Research article
Assessment of the Heavy Metals in Different Organs of The Commercially Significant Fish Species Available in Coastal Bangladesh
1 Department of Knowledge Management for Development, Young Power in Social Action, Chittagong, Bangladesh.
2 Department of Zoology, Gachbaria Government College, Chandanaish, Chittagong, Bangladesh.
*Corresponding Author: Prabal Barua, 1 Department of Knowledge Management for Development, Young Power in Social Action, Chittagong, Bangladesh.
Citation: Barua P, Barua C. (2024). Assessment of the Heavy Metals in Different Organs of The Commercially Significant Fish Species Available in Coastal Bangladesh, Pollution and Community Health Effects, BioRes Scientia Publishers. 2(1):1-15. DOI: 10.59657/2993-5776.brs.24.015
Copyright: © 2024 Prabal Barua, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 03, 2024 | Accepted: February 19, 2024 | Published: February 24, 2024
Abstract
Coastal resources offer the chance to utilize the coast in a variety of ways even in a dangerous environment. Because of the tension that comes from both home and industrial effluent, the pollution problem is severe here. The goal of the current study was to determine whether the extensively exposed heavy metals had any discernible harmful effects on the various organs of the commercially significant marine fish that were harvested from Bangladesh's Bengal Marine Bay, which is close to Chattogram City. The concentrations of three heavy metals—lead, arsenic, and chromium—were measured in the kidney, liver, muscle, and gills of three common fish species—Herpodon nehereus, Pampus chinensis, and Hilsa ilisha—in this investigation. A mass spectrometer was used to measure and examine each heavy element. The results showed that Herpodon nehereus had the greatest quantities of all three heavy metals, which were not statistically different from those found in Pampus chinensis and Hilsa ilisha. The fish under examination had the highest lead concentration in their kidneys and gills, but there was a notable fluctuation in their muscles and liver. When it came to chromium, the situation was rather different. It was discovered that the gills had the largest concentration, while the other three organs showed only negligible accumulation. The organs most exposed to arsenic were the kidneys and livers, with minimal exposure to muscles and a minor build-up in the gills. Of the three heavy metals, the fish under examination had the highest accumulation trend of arsenic, followed by lead, and the lowest exposure to chromium. The concentrations of lead and chromium were determined to be safe for human consumption, however the values of arsenic accumulation were deemed critical for human consumption since they surpassed the minimum safe limits set by the WHO and FAO. The Chattogram coastline water may be severely arsenic-polluted as a result of companies nearby discharging their wastewater. The lead recorded readings were getting closer to the safety values. It is imperative that action be done to minimize human discharges into coastal waters, since excessive pollution will not only harm aquatic life but also increase the likelihood of socioeconomic catastrophes.
Keywords: atomic absorption; spectroscopy; fish organs; bioaccumulation; socioeconomic catastrophes
Introduction
Fish is a common animal-source food consumed by millions of people in Bangladesh. It accounts for about 60% of animal protein intake, or 18 kg per person annually, and is consumed far more frequently than any other animal-source food (Belton et al., 2014). A greater proportion of the population eats fish since it is more affordable, readily available, and palatable than other protein sources like goat and chicken meat. It is also safer and healthier (Astawan and Ikan, 2004). For some cultures, such as those who don't eat red meat, the undernourished, the immunocompromised, pregnant women, and nursing moms, fish is the main source of protein. According to FAO (1999), fish meat is primarily made up of water (66–81%), protein (16–21%), carbohydrates (0.2–25%), and ash (1.2–1.5%). Because it contains a significant amount of Essential Amino Acids (EAA) and polyunsaturated fatty acids, such as omega-3 and omega-6, it is thought to have a high biological value. Additionally, fish offers the minerals required for good health (Gjedrem et al., 2012). According to Kris-Etherton et al. (2002), the American Heart Association advised consuming fish at least twice a week to fulfill daily requirements for protein and polyunsaturated fatty acids.
