Original article
Audit on Obtaining Chemotherapeutic Drugs Administration Consent from Patients Admitted for Chemotherapy at Day Care Oncology in a Tertiary Care Hospital Karachi, Pakistan
1Staff medical officer supervisor at day care oncology department of oncology the aga khan university hospital.
2Medical officer day care oncology department of oncology the aga khan university hospital.
*Corresponding Author: Dr. Arifa Aziz,Staff medical officer supervisor at day care oncology department of oncology the aga khan university hospital.
Citation: Aziz A, Zubair S. (2024). Audit on Obtaining Chemotherapeutic Drugs Administration Consent from Patients Admitted for Chemotherapy at Day Care Oncology in a Tertiary Care Hospital Karachi, Pakistan. International Journal of Medical Case Reports and Reviews, BioRes Scientia Publishers. 3(3):1-5. DOI: 10.59657/2837-8172.brs.24.055
Copyright: © 2024 Arifa Aziz, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 02, 2024 | Accepted: April 25, 2024 | Published: May 04, 2024
Abstract
Objective: It was observed multiple times that incomplete consent forms are present in confidential files and consent not taken from the clinic. Patients who are coming for chemotherapy at day care oncology chemotherapy unit for the first cycle of chemotherapy should have complete consent forms. If the consent for chemotherapy not available in the file, then day care physician will take the consent prior to administration of chemotherapeutic drugs leading to delays in the process of chemotherapy administration therefore in the meeting of Comprehensive unit-based safety program (CUSP ) it was suggested to conduct an audit to check for properly obtained consents before administering the chemotherapeutic drug in initial patients referred from the clinics to day care oncology for chemotherapy administration.
Method: Performa for data collection was designed by daycare physicians to check for properly obtained consents before administering the chemotherapeutic drug in patients referred from the clinics. Data was collected from 15th February 2024 till 29th February 2024 in admitted patients at daycare oncology. Including all patients of both genders coming to day care oncology for chemotherapy administration, sample size is 100.
Results: Total consents forms signed from clinics is 70% and out of this only 30% of the forms were completely filled, use of abbreviations was observed in 30 % of these forms. The percentage of clauses not marked is 63 %. Missing consultant name, designation, and signature seen in 17 % of the incomplete consent forms. In 20% of the patients’ confidential files both clauses and consultant name, designation, signature were missing.
Conclusion:The majority of the consent’s forms obtained in the clinic were incomplete. All incomplete consents forms were completed in day care to avoid errors and over writing new consent forms need to be taken by day care physicians before administering chemotherapy. The new consents were also obtained if not available in confidential file on behalf of the respective consultants by the day care physicians and signed by the administering nurses.
Keywords: consent form; high alert medication; incomplete consent form; delay in chemotherapy administration process
Introduction
In chemotherapy unit we are dealing with high alert medications therefore patient’s consent is mandatory before administration of chemotherapeutic drugs to ensure smooth running of day care chemotherapy administration process to avoid delays, consents are supposed to obtain in clinic by consultant after explaining disease process and treatment plan and side effects to the patients and their attendants and if patients agree for treatment consent obtained. Oncology nurses have instructions to check consent form for allowing administration of drugs. Incomplete consent forms were identified in confidential files of patients, this includes missing witness signatures, use of abbreviations or protocol names instead of writing full name of the drugs and consultant’s full name, pneumonic are also missing or no consent taken at all from clinics and for all incomplete and missing consent forms are obtained in day care physicians. It was decided in Comprehensive unit-based safety program (CUSP) meeting held monthly to identify area where improvement is required aim of this meeting is to improve services in day care oncology. This initiative encourages the front-line staff to come forward and work on the issues identified by them on ground through quality improvement projects. CUSP also promotes a culture of safety by incorporating best practices into daily routine, encouraging accountability, and promoting system-wide improvement in patient safety and teamwork. Therefore, management decided to conduct an audit and identify the reasons behind these observations and to check for properly obtained consents before administering the chemotherapeutic drugs in initial patients referred from the clinics. The consent form includes the name of the drugs used in chemotherapy protocol, signature of the witnesses and proper date with side effects explained to the patient was checked.
Methodology
Proforma for the audit of consent form availability in confidential file was prepared by day care physician, retrospective data was collected from confidential files of patients, consent proforma includes name of the drug / chemotherapy protocol, signature of the witnesses and proper date, with side effects of chemotherapeutic drugs were explained to the patient prior taking consent was assessed. Consent forms of all initial patients admitted for day one of chemotherapy in day-care oncology were checked in patients’ confidential file. Hundreds of patient’s files were checked, and data collected from 15th February 2024 till 29th February 2024.
Result
Total consents forms signed from clinics was 70% and out of this only 30% of the forms were properly filled, remaining 30%. consent forms were filled by day care physicians. Use of abbreviations was observed in 30 % of these forms. The percentage of clauses not marked was 63 %. Missing consultant name, designation, and signature in 17 % of files incomplete consent forms were present. In 20% of the patients file clauses and consultant name, designation, signature both were missing.
