. coli and Klebsiella pneumoniae that were isolated from urinary tract infection patients [97]. Additionally, they showed how "bacteriophage adaptation" studies may be used to increase the lytic activity of bacteriophage cocktails.
Additionally, Galtier et al. (2016) discovered three virulent phages from wastewater that targeted the strain of UPEC that was resistant to antibiotics. In vitro and in vivo characterizations of phage efficacy have been conducted, and it has been observed that intestinal transmission of UPEC can be considerably inhibited by a single phage cocktail dosage. Numerous trials, like this one, give patients hope that bacteriophage therapy may be used to treat acquired E. coli urinary tract infections [98].
According to Dufour et al. (2016), the bacteriophage LM33-P1 is effective exclusively against strains of E. coli O25b that are extremely resistant to fluoroquinolones and β-lactam antibiotics. Based on multiple animal models (pneumonia, sepsis, and urinary tract), the bacteriophage LM33-P1 is extremely effective both in vitro (short eclipse and latency periods of 7 and 9 min, respectively, burst magnitude 320 pfu, rapid adsorption) [99]. Besides, Dufour et al. (2016) demonstrated that lipopolysaccharide dependence mediated the LM33-P1 phage's unique and mutually exclusive association with O25b strains. According to Slobodníková et al. (2021), phage therapy is a potentially effective treatment for various infections. The recently obtained phages have shown excellent effectiveness against several clinical strains of E. coli that are not associated with urinary tract infections in the surrounding area [100]. Phage has demonstrated therapeutic promise for treating urinary tract infections brought on by E. coli strains with varying clonality profiles and antibiotic resistance, according to in vitro research (Slobodníková et al., 2021). Following the legalization of phage therapy under health legislation in Slovakia, this new phage cocktail has the potential to fill a gap in the therapeutic arsenal for treating urinary tract infections in patients infected with polyresistant strains of E. coli allergic to antibiotics. are taking medicines or have repeated or persistent urinary tract infections. Subsequently, the therapeutic potential of the freshly isolated phages could also be confirmed in vivo as part of experimental therapy.
Sanchez et al. (2022) also reported the development of phage cocktails that lyse modern strains of E. coli isolated from the urine of spinal cord injury (SCI) patients and exhibit potent biofilm-forming properties [101]. Biofilm formation is an important virulence determinant that facilitates E. coli pathogenesis in the urinary tract and is associated with increased bladder function and higher rates of antimicrobial resistance and recurrence after primary UTI [102]. They developed and characterized a new strategy to combat E. coli biofilms. They found that biofilm formation by different strains of E. coli varies in complex media and urine, some phage is lytic and retains the ability to kill biofilm in urine, the phage cocktails in our library target most E. coli isolated from our patient population, broad host phage cocktails are highly effective in both CAUTI models and phage cocktails act synergistically with antibiotics. They also showed that phage cocktails retained their anti-biofilm activity against older biofilms grown in human urine. It has been shown that individual phage activity decreases in older biofilms.
ii) Neonatal Meningitis and Sepsis
The disease brought on by pathogenic E. coli and similar bacteria usually causes sepsis (blood poisoning) if it occurs at birth or within the first two days after birth. If it occurs in children older than 48 hours, it is more likely to cause meningitis. Neonatal meningitis (NM) and sepsis caused by E. coli remain major public health problems in developing and middle-income countries. Treatment with antibiotics has always been a routine treatment for this infection. However, due to the emergence of drug-resistant bacteria, the effectiveness of antibiotics has diminished. Currently, E. coli exhibit varying degrees of resistance to third-generation cephalosporins [103]. An alternative approach, phage therapy, has the potential to circumvent antibiotic resistance by lysing pathogenic bacteria. According to Barrow et al. (1998), the bacterial strain E. coli K1+ was utilized to treat hens for sepsis and meningitis-like diseases using a lytic bacteriophage previously obtained from wastewater that binds to the envelope antigen K1 [104]. Protection was also achieved when phage administration was delayed until disease symptoms appeared. The phage could multiply in the blood. In colostrum-poor newborn calves fed, intramuscular phage inoculation delayed the appearance of the bacterium in the blood and increased lifespan. With some caveats, this approach offers considerable potential for treating bacterial diseases.
The report by Pouillot et al. (2012) indicated that bacteriophages are a potential therapeutic for sepsis and meningitis caused by multidrug-resistant and widespread E. coli [105]. They evaluated phage therapy in experimental infections caused by S242, a lethal strain of neonatal meningitis E. coli belonging to the globally distributed clone O25b: H4-ST131 that produces the extended-spectrum beta-lactamase CTX-M-15. The lytic phage active against S242 was isolated from ambient water. After determining the in vitro and ex vivo stability and pharmacokinetic properties of the phage in young rats, they evaluated the therapeutic efficacy of a single dose of 108 PFU in sepsis and meningitis models with 100% mortality. The phage (EC200PP) was partially neutralized by human serum. In contrast to high phage concentrations in the spleen and kidney, low titers were observed in urine and the central nervous system. In a model of sepsis, EC200PP administered 7 hours or 24 hours after infection resulted in 100% and 50% pup survival, respectively. In a meningitis model, EC200PP was administered 1 hour or 7 hours after infection and saved 100% of the animals. The more delayed treatments were related to the selection of phage-resistant S242 mutants (Table 3). However, a representative mutant was very sensitive to the destructive activity of serum and was virulent in an animal model.