One of the top fish-producing nations in the world, Bangladesh produced 42.77 lakh MT of fish in 2017–18, with aquaculture output accounting for a portion of that total 24.05 lakh MT, or 56.24 percent, of the total production. Bangladesh has emerged as one of the world's leading fish producers, having achieved fish self-sufficiency for the first time in 2014. Bangladesh came in third place, behind China and India, in the FAO's 2013–2014 assessment on inland water body fish output (FAO, 2016). Over the past ten years, this sector has experienced average growth of 5.26 percent. It's a significant accomplishment for the nation. Such success is the product of the current government's persistent efforts for the nation's fishing industry. The government will place a high priority on the preservation of jatka (little hilsa), expanding shrimp farming, and safeguarding natural fish breeding areas and the tolerant gathering of marine fish (DoF, 2018)
There are 475 species of finfish in Bangladesh's coastal waters, offering a variety of fishing opportunities (Mazid, 2005). Just 100 fish species—among them the hilsa, pomfret, chanda, tuna, marine catfish, marine eel, jawfish, ribbonfish, bombay duck, etc.—are significant from a commercial standpoint (Quader, 2010). Bangladesh's economy is significantly influenced by its maritime fisheries. More than 11% of Bangladesh's population makes their living either directly or indirectly from this industry (Fisheries Statistical Yearbook of Bangladesh, 2013). With a growth rate of 2.71%, the overall production of marine fisheries is 6.55 lakh MT, contributing 15.31% to the total production of fish (DoF, 2018). Currently, the marine fisheries industry makes up over 18% of the nation's total fish production. Bangladesh ranked 11th in the world and produced 1,13,200 tons of fish from marine and coastal sources, according to the FAO data (FAO, 2016). Concerning the preservation, conservation, and biodiversity of marine and coastal resources, Prime Minister "Seikh Hasina" likewise places the highest importance. She established sanctuaries on Saint Martin Island and the Sundarbans, a well-known mangrove forest, with the goal of preserving and enhancing the region's biodiversity and fisheries. In order to safeguard the maritime flora and fauna's breeding grounds and boost marine fish output, the government has also established a marine reserve spanning 698 square kilometers in the Bay of Bengal (DoF, 2018).
Because fish and fisheries products have a high protein content and little to no carbohydrate and fat value, they are valuable not just nutritionally but also as a source of foreign exchange for many countries worldwide. Bangladesh's export earnings are mostly derived from the fisheries and aquaculture sectors, which account for 2.7% of export earnings, 3.74% of the country's GDP, and 22.23% of the agricultural sector (FAO, 2018). Bangladesh's marine fish exports are growing daily as a result of the large global market, which includes the USA, UK, Japan, Belgium, Netherlands, Thailand, Germany, China, France, Canada, Spain, and Italy. Bangladesh has 129 fish processing industries, but only 62 of those facilities are approved by the EU. For fish to be accepted in international trade and to prevent consumer health issues, it is crucial to maintain and monitor fish production and quality (DoF, 2011).
Bangladesh's national fish is the hilsa ilisha. The saying "Hilsa is the king of fishes" is said to exist. The most significant open-water single-species fishery in Bangladesh is hilsa, which is found in almost all of the country's main river systems, estuaries, and oceans. Hilsa accounts for 12.09 percent of the nation's total fish production. From 1.99 lakh MT in 2003–04 to 5.17 lakh MT in 2017–04, the total production of hilsa rose.
The common name for Harpodon nehereus is "Bombay duck." In the vicinity of Bangladesh's shore, this fish is referred to as "Lotiya Machh" or "Litta" locally (Sarker et al., 2017). One of the most popular and easily accessible estuary fish along the Chattogram coast is the bombay duck, which is found in shallow inshore water. It is the most palatable fish and offers customers an excellent amount of nutrition. Approximately 1.78% of the nation's total fish production comes from the bombay duck. About 75085 MT of Bombay duck fish are produced annually in the marine fisheries sector (DoF, 2018). A significant amount of Bombay duck is turned into rope-dried products, and dried Bombay duck is important for trade in south and south-east Asia, claim Rahman and Chowdhury (1988).
The muddy bottom along seashores is home to Pampus chinensis, sometimes known as pomfret. They are highly sought-after in the market and are considered to be pretty tasteful. Locals refer to this fish as "chanda" or "rupchanda" fish. The Rup, Fali, Hali, and Parastromateus niger are the prevalent species found in the Bay of Bengal. A sizeable amount of the total fishery resources gathered from the Bay of Bengal are contributed by the promfrets. Pampus chinensis accounts for about 0.28% of Bangladesh's total fish production. About 11899 MT of Pampus chinensis fish are produced annually in the maritime sector (DoF, 2018).
