Research Article
Atopic Dermatitis in Senegal: Epidemiological, Clinical, and Therapeutic Features
- Diatta Boubacar Ahy *
- Arahou Teba
- Patrice Mendy
- Ndiague Fall
- Pie Nibirantije
- Ndiaye Coumba
- Niar Ndour
- Ndiaye Mame
- Diadie Saer
- Diop Assane
- Ndiaye Maodo
- Diallo Moussa
- Ly Fatimata
- Niang Suzanne Oumou
Department of Dermatology, Dakar Cheikh Anta Diop University, Dakar, Senegal.
*Corresponding Author: Diatta Boubacar Ahy, Department of Dermatology, Dakar Cheikh Anta Diop University, Dakar, Senegal.
Citation: Diatta B Ahy, Teba A, Mendy P, Fall N, Nibirantije P, et al. (2023). Atopic Dermatitis in Senegal: Epidemiological, Clinical, and Therapeutic Features. Dermatology Research and Reports, BioRes Scientia Publishers. 2(1):1-6. DOI: 10.59657/2993-1118.brs.23.008
Copyright: © 2023 Diatta Boubacar Ahy, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 28, 2023 | Accepted: August 12, 2023 | Published: August 16, 2023
Abstract
Introduction: Atopic dermatitis (AD) is a chronic inflammatory disease that is on the rise in industrialized countries. It is underestimated in Africa. This study aimed to describe the epidemiological, clinical, therapeutic, and evolutionary profile of atopic dermatitis, and to identify its impact on patients' quality of life.
Methodology: We conducted a retrospective multicenter descriptive-analytical cross-sectional study in three dermatology referral departments in Dakar for over 3 years. We included all patients who consulted us for atopic dermatitis.
Results: We identified 301 cases of atopic dermatitis, representing a hospital frequency of 0.6%. The mean age was 17 years. Pruritus was the main functional symptom. According to SCORAD, atopic dermatitis was mild in 50 cases (16.6%), moderate in 226 cases (75.10%), and severe in 25 cases (8.3%). AD was associated with seborrheic dermatitis in 1.9% and allergic contact dermatitis in 1.6%. Skin infections included staphylococcal in 8.97%, herpetic in 3.03% and scabies in 1.6%. Topical steroids and skin infections were the first-line treatment, in combining with therapeutic education. Methotrexate and azathioprine were used in 3 cases. Complete response was observed in 76.6% of patients.
Conclusion: Atopic dermatitis is a common chronic inflammatory disease in Senegal. Early management and therapeutic education can prevent complications and reduce the psychological and social impact.
Keywords: epidemiology; atopic dermatitis; senegal
Introduction
Atopic dermatitis (AD) is a chronic, pruritic inflammatory dermatosis with a multifactorial pathogenesis. It is part of atopic disease, which refers to polygenic hereditary traits that include atopic dermatitis, allergic rhinitis, asthma, and allergic conjunctivitis [1,2]. AD is a public health burden due to its high prevalence in both industrialized and developing countries [3]. AD affected at least 230 million people worldwide in 2019. Its prevalence is estimated at between 15% to 25% in children and 1% to 10% in adults [5].
Atopic dermatitis remains an underestimated pathology in Sub-Saharan Africa, with an estimated prevalence of 9.2% [5]. It is a chronic disease whose symptoms can lead to insomnia, absenteeism from school and work, and acute and chronic complications such as staphylococcal infections or a worsening of erythroderma with the use of medicinal plants in Africa [6]. Atopic dermatitis appears to be common in Africa, although few cases have been reported in the literature. It is for this reason that we carried out this study, intending to describe the clinical, therapeutic, and evolutionary-epidemiological features of atopic dermatitis, and assess the severity and the impact of atopic dermatitis on patients' quality of life.
Patients and Methods
This is a multicenter cross-sectional study over 03 years from January 1, 2019, to December 31, 2021. It was carried out in the dermatology departments of Hospital Aristide Le Dantec, Institute Social Hygiene, and Albert Royer Children Hospital. We included all patients presenting with atopic dermatitis. The diagnosis of atopic dermatitis was based on the diagnostic criteria of the United Kingdom Working Party [7]. The presence of atopic equivalents such as asthma, allergic conjunctivitis, or allergic rhinitis. The existence of pruritus and cutaneous signs such as eczema in the folds, extension surfaces of limbs, convex areas, cutaneous xerosis, and minor signs of atopy (periorbital hyperpigmentation, Dennie-Morgan double fold, keratosispilaris, palmo-plantar hyperlinearity, folliculare czematitis, achromiante czematitis, pulpitis, cheilitis). Pneum-allergen sensitization was sought using pneum-allergen prick tests. Data were collected using a survey form. The parameters studied were epidemiological, clinical, therapeutic, and evolutionary data. Severity was assessed by SCORAD [8,9] and impact on quality of life by CLDQI / DLQI [10]. Data entry was performed using Sphinx version 5.0 and analysis was performed using SPSS version 20.