Bacteriophage therapy was also mentioned in a report by Eid et al. (2022) as an alternate biocontrol method against the spread of multidrug-resistant E. coli in broiler chickens [106]. They investigated the ability of bacteriophages to inhibit and lyse biofilms previously formed by E. coli. In addition, experimental studies were conducted using the bacteriophage E. coli O78 for the prevention and control of colibacillosis in day-old chicks. The experimental infection result showed that the phage performance indices showed a significant increase in body weight, weight gain, and better FCR than antibiotic-treated infected bacteriophage and antibiotic-treated infected bacteriophage in both treated and challenged groups. In the end, their findings demonstrated that bacteriophages are a helpful substitute for antibiotics, especially when it comes to the management and prevention of illnesses that are resistant to several drugs. The combination of antibiotic and bacteriophage therapy has also been shown to be effective in reducing and rationalizing antibiotics used to treat bacterial diseases.
iii) E. coli Pneumonia
Pathogenic extraintestinal coliforms (ExPEC) have been associated with respiratory pneumonia (VAP), a common and life-threatening nosocomial infection [107]. The most dominant phylogenetic group is B2 with strains possessing large numbers of virulence factor genes such as sfa and iron, which are essential for iron adhesion and uptake [108]. The use of bacteriophages in the treatment of VAP is an interesting alternative to conventional antibiotic therapies [109].
In 2015, Doufour's team published the first study on the effective treatment of two bacteriophages (536 P1 and 536 P7) recovered from wastewater that was used to treat E. coli pneumonia. They showed that a combination of antibiotic therapy and phage therapy resulted in 100% survival in VAP-infected mice. Phage.536_P7 treatment alone could not save the majority of mice, but a variant created by adapting this phage to E. coli significantly increased the effectiveness of in vivo therapy. Phage.536_P7 treatment alone could not save the majority of mice, but a variant created by adapting this phage to E. coli significantly increased the effectiveness of in vivo therapy. The reports from [110], confirmed the efficacy of bacteriophage treatment of pneumonia caused by multidrug-resistant K. pneumoniae. The lytic phage, KLPN1, has been shown to infect and lyse coat-like K2 strains, and genomic sequence analysis revealed that the phage expresses proteins (eg. G. lysine, holin) that have potential use in phage therapy [109].
Table 3: Summary on reports of phage therapy in pathogenic E. coli.
| Diseases | Phage/s applied | Effectiveness | |
| Urinary tract infection | Single phage/T4 | Bacterial inoculum rendered untreated mice 100 |
Conclusion and Future Perspectives
The recently rediscovered field of phage therapy promises to have a wide range of positive effects on science and agriculture, veterinary medicine, and medicine, including a potential solution to counteract the increasing prevalence of antibiotic-resistant pathogens. The potential of combining antibiotic therapy with phage therapy, using phage cocktails, or using phage protein products could be the best area for the effective treatment of phage infections. Given the usefulness and variety of applications of phage therapy, this area of research is urgently needed. This review article summarizes various considerations related to phage therapy against pathogenic E. coli bacteria and its potential benefits. In the age of the global antibiotic crisis, phage therapy has become a potential alternative that has already confirmed clinical cases of success.
In a future perspective, phage therapy is expected to be applied in clinical cases of patients who experienced the failure of antibiotic treatments. Furthermore, unlike antibiotic therapy, it is anticipated that phage preparations for therapeutic applications will be created in a personalized manner by creating phage cocktails that may postpone the development of bacterial resistance to phages. There may be strong selective pressure put on the emergence of resistant bacteria if phages are widely used in the future as therapeutic and environmental control agents. Still, it seems improbable that no phage will be available in nature to infect a bacterium that has become resistant to a previous phage. It is hoped that these entities (phages), which are abundantly present in the biosphere, could provide answers to many of the questions that people are currently grappling with due to the quick advancements in the fields of molecular biology and biotechnology.
Declarations
Ethics Approval and Consent to Participate:
Not applicable for that section.
Consent for Publication:
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, concerning intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Availability of Data and Materials:
Not applicable for that section.
Competing Interests:
We hereby declare that the disclosed information is correct and that no other situation of real, potential, or apparent conflict of interest is known to us. We undertake to inform you of any change in these circumstances, including if an issue arises during the course of the meeting or work itself.
Funding:
There has been no significant financial support for this work that could have influenced its outcome.
Authors' Contributions:
Bacteriophage-based interventions in the therapy of antimicrobial-resistant pathogenic Escherichia coli. All works in this paper are contributed by us and it towards our work. The authors confirm sole responsibility for the following: Idea development, Literature review, Source citation, Review analysis, and Manuscript preparation.
Acknowledgments:
Not applicable for that section.