The marine ecosystem is currently continuously contaminated by chemical contaminants originating from both lithogenic and anthropogenic sources. Unsettling quantities of chemicals and heavy metals in aquatic habitats have been the consequence of anthropogenic causes such as shipping, urbanization, agriculture, and industrial activities during the past few decades (Rahman and Chowdhury, 1988). Crude oil spills can happen from tankers, offshore platforms, drilling rigs, wells, and refinery releases. They can also happen from refined petroleum products (such diesel, gasoline, and other products) and their byproducts, as well as from heavy fuel used by huge ships. An oil spill into water has a negative impact on the environment and its components. Fish are the marine animal species that are most unable to escape the negative impacts of these pollutants. The absence of natural metal removal systems exacerbates this disease. As a result, metals move throughout the aquatic environment, often having negative impacts, from one compartment to another, including the biota. Toxins are concentrated in the organs of marine life, including fish, and over time, these toxins can build up and ultimately cause the creatures' deaths (Bhattacharya et al., 1994). The gills, skin, kidney, digestive system, muscle, and liver of fish are the organs that absorb heavy metals (Annabi et al., 2013). One major cause of food contamination and a health risk is heavy metals. Arsenic, cadmium, lead, mercury, and chromium exposure pose the greatest risks to human health. It has been demonstrated that consuming food containing higher levels of heavy metals than is advised can have detrimental effects on health, including kidney disease, neurological damage, reduced cognitive function, heart disease, gastrointestinal disorders, bone fractures, cancer, and death (Flora et al., 2008).
With an estimated population of about 8.5 million, Chattogram is a commercial hub and the industrial nerve center of Bangladesh. However, because of nearby activities, the Chattogram coastline is susceptible to high levels of heavy metal pollution. The Chattogram coast of the Bay of Bengal is home to a diverse range of businesses, each of which releases a unique spectrum of effluents into the terrestrial and aquatic ecosystems that contain heavy metals and other dangerous pollutants (Manwar, 2001). The fact that fish larvae and juveniles develop in the near-shore zone is more significant.
Fish are among the indicators found in bodies of water, especially when it comes to identifying the deadly risks resulting from human activity. There are five primary pathways by which heavy metals can enter fish: through food, non-food particles, gills, oral water ingestion, and the skin. Following absorption, the pollution hazard is transported via the bloodstream to the liver, where it undergoes processing and/or storage. According to Nussey et al. (2000), pollutants that are changed in the liver can either be kept there, eliminated in bile, transferred to other excretory organs for elimination, like the kidneys or gills, or stored in fat, which is an additional type of hepatic tissue. A marine fish's internal organs get various amounts of heavy metals depending on the chemical composition of their surroundings. The choice of aquatic biota is influenced by a number of variables, including the organism's capacity to accumulate heavy metals, motility, and economic worth. Because of their large size, which makes them clearly identifiable, their capacity to accumulate metals, their long lifespan, ease of sample collection, and their ideal size for analysis, fish are frequently used as biota. Fish can therefore be used as sentinel species and bio-monitors of heavy metal pollution and water quality since they provide information about the risks to both humans and the aquatic ecosystem (Authman et al., 2015).
The objective of this investigation was to evaluate the concentrations of specific heavy metals in various fish organs obtained from the Chattogram coastal region in Bangladesh. The significance of fish contamination and the dangerous toxic effects of these heavy metals on public health were examined.
Materials and Methods
Study Areas
The samples (three individuals of each three species) were collected from the caught fishes by fishermen’s nets from the Chattogram coast of Bay of Bengal and some were collected from local market at three different times. Then the collected fish species were transported to the laboratory of Bangladesh Council for Science and Industrial Research, Chittagong Campus. Fish samples were stored in plastic bags at -20º C until dissection. The total length (cm) and weight (g) of fishes was measured in every case.