Results
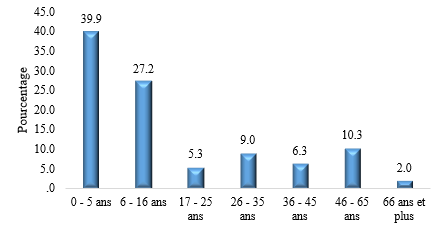
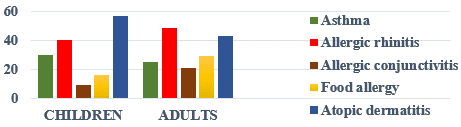
We recorded 301 cases of atopic dermatitis, representing a hospital frequency of 0.6%. The mean age was 17 years, with extremes ranging from 6 months to 74 years. The study population comprised children in 202 cases (67%) and adults in 99 cases (33%). Figure 1 shows the age distribution of patients. Patients were female in 159 cases and male in 142 cases, giving a sex ratio of 0.9. Pruritus was the main reason for consultation. Patients lived in urban areas in 280 cases (93%). There was an atopic equivalent in 137 cases (45.5%). Figure 2 illustrates the distribution of patients according to atopic equivalents. The predominant elementary lesions were cutaneous xerosis in 280 cases (93%), erythema in 247 cases (82.8%), and oozing in 28 cases. Table 1 shows the distribution of patients according to the elementary lesion.
Table 1: Distribution of patients according to elementary lesion
Enfant Number (%) | Adulte Number (%) | ||
| Cutaneous xerosis | 187 (92,5) | 93 (93,9) | 280(93) |
| Erythema | 167 (82,6) | 80 (80,8) | 247(82) |
| Vesicles | 28 (13,8) | 15 (15) | 43(14,2) |
| oozing | 26 (12,8) | 18 (18) | 44(14,6) |
| Œdema | 60 (29,7) | 29 (29) | 89(29,5) |
| Lichenification | 30 (14,8) | 21 (21) | 51(16,9) |
| Prurigo | 12 (5,9) | 3 (3) | 15(4,9) |
| Double palpebral folds | 26 (12,8) | 13 (13) | 39(12,9) |
| Keratosis pilaris | 30 (10) | 29 (29) | 59(19,6) |
| Periorbital hyperpigmentation | 20 (10) | 24 (24) | 44(14,6) |
| Dyshidrosis | 2 (1) | 13 (13) | 15(4,9) |
Table 2: Distribution of patients according to the topography of the lesions
| Topography | Number | Percentage (%) |
| Extension face limbs | 180 | 60 |
| Pleats | 163 | 54,15 |
| Trunk | 153 | 50,8 |
| Face | 143 | 47,5 |
| Hands and feet | 51 | 16,9 |
| Scalp | 43 | 14,28 |
| Buttocks | 16 | 5 |
| Genitals | 6 | 0,02 |
Figure 1: Distribution of patients according to age groups
Figure 2: Distribution of patients according to a topic
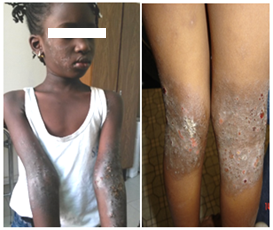
Figure 3: Atopic eczema in children
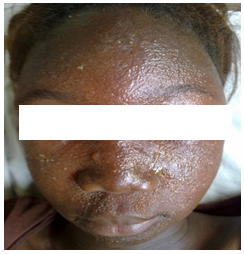
Figure 4: facial allergic contact dermatitis associated with atopic dermatitis
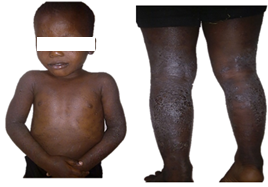
Figure 5: severe atopic dermatitis in a boy
The topography of lesions was the large folds of the limbs in 180 cases (60%), the face in 143 cases (47.5%), and the trunk in 153 cases (50.8%). Table 2 shows the distribution of patients according to lesion topography. According to SCORAD, atopic dermatitis was mild in 50 cases (16.6%), moderate in 226 cases (75.10%), and severe in 25 cases (8.3%). Table 3 shows the distribution of patients according to SCORAD. Complications included staphylococcal infection in 27 cases (9%), scabies in 5 cases (1.6%), herpetic superinfection in 3 cases (3%), and molluscum contagiosum in one case. Atopic dermatitis was associated with seborrheic dermatitis in 6 cases (2%), allergic contact dermatitis in 5 cases (1.6%), and Napkin dermatitis in 3 cases (0.3%). Concerning the impact on quality of life, the median DLQI in adults was 9, with extremes of 0 and 24. The impact on quality of life in adults was low in 21 cases (21.2%), moderate in 47 cases (47.5%), and severe in 31 cases (31.3%).