References
- Millar, M. R., Seale, J., Turton, J., Wilks, M., Costeloe, K., et al. (2016). ESBL-producing Enterobacteriaceae in 24 neonatal units and associated networks in the south of England: no clustering of ESBL-producing E. coli in units or networks. Journal of Antimicrobial Chemotherapy, 71(5):1174-1177.
Publisher | Google Scholor - Conte, M. P., Longhi, C., Marazzato, M., Conte, A. L., Aleandri, M., et al. (2014). Adherent-invasive E. coli (AIEC) in pediatric Crohn’s disease patients: phenotypic and genetic pathogenic features. BMC Research Notes, 7:1-12.
Publisher | Google Scholor - Clements, A., Young, J. C., Constantinou, N., & Frankel, G. (2012). Infection strategies of enteric pathogenic E. coli. Gut Microbes, 3(2):71-87.
Publisher | Google Scholor - Nataro, J. P. & Kaper, J. B (1998). Diarrheagenic E. coli. Clin. Microbiol. Rev. 11:142-201.
Publisher | Google Scholor - Pérez-Cobas AE, Artacho A, Knecht H, Ferrús ML, Friedrichs A, et al. (2013). Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS One. Public Library of Science; 8:e80201.
Publisher | Google Scholor - Loc-Carrillo, C., & Abedon, S. T. (2011). Pros and cons of phage therapy. Bacteriophage, 1(2):111-114.
Publisher | Google Scholor - Woolston J, Parks AR, Abuladze T, Anderson B, Li M, et al. (2013). Bacteriophages lytic for Salmonella rapidly reduce Salmonella contamination on glass and stainless-steel surfaces. Bacteriophage. 3:e25697.
Publisher | Google Scholor - Khan Mirzaei M, Nilsson AS. (2015). Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One, 10:e0118557.
Publisher | Google Scholor - Jaiswal, A. (2022). Phage Therapy: Genomics to Applications and Future Prospects. In Alternatives to Antibiotics: Recent Trends and Future Prospects. Singapore: Springer Nature Singapore. Pp. 109-145.
Publisher | Google Scholor - Melo, L. D., Veiga, P., Cerca, N., Kropinski, A. M., Almeida, C., et al. (2016). Development of a phage cocktail to control Proteus mirabilis catheter-associated urinary tract infections. Frontiers In Microbiology, 7:1024.
Publisher | Google Scholor - Dufour, N., Debarbieux, L., Fromentin, M., & Ricard, J. D. (2015). Treatment of highly virulent extraintestinal pathogenic E. coli pneumonia with bacteriophages. Critical Care Medicine, 43(6):e190-e198.
Publisher | Google Scholor - Kutateladze, M., & Adamia, R. (2010). Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends in Biotechnology, 28(12):591-595.
Publisher | Google Scholor - Abdelrahman, F., Easwaran, M., Das, R., Ahn, J., Patpatia, S., et al. (2022). Bacteriophages in Human Health.
Publisher | Google Scholor - Abedon S.T., Kuhl S.J., Blasdel B.G., Kutter E.M (2011). Phage Treatment of Human Infections. Bacteriophage. 1:66-85.
Publisher | Google Scholor - Haq, I. U., Chaudhry, W. N., Akhtar, M. N., Andleeb, S., and Qadri, I. (2012). Bacteriophages and their implications on future biotechnology: a review. Virol. J. 9:9.
Publisher | Google Scholor - Monk, A., Rees, C., Barrow, P., Hagens, S., and Harper, D. (2010). Bacteriophage applications: where are we now? Lett. Appl. Microbiol. 51:363-369.
Publisher | Google Scholor - Sillankorva, S., Pleteneva, E., Shaburova, O., Santos, S., Carvalho, C., et al. (2010). Salmonella Enteritidis bacteriophage candidates for phage therapy of poultry. J. Appl. Microbiol. 108:1175-1186.
Publisher | Google Scholor - Vogt RL, Dippold L (2005). E. coli O157:H7 outbreak associated with consumption of ground beef, June–July 2002. Public Health Reports, 120(2):174-178.
Publisher | Google Scholor - Mobley, Harry L. T.; Nataro, James P.; Kaper, James B. (2004). Pathogenic E. coli. Nature Reviews Microbiology, 2(2):123-140.
Publisher | Google Scholor - Jurczak-Kurek, A., Gąsior, T., Nejman-Faleńczyk, B., Bloch, S., Dydecka, A., et al. (2016). Biodiversity of bacteriophages: morphological and biological properties of a large group of phages isolated from urban sewage. Scientific Reports, 6(1):1-17.
Publisher | Google Scholor - Ferens, W. A., & Hovde, C. J. (2011). E. coli O157: H7: animal reservoir and sources of human infection. Foodborne Pathogens and Disease, 8(4):465-487.
Publisher | Google Scholor - Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., et al. (2005). Diversity of the human intestinal microbial flora. Science, 308(5728):1635-1638.
Publisher | Google Scholor - Thompson, Andrea. (2007). E. coli Thrives in Beach Sands. Live Science. Retrieved, 12:03.
Publisher | Google Scholor - Todar, K. (2007). Pathogenic E. coli. Online Textbook of Bacteriology. University of Wisconsin- Madison Department of Bacteriology, United States of America.