Dissection and Preparation for Digestion
Each of the collected fishes was dissected for its muscle, gill, liver and kidney tissues. All the final sample preparation was carried out according to the procedure described by UNEP Reference Methods (1984). Heavy Metal Analysis: Heavy metals (As, Pb and Cr) were analyzed in a graphite furnace (GBCGF 3000 with Zeeman background corrector) with an auto sampler. Apparatus A) Atomic Absorption Spectrophotometer (AAS) (Phillips AAS with double beam and deuterium background corrector). B) Hollow cathode or electrodeless discharge lamps. C) Microwave oven. D) Teflon digestion vessels 100ml, withstanding a pressure of at least 1.4 MPa. E) Volumetric flask. F) Funnels. G) Plastic bottles. H) Drying oven. Reagents A) Deionized water. B) Nitric acid 65% (w/w). C) Perchloric acid 30% (w/w). Procedure A) Pre-treatment: For analyzing metals, fish organs were preserved with 10% formalin solution after dissection. B) Drying: Fish organs were then dried in drying oven at 105°c to constant weight. The various organs of each species collected were pooled and milled with a mortar and pestle. They were put in dry labeled plastic containers and stored in desiccator until digestion. C) Digestion: 0.75 g dry fish organs are weighted into digestion vessel. Then 5 ml 65% HNO3 and 2ml 30% perchloric acid were added. Vessels were then closed and placed in holder. Vessel holder then placed in digestion chamber and exposed to defined program parameters 250 watt for 3 min, 630 watts for 5 min, 500 watts for 22 min and final 0 watts for 15 min. Then removed digestion vessels from digestion chamber and cooled thoroughly before opening them. Vessels were then opened transferred to 25 volumetric flask and dilute to mark with deionized water. Then solution transferred to plastic tube. D) AAS determination. The concentration of Pb, Cr and as were determined by flame techniques in AAS. E) Calibration curves: A calibration curve is used to determine the unknown concentration of an element. The instrument is calibrated using several solutions of known concentrations. A calibration curve is produced which is continually rescaled as more concentrated solutions are used the more concentrated solutions absorb more radiation up to a certain absorbance. The calibration curve shows the concentration against the amount of radiation absorbed. Calibration curves were plotted for each of metal standard solution. When sample solution fed into the instrument and the unknown concentration of the element then displayed on the calibration curve. All digested samples were analyzed three times for each metal. The instrument was calibrated with standard solutions prepared from commercial materials. Analytical blanks were run in the same way as the samples and determined using standard solutions prepared in the same acid matrix.
Data Analysis
Statistical analysis of data was carried out using SPSS statistical package program. One-way analysis of variance (ANOVA) and Duncan multiple range tests were used to assess whether metal concentrations varied significantly among species & organs. The comparative accumulation of Pb, Cr and as in each species was demonstrated by using Microsoft Excel software. A significance threshold of 5% was applied.
Results
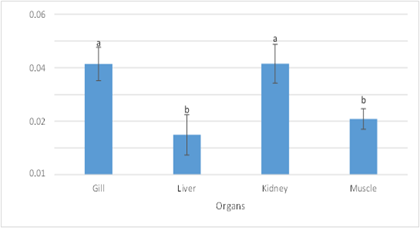
The heavy metal concentrations in three different fish species Herpodon nehereus, Pampus chinensis, and Hilsa ilisha are presented in the Figure 1. Of the three fish, the kidney had the highest estimated lead content (0.041467), while the gills had the second-best value (0.041422). Liver (0.014878 ppm) and muscle (0.020822 ppm) measured values were substantially varied from the two organs described above (Fig 1).
Figure 1: Lead Concentration in Fish Organs and Muscle
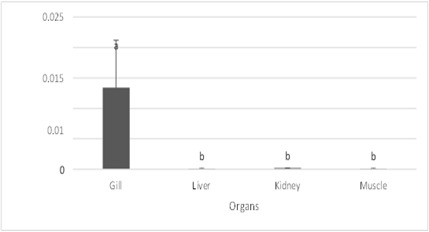
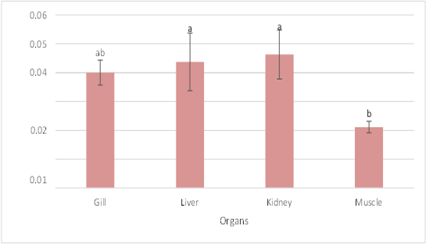
The gills had the highest concentration of chromium, measuring 0.013422 ppm, whereas the liver, kidneys, and muscles had far lower concentrations, averaging 0.000089 ppm which, when compared to Gill, are statistically substantially different at 0.000167 ppm and 0.000089 ppm, respectively. Kidney had the highest concentration of arsenic (0.046256 ppm), with liver having the lowest concentration (0.043722 ppm) statistically. The recorded value for the gills is in the middle of the range statistically when compared to the above three organs (0.040033 ppm), while the lowest value found in the muscle (0.021133 ppm) differs significantly from the previous two (Fig 2).