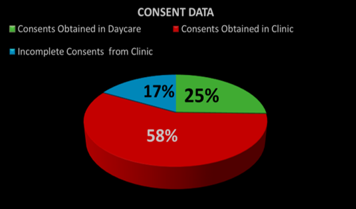
Audit Results of 20219 as shown in chart -1: 1-Consents forms obtained from clinic was 58%, 2- Consents forms obtained in day Care was 25 %, 3 -Incomplete Consents forms from clinic was 17 %.
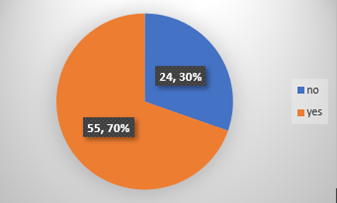
Chart-2: A total of 70 % incomplete consent forms were observed from clinics and 30% of the forms were properly filled.
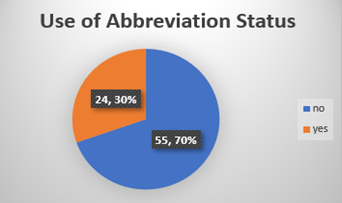
Chart-3: Out of the 70 % incompletely filled consent forms from clinic, 30 % had abbreviations in the form of consultant’s mnemonics or drug protocol.
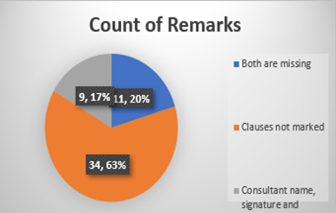
Chart -4: Unmarked clauses were observed in 63 % of the incomplete forms. Furthermore, in 17% of the forms, the consultant’s name, signature, or designation was left blank. Another 20 % had both the clauses and the consultant’s information missing.
Discussion
The informed consent for chemotherapy procedure has both legal and ethical importance. The common-law requirement that a patient consent to medical treatment has developed over time to include the disclosure of a patient's diagnosis, the nature of the proposed intervention, intended benefits, associated risks and adverse effects, and medically reasonable alternatives and their corresponding risks and adverse effects [1]. Consent from patient for chemotherapy is essential before starting chemotherapeutic drugs, the consent forms are incomplete missing witness signature, use of abbreviations which is not allowed on consent forms it is mandatory to write full name of the drugs, in few cases no consent is taken from clinics. During the CUSP meeting it was suggested to conduct an audit and check for properly obtained consents before administering the chemotherapeutic drugs in new patients referred from the clinics. The name of the drugs, signature of the witnesses and proper date, with side effects of chemotherapeutic drugs explained to the patient in the clinic before taking consents. Missing consents were checked and filled out during their daycare admission before drug administration, new consents were obtained on behalf of the respective consultants by the day care physicians and signed by the drug administering nurses all this process leads to delay in chemotherapy administration process. Patient when admitted for chemotherapy administration wants timely administration of chemotherapy with no delays [2]. This audit is worthwhile because competent adult patients have the basic right to agree or refuse an examination, inquiry, or treatment. Many doctors have a poor awareness of the law, and unpredictable or ineffective systems for getting permission prior to a procedure has been cited as a key contributor to litigation. Misconceptions about acquiring consent from adolescents and the idea of competence to provide informed consent are frequently encountered [3]. Chemotherapy and Immunotherapy fall into the category of high-risk high alert medications. Proper consent prior to administration and initiation of treatment is necessary and should ideally be obtained from the clinic [3,4]. In case of missing consent and change of drug regimen, consents are signed by the daycare physicians and witnessed by the administering nurses. Consents should be properly filled out and checked as they hold a key position in the entire process of chemotherapy infusion. The name of the chemotherapeutic drugs protocols, signature of the witnesses and proper date, with side effects of drugs explained to the patient prior to chemotherapeutic drug administration [5].
Conclusion
The majority of the consents were obtained in the clinic out of these mostly were incomplete forms. Consent forms checked and filled out by physicians in their daycare admission prior to chemotherapy leading to delays in chemotherapy administration process. New consents were also obtained on behalf of the respective consultants by the day care physicians and signed by the administering nurses.
Declarations
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgement
The author would like to thank Mr. Salman Faheem Associate in the department of oncology for data analysis and amber Hussain head nurse day care oncology and Anila Ali Bux registered nurse at day care oncology.
Way Forward
Nurse Navigator in oncology clinics is supposed to check all consent forms for chemotherapy administration before the patient leave the clinic and make sure that these consent forms are complete.
References
- Journal of Oncology Practice
Publisher | Google Scholor - Aziz A. (2016). Innovation in chemotherapy administration process. Indian J Cancer, 53:331-332.
Publisher | Google Scholor - Caldicott Y et al. (2003). The ethics of private examinations – teaching tomorrow's doctors. BMJ, 326: 97-101.
Publisher | Google Scholor - Department of Health. Reference guide to consent for examination or treatment. Leeds: DoH, 2001.
Publisher | Google Scholor - Kravitz RL, Melnikov J. (1999). Engaging patients in medical decision making. BMJ, 323: 584-585.
Publisher | Google Scholor