Publisher | Google Scholor - Liu, L., Oza, S., Hogan, D., Chu, Y., Perin, J., et al. (2016). Global, regional, and national causes of under 5 mortalities in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet, 388(10063):3027-3035.
Publisher | Google Scholor - Kaper, J. B., Nataro, J. P., & Mobley, H. L. (2004). Pathogenic E. coli. Nature Reviews Microbiology, 2(2):123-140.
Publisher | Google Scholor - Croxen, M. A., Law, R. J., Scholz, R., Keeney, K. M., Wlodarska, M., et al. (2013). Recent advances in understanding enteric pathogenic E. coli. Clinical Microbiology Reviews, 26(4):822-880.
Publisher | Google Scholor - Gomes, T. A., Elias, W. P., Scaletsky, I. C., Guth, B. E., Rodrigues, J. F., et al. (2016). Diarrheagenic E. coli. Brazilian Journal of Microbiology, 47:3-30.
Publisher | Google Scholor - Miko, A., Pries, K., Haby, S., Steege, K., Albrecht, N., et al. (2009). Assessment of Shiga toxin-producing E. coli isolates from wildlife meat as potential pathogens for humans. Applied and Environmental Microbiology, 75(20):6462-6470.
Publisher | Google Scholor - Sack RB. (2011). The discovery of cholera -like enterotoxins produced by E. coli causing secretory diarrhea in humans. The Indian Journal of Medical Research; 133(2):171-180.
Publisher | Google Scholor - Khalil, I. A., Troeger, C., Blacker, B. F., Rao, P. C., Brown, A., et al. (2018). Morbidity and mortality due to shigella and enterotoxigenic E. coli diarrhea: the Global Burden of Disease Study 1990–2016. The Lancet Infectious Diseases, 18(11):1229-1240.
Publisher | Google Scholor - Troeger, C., Colombara, D. V., Rao, P. C., Khalil, I. A., Brown, A., et al. H. (2018). Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrheal diseases in children younger than 5 years. The Lancet Global Health, 6(3):e255-e269.
Publisher | Google Scholor - Esmaeili Fard Barzegar, K., Najar-peerayeh, S., Mazaheri Nezhad Fard, R., & Bakhshi, B. (2021). Isolation of a bacteriophage with lytic activity on enteropathogenic Escherichia coli from a waste water source in Tehran. Pathobiology Research, 24(3):0-0.
Publisher | Google Scholor - Schuetz, A. N. (2019). Emerging agents of gastroenteritis: Aeromonas, Plesiomonas, and the diarrheagenic pathotypes of E. coli. In Seminars in Diagnostic Pathology, 36(3):187-192.
Publisher | Google Scholor - Jensen, Betina Hebbelstrup; Olsen, Katharina E. P.; Struve, Carsten; Krogfelt, Karen Angeliki; Petersen, Andreas Munk, (2014). Epidemiology and Clinical Manifestations of Enteroaggregative E. coli. Clinical Microbiology Reviews, 27(3):614-630.
Publisher | Google Scholor - Huang, D. B., Mohanty, A., DuPont, H. L., Okhuysen, P. C., & Chiang, T. (2006). A review of an emerging enteric pathogen: enteroaggregative E. coli. Journal of Medical Microbiology, 55(10):1303-1311.
Publisher | Google Scholor - Servin AL (2005): Pathogenesis of Afa/Dr diffusely adhering E. coli. Clinical Microbiology Review, 18:264-292.
Publisher | Google Scholor - Mansan-Almeida, R., Pereira, A. L., & Giugliano, L. G. (2013). Diffusely adherent E. coli strains isolated from children and adults constitute two different populations. BMC Microbiology, 13:1-14.
Publisher | Google Scholor - Cabrera-Sosa, L., & Ochoa, T. J. (2020). E. coli diarrhea. In Hunter's Tropical Medicine and Emerging Infectious Diseases, 481-485.
Publisher | Google Scholor - Nicolle, L. E. (2008). Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urologic Clinics of North America, 35(1):1-12.
Publisher | Google Scholor - Justice, S. S., Hunstad, D. A., Seed, P. C., & Hultgren, S. J. (2006). Filamentation by E. coli subverts innate defenses during urinary tract infection. Proceedings of the National Academy of Sciences, 103(52):19884-19889.
Publisher | Google Scholor - Ehrlich G, Hu F, Shen K, Stoodley P, Post J, (2005). Bacterial plurality as a general mechanism driving persistence in chronic infections. Clinical Orthopedics and Related Research, (437):20-24.
Publisher | Google Scholor - Croxen, M; Finlay, B B. (2010). Molecular mechanisms of E. coli pathogenicity. Nature Reviews. Microbiology, 8(1):26-38.
Publisher | Google Scholor - Gould, D. (2010). Causes, prevention and treatment of E. coli infections. Nursing Standard, 24(31):50.
Publisher | Google Scholor - Zhang, H., Yang, Z., Zhou, Y., Bao, H., Wang, R., et al. (2018). Application of a phage in decontaminating Vibrio parahaemolyticus in oysters. International Journal of Food Microbiology, 275:24-31.