Figure 2: Chromium Concentration in Fish Organs and Muscle
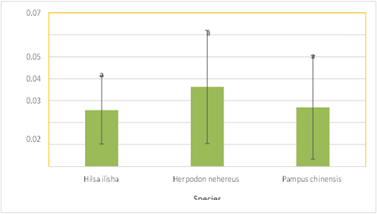
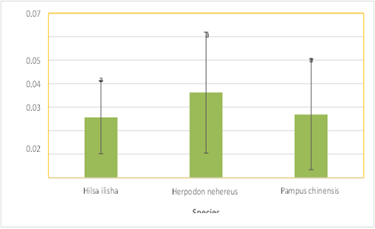
The results showed that Herpodon nehereus had the highest lead readings (0.036250 ppm), followed by Pampus chinensis (0.026983 ppm) and Hilsa ilisha (0.025708 ppm), in that order. There was no statistically significant variation in the acquired data set. It was discovered that the concentration of chromium in various fish species was less than the values of lead, with Herpodon nehereus exhibiting the highest concentration (0.004725 ppm). The readings for the other two species, Hilsa ilisha and Pampus chinensis, respectively, were 0.001225 ppm and 0.004375 ppm, and they did not differ substantially (Fig 3).
Figure 3: ArsenicConcentration in Fish Organs and Muscle.
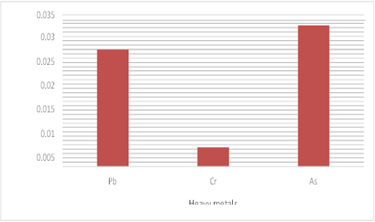
Additionally, Herpodon nehereus has the highest measured concentration of arsenic (0.049258 ppm), followed by Pampus chinensis (0.032567 ppm) and Hilsa ilisha (0.031533 ppm). It was discovered that arsenic was more concentrated than lead and chromium. According to the recorded readings in Hilsa Ilisha, arsenic has a greater concentration above the recommended value of 0.01ppm (WHO/ FAO,2005). The mean value of as is 0.031533ppm. Of the three heavy metals under investigation, chromium had the lowest mean average value (0.001225ppm), which is significantly less than the standard value (0.1ppm) of chromium (WHO/ FAO,2005). The observed average lead concentration of 0.025708ppm is likewise less than the WHO/FAO guideline threshold of 0.3ppm (2005).
Figure 4: Lead Concentration in Different Marine Fish Species.
According to the Herpodon nehereus recorded data, arsenic (As) has a greater concentration than the recommended value of 0.1 ppm, with a mean value of 0.049258 ppm. Of the three heavy elements under investigation, chromium had the lowest mean average value (0.004725 ppm). Lead was found to have an average value of 0.031533 ppm.
The levels of Pb and Cr are below the WHO/FAO standard limit of 0.3 ppm and 0.1 ppm, respectively, published in 2005. Different Heavy Metal Concentrations in Pampus chinensis: The data showed that arsenic (As) had the highest concentration in Pampus chinensis, with a mean value of 0.032567 ppm, greater above the advised value of 0.01 ppm. Of the three heavy metals under investigation, chromium had the lowest mean average value (0.004375 ppm), much below the recommended level of 0.1 ppm. The detected average lead concentration of 0.032567 ppm is likewise less than the standard value (Fig 4) of 0.3 ppm provided by WHO/FAO (2005).
Figure 5: Different Heavy Metals Concentration in Pampus chinensis.
Of the three heavy metals examined, arsenic was found to be more prevalent in each of the three species. The findings showed that it is urgent to take the required action since the levels of arsenic in all three fish cases were greater than the permissible levels for human consumption. Lead and chromium reported values are within acceptable bounds.
Figure 6: Lead Concentration in Different Marine Fish Species.
The kidney tissues contain the majority of the heavy metals within the organ. Even while the amount of arsenic in muscles was discovered to be more than the safety limits, the concentration in muscles is the lowest, which is a good discovery because we eat muscles on a regular basis.