Table 3: Distribution of patients according to severity of the SCORAD
Atopic dermatitis SCORAD | Children Number (%) | Adults Number(%) | |
| Mild AD | 36 (17,8) | 14(14,1) | 50(16,6) |
| Moderate AD | 155 (76,7) | 71(71,7) | 226(75,1) |
| Severe AD | 11 (5,4) | 14(14,1) | 25(8,3) |
| 202(100) | 99(100) | 301(100) |
Table 4: distribution of patients according to the treatments used
Children Number (%) | Adults Number (%) | ||
| Topical steroids | 193(95,5) | 91(91,9) | 284(94,3) |
| Moisturizers | 200(99) | 96(96,9) | 296(98,3) |
| Antihistamine | 150(74,2) | 85(85,8) | 235(78) |
| Topical antibiotic | 13(6,4) | 8(8) | 21(6,9) |
| Immunosuppressants | 3(1,4) | 2(2) | 5(1,6) |
In children, the mean CDLQI score was 7.97, with extremes from 0 to 19. AD had a weak effect on children's quality of life in 34 cases (32.1%), a moderate effect in 44 cases (41.5%), and a strong effect in 28 cases (26.4%). All patients had received topical steroids and moisturizers. Table 4 illustrates the distribution of patients according to the different drugs administered. After one month of treatment, the evolution was favorable in 230 cases (76.4%), stationary in 32 cases (10.6%), and persistent in 13 cases (4.3 %).
Discussion
We report 301 cases of atopic dermatitis in pigmented photo types, representing a hospital frequency of 0.6%. In Senegal, there has been an increase in the hospital prevalence of atopic dermatitis compared with previous publications in 2007 [11]. The 0-5 age group was the most represented in our study, confirming the early-onset of AD in infants [12]. A female predominance is often reported, and was also observed in our study [9].
This predominance seems to be explained by hormonal factors [13]. In vitro, estradiol has been shown to exacerbate Th2 inflammation and is a direct promoter of mastcell degranulation. Dihydrotestosterone, on the other hand, tends to reduce Th2 inflammation and thus inhibit the development of atopy [13]. Environmental factors play a key role in the onset of atopic dermatitis. These include air pollution, lifestyle and stress. In our study, 52.8% of the population lived in urban areas. Some authors report a significantly higher risk of atopic dermatitis in an urban than in a rural environment [14].
The clinical presentation of atopic dermatitis was polymorphous in our study. Mild to moderate atopic dermatitis was present in 91.7% of patients, according to SCORAD, in both children and adults. Severe forms were mainly found in adults. The use of traditional medicinal plants in 8 cases was the main aggravating factor. Due to their widespread use in Senegal, medicinal plants contribute to the aggravation of atopic dermatitis symptoms through immunoallergic or toxicphenomena [6].
Pruritus was frequent and was the cause of insomnia in 51.16% of atopic patients. In the literature, it concerns more than 80% of children treated for atopic dermatitis. [15]. In 46.3% of cases, pruritus had a significant impact on children's quality of life, with insomnia, school absenteeism, sadness and stress in adults. Authors in Africa have also reported a considerable deterioration in the quality of life of atopic children, with pruritus in 65%, sadness in 38.3%, insomnia in 90% and a drop in school performance in 10% [16]. Cutaneous xerosisis the most frequent elementary lesion in our study, sometimes isolated in atopic patients. It can sometimes be the only manifestation of atopic dermatitis [17].
In our stady, we noted many minor signs that seem to be more frequent on pigmented skin, such as periorbital hyperpigmentation, keratosispilaris, Dennis Morgan's double fold, post-inflammatory hyperpigmentation and achromy eczematitis[18,19].Staphylococcal infection was noted in 8.9% of cases, and is one of the most frequent complications in atopic skin [20]. Other complications were scabies, herpetic infection and Molluscum contagiososum [20].
Therapeutically, topical steroids and moisturizers were the first-line treatment in our series, as was therapeutic education, with clinical remission in 76.4% of cases. The immunosuppressants used were azathioprine and methotrexate, due to their availability and accessibility.