Publisher | Google Scholor - Maurer, J. J. (2011). Rapid detection and limitations of molecular techniques. Annual Review of Food Science and Technology, 2:259-279.
Publisher | Google Scholor - Johnson, J. R., Kuskowski, M. A., Menard, M., Gajewski, A., Xercavins, M., et al. (2006). Similarity between human and chicken E. coli isolates in relation to ciprofloxacin resistance status. The Journal of Infectious Diseases, 194(1):71-78.
Publisher | Google Scholor - Perfeito, Lília; Fernandes, Lisete; Mota, Catarina; Gordo, Isabel. (2007). Adaptive Mutations in Bacteria: High Rate and Small Effects. Science, 317(5839):813-815.
Publisher | Google Scholor - Salyers, A. A., Gupta, A., & Wang, Y. (2004). Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends in microbiology, 12(9), 412-416.
Publisher | Google Scholor - Paterson DL, Bonomo RA (2005). Extended-spectrum beta-lactamases: a clinical update. Clinical Microbiology Reviews, 18(4):657-686.
Publisher | Google Scholor - Weinbauer, M. G. (2004). Ecology of prokaryotic viruses. FEMS Microbiology Reviews, 28(2), 127-181.
Publisher | Google Scholor - Mansour, N. M. (2017). Bacteriophages are natural gift, could we pay further attention. Bacteriology Reviews, 40:793-802.
Publisher | Google Scholor - Rakhuba DV, Kolomiets EI, Dey ES, Novik GI. (2010). Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Polish Journal of Microbiology, 59:145-155.
Publisher | Google Scholor - Motlagh AM, Bhattacharjee AS, Goel R. (2016). Biofilm control with natural and genetically modified phages. World Journal of Microbiology and Biotechnology; 32:67.
Publisher | Google Scholor - Labrie, S. J., Samson, J. E., & Moineau, S. (2010). Bacteriophage resistance mechanisms. Nature Reviews Microbiology, 8(5):317-327.
Publisher | Google Scholor - Minot, S., Sinha, R., Chen, J., Li, H., Keilbaugh, S. A., et al. (2011). The human gut virome: inter individual variation and dynamic response to diet. Genome Research, 21(10):1616-1625.
Publisher | Google Scholor - Czajkowski, R. (2019). May the phage be with you? Prophage-like elements in the genomes of soft rot Pectobacteriaceae: Pectobacterium spp. and Dickeya spp. Frontiers in Microbiology, 10:138.
Publisher | Google Scholor - Stone, E., Campbell, K., Grant, I., & McAuliffe, O. (2019). Understanding and exploiting phage–host interactions. Viruses, 11(6):567.
Publisher | Google Scholor - Leprince, A.; Nuytten, M.; July, E.; Tesseur, C.; Mahillon, J. (2022). Getting Outside the Cell: Versatile Holin Strategies Used by Distinct Phages to Leave Their Bacillus Thuringiensis Host. Journal of Virology, 96:e00696-722.
Publisher | Google Scholor - Casjens, S. R., & Gilcrease, E. B. (2009). Determining DNA packaging strategy by analysis of the termini of the chromosomes in tailed-bacteriophage virions. Bacteriophages: Methods and Protocols, Molecular and Applied Aspects, 91-111.
Publisher | Google Scholor - Kaliniene, L., Šimoliūnas, E., Truncaitė, L., Zajančkauskaitė, A., Nainys, J., et al. (2017). Molecular analysis of Arthrobacter myovirus vB_ArtM-ArV1: we blame it on the tail. Journal of Virology, 91(8), e00023-17.
Publisher | Google Scholor - Giri, N. (2021). Bacteriophage structure, classification, assembly and phage therapy. Biosciences Biotechnology Research Asia, 18(2):239-250.
Publisher | Google Scholor - Abedon ST, Thomas-Abedon C, Thomas A, Mazure H. (2011). Bacteriophage prehistory: Is or is not Hankin, 1896, a phage reference? Bacteriophage; 1:174-178.
Publisher | Google Scholor - Summers WC. (1999). Félix d’Herelle and the origins of molecular biology. New Haven, CT: Yale University Press.
Publisher | Google Scholor - d'Herelle, F. (1931). An address on bacteriophagy and recovery from infectious diseases. Canadian Medical Association Journal, 24(5):619.
Publisher | Google Scholor - Smith HW, Huggins MB. (1983). Effectiveness of phages in treating experimental Escherichia coli diarrhea in calves, piglets and lambs. J Gen Microbiol. 129:2659-2675.
Publisher | Google Scholor - Sulakvelidze, A., & Barrow, P. (2005). Phage therapy in animals and agribusiness. Bacteriophages: biology and applications, 335-380.
Publisher | Google Scholor - Sulakvelidze, A., & Morris, J. G. (2001). Bacteriophages as therapeutic agents. Annals of medicine, 33(8):507-509.
Publisher | Google Scholor - Sulakvelidze, A., Alavidze, Z., & Morris Jr, J. G. (2001). Bacteriophage therapy. Antimicrobial agents and chemotherapy, 45(3):649-659.