The distribution of As, Cr, and Pb in the gills, liver, muscle, and kidney of several fish species, including Herpodon nehereus, Pampus chinensis, and Hilsa ilisha, that were caught off the Chattogram coast of the Bay of Bengal, was usefully shown by this study. These fish species were chosen because they are the most popular and significant marine fish for Bangladeshi commerce. Because they are frequently harmful elements found in industrial discharges and have complex biological reactions even at low concentrations, Cr, Pb, and as were selected. The correlations between the heavy metal concentrations in Hilsa ilisha, Pampus chinensis, and Herpodon nehereus were also examined in this study. Regarding heavy metals (Pb, As, and Cr), it was found that there was no statistically significant variation for any of the three fish species (Table 1).
Table 1: Heavy Metal Concentrations in Different Fish Species
| Species | Lead(Pb) | Chromium (Cr) | Arsenic (As) |
| Hilsa ilisha | 0.025708a | 0.001225a | 0.031533a |
| Herpodon nehereus | 0.036250a | 0.004725a | 0.049258a |
| Pampus chinensis | 0.026983a | 0.004375a | 0.032567a |
| WHO limit | 0.3 ppm | 0.1 ppm | 0.01 ppm |
A statistical analysis was done to see how the quantities of heavy metals in various fish species' organs correlated. In several fish species' organs, the presence of three heavy metals differed statistically (Table 2).
Table 2: Heavy Metal Concentration in Different Fish Organs
| Organs | Lead(Pb) | Chromium (Cr) | Arsenic (As) |
| Gill | 0.041422a | 0.013422a | 0.040033ab |
| Liver | 0.014878b | 0.000089b | 0.043722a |
| Kidney | 0.041467a | 0.000167b | 0.046256a |
| Muscle | 0.020822b | 0.000089b | 0.021133b |
Discussion
In coastal water environments, it has been shown that both essential and non-essential metals accumulate along the trophic chain. Although non-essential metals have no metabolic use, they can be hazardous to humans even at very low amounts due to their bioaccumulation in fish (Anwar et al., 2009). The concentration of heavy metals in fish is significant for managing the environment as well as for human consumption. The analysis results for the metal concentration ranges in fish samples showed the following: As: 0.031533-0.049258 ppm; Pb: 0.025708–0.036250 ppm; and Cr: 0.001225–0.004725 ppm. The following order of decline in heavy metal concentrations was observed in Herpodon nehereus, Pampus chinensis, and Hilsa ilisha: As> Pb> Cr. There were differences in heavy metal concentrations both within and across species. We discovered that, of the various heavy metal concentrations that were detected, the concentration of arsenic was significantly higher in various experimental fishes than in any other value, whereas the concentration of Cr was lowest across all experimental fishes (Table 1). The work of (Minareci et al., 2009) was being contradicted by this finding.
In muscle tissue of fish species gathered from the northeast coast of India, the concentrations of Cu, Zn, Mn, Fe, Cr, Pb, and as were measured by Bhupander et al. (2012). They discovered that Arius sp. and Harpadon nehereus (0.91 µg g-1) had higher concentrations of arsenic buildup. Formio niger (0.93 µg g-1) and 1.22 µg g-1. Nonetheless, Hilsa ilisa collected lower quantities (0.05 µg g-1) which is comparable to our investigation. There's a chance that the Chattogram coastline is seriously arsenic contaminated. A large industrial and siderurgic plant close to the coast may be the source of its effluent discharges, and agro-industrial activities—primarily runoff from phosphate fertilizer-treated agricultural soils and other waste—may also be linked to it. In most cases, the maximum allowed quantities of arsenic in fish tissues surpassed the WHO and FAO guideline, indicating poor water quality in the Chattogram coastal area. Three fish species have an average muscle concentration of 0.021133, which is greater than the WHO/FAO guideline standards for arsenic (Figure 5). If arsenic concentrations are high enough, it poses a serious threat to life and is unknown to play any role in biological processes. Fish absorb arsenic from their GI tract and then it enters their circulation after being consumed. According to Kakkar and Jaffery (2005), arsenic quickly leaves the circulation and travels to other tissues.