Conclusion
Atopic dermatitis is a chronic inflammatory dermatosis in increasing in Dakar. Clinical manifestations are polymorphous, dominated by atopic eczema and cutaneous xerosis. Minor signs such as pulpitis, cheilitis, keratosispilaris and prurigo are common on pigmented skin. With the advent of new therapies such as calcineurin inhibitors and biotherapies, chronic inflammatory flare-ups can be relieved and controlled as effectively as possible. Therapeutic education remains essential to reduce the psychosocial impact.
References
- Catteau B. (2002). Atopic dermatitis: current epidemiology and clinical data.French J Allergol Clin Immunol, 42:373-377.
Publisher | Google Scholor - Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, et al. (2013). Assessment of clinical Signs of atopic dermatitis: A systematic review and recommendation. J Allergy Clin Immunol, 132:1337-1347.
Publisher | Google Scholor - Ezzedine K, Kechichian E. (2017). Épidémiologie de la dermatite atopique. Ann Dermatol Vénéréol, 144:4‑7.
Publisher | Google Scholor - Torres T, Ferreira EO, Gonçalo M, Mendes-Bastos P, Selores M, Filipe P. (2019). Update on Atopic Dermatitis. Acta Médica Port, 32(9):606-613.
Publisher | Google Scholor - Ahogo KC, Kouassi YI, Gbery IP, Azagoh KR, Kouame IY, Kouassi KA, et al. (2017). Atopic dermatitis in children: Epidemiological and clinical aspects in Côte d’Ivoire. Our Dermatol Online, 8(1):25-27.
Publisher | Google Scholor - Niang SO, Diatta BA, Tine Y, Diadié S, Diop K, Ndiaye C, Ndour N, Deh A, Ndiaye M. (2020). Management of delayed hypersensibilities in medicinal plants. Rev fr allergol, 60:275-276.
Publisher | Google Scholor - Williams HC, Burney PG, Pembroke AC, Hay RJ. (1994). The U.K. Working Party's Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol, 131(3):406-416.
Publisher | Google Scholor - Barbarot S, Aubert H, Stalder J-F. (2016). Quel est l’intérêt d’utiliser un score d’auto-évaluation dans la dermatite atopique en pratique courante ? Ann Dermatol Vénéréol, 143(12):305.
Publisher | Google Scholor - Dammak A, Guillet G. (2011). Dermatite atopique de l’enfant. J Pédiatrie Puériculture, 24(2):84‑102.
Publisher | Google Scholor - Finlay AY, Khan GK. (1994). Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol, 19(3):210‑216.
Publisher | Google Scholor - Niang SO. (2007). Dermatite atopique à Dakar. Guinée Médicale.
Publisher | Google Scholor - Teclessou JN, Mouhari-Toure A, Akakpo AS, Saka B, Boukari OBT, Moise Elegbede Y, et al. (2016). Facteurs de risque et manifestations allergiques associés à la dermatite atopique à Lomé (Togo) : étude portant sur 476 enfants de 0 à 15 ans. Ann Dermatol Vénéréol, 143(4):31‑32.
Publisher | Google Scholor - Sacotte R, Silverberg JI. (2018). Epidemiology of adult atopic dermatitis.Clin Dermatol, 36(5):595‑605.
Publisher | Google Scholor - Schram ME, Tedja AM, Spijker R, Bos JD, Williams HC, Spuls PhI. (2010). Is there a rural/urban gradient in the prevalence of eczema? A systematic review: Eczema in urban vs. rural areas. Br J Dermatol, 162(5):964‑973.
Publisher | Google Scholor - Chang Y-S, Chiang B-L. (2018). Sleep disorders and atopic dermatitis: A 2-way street? J Allergy Clin Immunol, 142(4):1033‑1040.
Publisher | Google Scholor - Kouassi YI, Ahogo KC, Bih OF, Kouassi KA, Kourouma HS, Allou AS, et al. (2019). Évaluation de la qualité de vie des enfants atteints de dermatite atopique par le score CDLQI en milieu tropical africain. Ann Dermatol Vénéréol, 146(12):187.
Publisher | Google Scholor - Yew YW, Thyssen JP, Silverberg JI. (2019). A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol, 80(2):390‑401.
Publisher | Google Scholor - Nnoruka EN. (2004). Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol, 43(10):739‑744.
Publisher | Google Scholor - Vachiramon V, Tey HL, Thompson AE, Yosipovitch G. (2012). Atopic Dermatitis in African American Children: Addressing Unmet Needs of a Common Disease: Atopic Dermatitis in African American Children. Pediatr Dermatol, 29(4):395‑402.
Publisher | Google Scholor - Braun C, Vocanson M, Lina G, Nicolas JF, Nosbaum A. (2020). Rôle de la dysbiose cutanée dans la dermatite atopique. Rev Fr Allergol, 60(2):78‑82.
Publisher | Google Scholor