Publisher | Google Scholor - Lin, D. M., Koskella, B., & Lin, H. C. (2017). Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World journal of gastrointestinal pharmacology and therapeutics, 8(3):162.
Publisher | Google Scholor - Petrovic Fabijan, A., Iredell, J., Danis-Wlodarczyk, K., Kebriaei, R., & Abedon, S. T. (2023). Translating phage therapy into the clinic: Recent accomplishments but continuing challenges. Plos Biology, 21(5):e3002119.
Publisher | Google Scholor - Cui Z, Feng T, Gu F, Li Q, Dong K, et al. (2017). Characterization and complete genome of the virulent Myoviridae phage JD007 active against a variety of Staphylococcus aureus isolates from different hospitals in Shanghai, China. Virology Journal, 14(1):26.
Publisher | Google Scholor - Dedrick, R. M., Guerrero-Bustamante, C. A., Garlena, R. A., Russell, D. A., Ford, K., et al. (2019). Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nature medicine, 25(5):730-733.
Publisher | Google Scholor - García, R., Latz, S., Romero, J., Higuera, G., García, K., et al. (2019). Bacteriophage production models: An overview. Frontiers in Microbiology, 10:1187.
Publisher | Google Scholor - Pirnay, J. P., De Vos, D., Verbeken, G., Merabishvili, M., Chanishvili, N., et al. (2011). The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharmaceutical Research, 28:934-937.
Publisher | Google Scholor - Adesanya, O., Oduselu, T., Akin-Ajani, O., Adewumi, O. M., & Ademowo, O. G. (2020). An exegesis of bacteriophage therapy: An emerging player in the fight against antimicrobial resistance. AIMS Microbiology, 6(3):204.
Publisher | Google Scholor - Azeredo, J., Pirnay, J. P., Pires, D., Kutateladze, M., Dabrowska, K., et al. (2021). Phage therapy. Wiki Journal of Medicine, 8(1):1-18.
Publisher | Google Scholor - Vandersteegen, K., Mattheus, W., Ceyssens, P. J., Bilocq, F., De Vos, D., et al. (2011). Microbiological and molecular assessment of bacteriophage ISP for the control of Staphylococcus aureus. PLoS One, 6(9):e24418.
Publisher | Google Scholor - Chibani-Chennoufi, S., Sidoti, J., Bruttin, A., Kutter, E., Sarker, S., et al. (2004). In vitro and in vivo bacteriolytic activities of E. coli phages: implications for phage therapy. Antimicrobial Agents and Chemotherapy, 48(7):2558-2569.
Publisher | Google Scholor - Dissanayake, U., Ukhanova, M., Moye, Z. D., Sulakvelidze, A., & Mai, V. (2019). Bacteriophages reduce pathogenic E. coli counts in mice without distorting gut microbiota. Frontiers in Microbiology, 10:1984.
Publisher | Google Scholor - De Gunzburg, J., Ghozlane, A., Ducher, A., Le Chatelier, E., Duval, X., et al. (2018). Protection of the human gut microbiome from antibiotics. The Journal of infectious diseases, 217(4):628-636.
Publisher | Google Scholor - Vahedi, A., Soltan Dallal, M. M., Douraghi, M., Nikkhahi, F., Rajabi, Z., et al. (2018). Isolation and identification of specific bacteriophage against enteropathogenic E. coli (EPEC) and in vitro and in vivo characterization of bacteriophage. FEMS Microbiology Letters, 365(16):136.
Publisher | Google Scholor - Zhao, J., Liu, Y., Xiao, C., He, S., Yao, H., et al. (2017). Efficacy of phage therapy in controlling rabbit colibacillosis and changes in cecal microbiota. Frontiers in Microbiology, 8:957.
Publisher | Google Scholor - Hernandes, R. T., Elias, W. P., Vieira, M. A., & Gomes, T. A. (2009). An overview of atypical enteropathogenic E. coli. FEMS microbiology letters, 297(2):137-149.
Publisher | Google Scholor - Dalmasso, M., Strain, R., Neve, H., Franz, C. M., Cousin, F. J., et al. (2016). Three new Escherichia coli phages from the human gut show promising potential for phage therapy. PloS one, 11(6):e0156773.
Publisher | Google Scholor - Dalmasso, M., Hill, C., & Ross, R. P. (2014). Exploiting gut bacteriophages for human health. Trends in Microbiology, 22(7):399-405.
Publisher | Google Scholor - Bruttin, A., & Brüssow, H. (2005). Human volunteers receiving E. coli phage T4 orally: a safety test of phage therapy. Antimicrobial Agents and Chemotherapy, 49(7):2874-2878.
Publisher | Google Scholor - Sheng, H., Knecht, H. J., Kudva, I. T., & Hovde, C. J. (2006). Application of bacteriophages to control intestinal E. coli O157: H7 levels in ruminants. Applied and Environmental Microbiology, 72(8):5359-5366.
Publisher | Google Scholor - Sarker, S. A., McCallin, S., Barretto, C., Berger, B., Pittet, A. C., et al. (2012). Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology, 434(2):222-232.