The high as concentrations in the kidney and liver, at 0.046256 ppm and 0.043722 ppm, respectively, were another significant finding of this investigation. It was discovered that the amounts of arsenic in the kidney and liver were significantly different from those in the gills and muscles. The eating habits of fish species that consume decomposed organic waste may have something to do with this. Fish are typically exposed to high levels of pollution because they have the ability to develop detoxifying mechanisms that sequester pollutants, rendering the metal comparatively inert and non-toxic to the organism (Weis, 1989). The kidney had the greatest estimated lead concentration (0.041467 ppm) out of the three fish, while the gill had the second-best result (0.01422 ppm). Figure 3 shows a substantial difference between the reported values in the kidney and gill (0.014878 ppm) and the muscle (0.020822 ppm). In the human body, lead replaces calcium in the bones as it builds up (DHSS, 1980). Oza and Muralidharan (2018) discovered that the lead levels in gill exceeded the uppermost allowable limits and liver tissues of the monsoon-season fish Harpodon nehereus in the west coast of India. It was also discovered that, in all the chosen tissues, it exceeded the FAO's permissible limits for human consumption during the pre-monsoon season. Higher lead concentrations were found in O's gills than in its muscles, according to a study by Kebede and Wondimu (2004). Nilotinus. Because of their physiological and anatomical characteristics that optimize water absorption efficiency, fish kidneys are thought to be the target site for contaminant uptake. The present study's results contradict the findings of Farombi et al. (2007), who stated that the liver had a higher lead content than the gills and kidney.
The concentration of chromium in the gills of all three marine fish species was found to be the greatest (0.013422 ppm), whereas the concentration in the liver, kidneys, and muscles was found to be the lowest (0.000089 ppm, 0.000167 ppm, and 0.000089 ppm, respectively), and these results were statistically distinct from the concentration in the gills. Fish generally store chromium in their gills, liver, kidney, and bones, according to an MSDS (2006) report. A significant factor in the overall metal levels of the gill may also be the adsorption of metals onto the gill surface (Canli and Furness, 1993). This observation is consistent with the findings of Amundsen et al. (1997) regarding freshwater fish species found in the border region between Russia and Norway.
The information in Table 2 demonstrates that, among the many tissues of marine fish, the kidney had the highest amounts of metals in the following order: kidney > liver > gill > muscle. The kidney's overall mean concentration of three metals (measured in mg/kg on a wet weight basis) was 0.031 ppm, while muscular tissues had values of only 0.0140 ppm. The findings of Taghavi Jelodar et al. (2011) were contradictory. They discovered that the mean concentrations of Cr and Pb in the muscle of Liza aurata from the southern Caspian Sea were, respectively, 1070 and 2600 µg/kg, greater than those in other organs. In another study conducted by Hossein Khezri et al. (2014) in the Persian Gulf close to the Iranian coast, Cr and Pb concentrations in the fish muscle were found to range between 36-264–1188 g/kg and 107 g/kg, respectively. As the portion of the fish that is edible, muscles frequently have the lowest quantities (Chen et al., 2004; Alcorlo et al., 2006). This has particular significance for human health. Goel (1996) states that heavy metals are absorbed by the digestive system and accumulate there. Food, dust, and water, therefore the kidneys may contain a high concentration. even though a lot of species can withstand elevated levels of necessary or non-essential metals by storing them in inactive locations like fish gut, scales, gills, bone, or exoskeleton.
Arsenic accumulation in the liver was found to be high in Herpodon nehereus (0.271991 ppm), whereas the kidney revealed high concentrations in Pampus chinensis and Hilsa ilisha (0.249 ppm and 0.02243 ppm) (Appendices E and G). The liver tissue of fishes is known to undergo significant metallothionein induction. According to a paper, the liver of Oreochromis niloticus synthesizes metallothionein, a protein that binds to metals, when exposed to heavy metals. In fish species, the liver and kidneys have the greatest quantities of most metals or metalloids that have been investigated to date (Cheung et al., 2004).
The physiological functions of each organ in a fish can be used to explain variations in the levels of accumulation in those organs. The accumulation of differences in various organs may be influenced by a number of factors, including behavior, ecological requirements, swimming habits, and regulatory ability among different fish species (Kalay et al., 1999). Additionally, a key factor in the accumulation process is typically the chemical makeup of the metals, including their pH and ionic strength. Since there isn't much room left to bind heavy metals in acidic environments, more heavy metals stay in the soluble phase. Hydrogen ions are sufficient to fill many of the negatively charged surfaces. According to Ishaq et al. (2011), the soluble form of metals is assumed to be more harmful due to its ease of transportation and increased availability to aquatic organisms.