Publisher | Google Scholor - Sarker, S. A., Sultana, S., Reuteler, G., Moine, D., Descombes, P., et al. (2016). Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. E Bio Medicine, 4:124-137.
Publisher | Google Scholor - Malik S, Sidhu PK, Rana J, Nehra K (2020). Managing urinary tract infections through phage therapy: a novel approach. Folia Microbiologica. 65:217-231.
Publisher | Google Scholor - de Miguel T, Rama JLR, Sieiro C, Sánchez S, Villa TG. (2020). Bacteriophages and lysins as possible alternatives to treat antibiotic-resistant urinary tract infections. Antibiotics. 9:466.
Publisher | Google Scholor - Chegini, Z., Khoshbayan, A., Vesal, S., Moradabadi, A., Hashemi, A., et al. (2021). Bacteriophage therapy for inhibition of multi drug‐resistant uropathogenic bacteria: a narrative review. Annals of Clinical Microbiology and Antimicrobials, 20(1):1-13.
Publisher | Google Scholor - Pires DP, Melo LD, Boas DV, Sillankorva S, Azeredo J. (2017). Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr Opin Microbiol. 39:48-56.
Publisher | Google Scholor - Chegini Z, Khoshbayan A, Moghadam MT, Farahani I, Jazireian P, et al. (2020). Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann Clin Microbiol Antimicrob. 19:1-17.
Publisher | Google Scholor - Perepanova, T. S., Darenkov, A. F., Kagan, E. V., Kotliarova, G. A., Kondrat'eva, E. M., et al. (1994). The clinical efficacy of the new drug agent ligenten in urology patients. Urologiia i Nefrologia, (3):14-17.
Publisher | Google Scholor - Sybesma, W., Zbinden, R., Chanishvili, N., Kutateladze, M., Chkhotua, A., et al. (2016). Bacteriophages as potential treatment for urinary tract infections. Frontiers in Microbiology, 7:465.
Publisher | Google Scholor - Galtier, M., De Sordi, L., Maura, D., Arachchi, H., Volant, S., et al. (2016). Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environmental Microbiology, 18(7):2237-2245.
Publisher | Google Scholor - Dufour, N., Clermont, O., La Combe, B., Messika, J., Dion, S., et al. (2016). Bacteriophage LM33_P1, a fast-acting weapon against the pandemic ST131-O25b: H4 Escherichia coli clonal complex. Journal of Antimicrobial Chemotherapy, 71(11):3072-3080.
Publisher | Google Scholor - Slobodníková, L., Markusková, B., Kajsík, M., Andrezál, M., Straka, M., et al. (2021). Characterization of Anti-Bacterial Effect of the Two New Phages against Uropathogenic E. coli. Viruses, 13(7):1348.
Publisher | Google Scholor - Sanchez, B. C., Heckmann, E. R., Green, S. I., Clark, J. R., Kaplan, H. B., et al. (2022). Development of phage cocktails to treat E. coli catheter-associated urinary tract infection and associated biofilms. Frontiers in Microbiology, 1465.
Publisher | Google Scholor - Shah, C., Baral, R., Bartaula, B., & Shrestha, L. B. (2019). Virulence factors of uropathogenic E. coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiology, 19:1-6.
Publisher | Google Scholor - Zhao Z, Yu J, Zhang H, Li J, Li Z. (2018). Five-year multicenter study of clinical tests of neonatal purulent meningitis. Clin Pediatr (Phila). 57:389-397.
Publisher | Google Scholor - Barrow, P., Lovell, M., & Berchieri Jr, A. (1998). Use of lytic bacteriophage for control of experimental E. coli septicemia and meningitis in chickens and calves. Clinical Diagnostic Laboratory Immunology, 5(3):294-298.
Publisher | Google Scholor - Pouillot, F., Chomton, M., Blois, H., Courroux, C., Noelig, J., et al. (2012). Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b: H4-ST131 E. coli strain producing CTX-M-15. Antimicrobial Agents and Chemotherapy, 56(7):3568-3575.
Publisher | Google Scholor - Eid, S., Tolba, H. M., Hamed, R. I., & Al-Atfeehy, N. M. (2022). Bacteriophage therapy as an alternative biocontrol against emerging multidrug resistant E. coli in broilers. Saudi Journal of Biological Sciences, 29(5):3380-3389.
Publisher | Google Scholor - Messika, J., Magdoud, F., Clermont, O., Margetis, D., Gaudry, S., et al. (2012). Pathophysiology of E. coli ventilator-associated pneumonia: implication of highly virulent extraintestinal pathogenic strains. Intensive Care Medicine, 38:2007-2016.
Publisher | Google Scholor - Lynch III, J. P., Clark, N. M., & Zhanel, G. G. (2013). Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert opinion on Pharmacotherapy, 14(2):199-210.
Publisher | Google Scholor - Cao, F., Wang, X., Wang, L., Li, Z., Che, J., et al. (2015). Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. BioMed Research International.
Publisher | Google Scholor - Nishikawa, H., Yasuda, M., Uchiyama, J., Rashel, M., Maeda, Y., et al. (2008). T-even-related bacteriophages as candidates for treatment of E. coli urinary tract infections. Archives of Virology, 153:507-515.
Publisher | Google Scholor - Rastogi, V., Yadav, P., Verma, A., & Pandit, J. K. (2017). Ex vivo and in vivo evaluation of microemulsion based transdermal delivery of E. coli specific T4 bacteriophage: A rationale approach to treat bacterial infection. European Journal of Pharmaceutical Sciences, 107:168-182.
Publisher | Google Scholor - Bull JJ, Otto G, Molineux IJ. (2022). In vivo growth rates are poorly correlated with phage therapy success in a mouse infection model. Antimicrob Agents Chemother. 56(2):949-954.
Publisher | Google Scholor - Aleshkin, A. V., Rubalskii, E. O., Volozhantsev, N. V., Verevkin, V. V., Svetoch, E. A., et al. (2015). A small-scale experiment of using phage-based probiotic dietary supplement for prevention of E. coli traveler's diarrhea. Bacteriophage, 5(3):e1074329.
Publisher | Google Scholor - Maura, D., Galtier, M., Le Bouguénec, C., & Debarbieux, L. (2012). Virulent bacteriophages can target O104: H4 enteroaggregative E. coli in the mouse intestine. Antimicrobial agents and chemotherapy, 56(12):6235-6242.
Publisher | Google Scholor - Nureye, D. Y., Assefa, S., & Mohammed, S. (2018). Bacteriophages and their applications: general aspects and a new insight in Ethiopia. Journal of Drug Delivery and Therapeutics, 8(6):278-284.
Publisher | Google Scholor - Leta, A., Yohannes, M., & Kassa, T. (2017). Assessment of therapeutic potential of bacteriophages to control E. coli infection in Swiss mice model. Ethiopian Journal of Applied Science and Technology, 8(2):73-83.
Publisher | Google Scholor - Gudina, I., Gizachew, Z., Woyessa, D., & Tefera, T. K. (2018). Isolation of bacteriophage and assessment of its activity against biofilms of uropathogenic E. coli in Jimma Town, South Western Ethiopia. American Journal of Current Microbiology, 6(1):52-66.
Publisher | Google Scholor - Hyman, P.; Abedon, S.T (2010). Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70:217-248.
Publisher | Google Scholor - Carascal, M.B.; Dela Cruz-Papa, D.M.; Remenyi, R.; Cruz, M.C.B.; Destura, R.V (2022). Phage Revolution Against Multidrug-Resistant Clinical Pathogens in Southeast Asia. Front. Microbiol. 13:820572.
Publisher | Google Scholor - Jault, P., Leclerc, T., Jennes, S., Pirnay, J. P., Que, Y. A., et al. (2019). Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. The Lancet Infectious Diseases, 19(1):35-45.
Publisher | Google Scholor - Vinner, G. K., Richards, K., Leppanen, M., Sagona, A. P., & Malik, D. J. (2019). Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics, 11(9):475.
Publisher | Google Scholor - Botka, T., Pantůček, R., Mašlaňová, I., Benešík, M., Petráš, P., et al. (2019). Lytic and genomic properties of spontaneous host-range Kayvirus mutants prove their suitability for upgrading phage therapeutics against staphylococci. Scientific Reports, 9(1):5475.
Publisher | Google Scholor - McCallin, S., Oechslin, F., Górski, A., Międzybrodzki, R., & Borysowski, J. (2019). Phage Therapy: A Practical Approach.
Publisher | Google Scholor - Tagliaferri, T. L., Jansen, M., & Horz, H. P. (2019). Fighting pathogenic bacteria on two fronts: phages and antibiotics as combined strategy. Frontiers in cellular and infection microbiology, 9:22.
Publisher | Google Scholor - Kakasis, A., & Panitsa, G. (2019). Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. International journal of antimicrobial agents, 53(1):16-21.
Publisher | Google Scholor - Ly-Chatain, M. H., Durand, L., Rigobello, V., Vera, A., & Demarigny, Y. (2011). Direct quantitative detection and identification of Lactococcal bacteriophages from milk and whey by real-time PCR: application for the detection of Lactococcal bacteriophages in Goat's raw milk whey in France. International journal of microbiology.
Publisher | Google Scholor - Chan BK, Abedon ST. (2012). Phage therapy pharmacology: phage cocktails. Adv Appl Microbiol. 78:1-23.
Publisher | Google Scholor - Van Belleghem, J. D., Dąbrowska, K., Vaneechoutte, M., Barr, J. J., & Bollyky, P. L. (2018). Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses, 11(1):10.
Publisher | Google Scholor - Santiago-Rodriguez TM, Hollister EB. (2019). Human virome and disease: high-throughput sequencing for virus discovery, identification of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses. 11(7):656.
Publisher | Google Scholor - Łusiak-Szelachowska, M., Żaczek, M., Weber-Dąbrowska, B., Międzybrodzki, R., Kłak, M., et al. (2014). Phage neutralization by sera of patients receiving phage therapy. Viral Immunology, 27(6):295-304.
Publisher | Google Scholor