Herpodon nehereus was found to exhibit the highest metal accumulation value when compared to the other two species (Table 1). Hilsa ilisha had the lowest concentration of the three heavy metals, while Herpodon nehereus had the highest level. The amounts of heavy metals were reduced in the following order: Hilsa ilisha > Pampus chinensis > Herpodon nehereus. Due to their higher trophic level within the food chain, Herpodon nehereus may have higher body concentrations of heavy metals due to bioaccumulation. Goel (1996) states that the bioaccumulation process of the heavy metal is attributed to the fish's intake of the metal through their diet. The meal is broken down, absorbed, and eliminated, but the heavy metal builds up in the organs of the aquatic animals. Because the assimilation efficiency of each trophic level is limited to 10%, in order to gain the same weight, a higher trophic level must consume ten times as much as its immediately preceding trophic level. When food moves from one trophic level to another, this causes the concentration of heavy metals to increase tenfold.
Arsenic (As) concentrations were greater in Hilsa Ilisha, with a mean value of 0.031533 ppm, than the recommended value of 0.01 ppm (WHO/FAO, 2005). The lead average recorded was 0.025708 ppm, which is likewise less than the WHO/FAO guideline value of 0.3 ppm (2005). According to a prior study (Chen et al., 2004), the liver has a greater arsenic level than the muscles. Since fish muscle does not come into close touch with arsenic and does not actively participate in detoxifying. According to research on fish toxicity, arsenic accumulates more in the gills since it is absorbed mostly by them during the aqueous phase (Ventura-Lima et al., 2009). While minimal amounts of chromium are essential for bodily functions such as metabolism and insulin cofactor, excessive amounts of the mineral can be harmful. An adult's daily chromium requirement is thought to be between 0.02 and 0.5 mg. According to WHO/FAO (2005), fish can have up to 0.1 parts per million of chromium. Of the three heavy metals under investigation, the mean average value of chromium in Hilsa ilisha was the lowest at 0.01225 ppm, well below the allowable limit (Table 1).
The readings found in Herpodon nehereus indicate that the concentration of arsenic (As) is higher than the acceptable level. Compared to the standard value of chromium, the mean average value of chromium was the lowest of the three heavy metals under investigation. Additionally, the average lead value was less than the benchmark value. The sample Pampus chinensis was found to have a mean as concentration of 0.032567 ppm, which is greater than the recommended level. Compared to the standard value of chromium, the mean average value of chromium was the lowest of the three heavy metals under investigation. Lead levels were found to be on average 0.032567 ppm (Table 1). The heavy metal accumulation pattern in the gills in this investigation was pb>As>Cr, however As>Pb>Cr was found in the liver, muscle, and kidney (Table 2). Lead concentrations in the kidney and gills differed markedly from those in the liver and muscle (Figure 3). There is no discernible difference between the kidney, liver, and gills for arsenic (Figure 5).
This study discovered that the amount of arsenic that accumulated in several fish organs was greater than the usual reference values advised by the FAO and WHO. The non-edible sections of the fish body contained high amounts. However, fish development may be hampered by this threatened concentration of metals. Because early life stages, such as hatching time, larval development, and juvenile growth, are more vulnerable than mature stages, fish development may be hampered by the presence of heavy metals in water (Weis and Weis 1989; Friedmann et al., 1996). Friedmann and colleagues (1996) demonstrated that juvenile walleye and Stizostedion vitreum development was hindered by even low levels of As. Furthermore, according to Weis and Weis (1989), both necessary and non-essential metals may affect fish embryonic development, resulting in abnormal development retardation, organ impairment, or even death. To gain a better understanding of the impact of metals on fish development and the current state of population dynamics, it is necessary to periodically evaluate fish growth and its correlation with metal concentration in the aquatic environment in the field.
Conclusion
Many fish species, including Herpodon nehereus, Pampus chinensis, and Hilsa ilisha, may be found in the Bay of Bengal, which is thought to have a high level of biodiversity. However, because shipbreaking yards and industrial effluents discharge large amounts of wastewater, the impact of wastes thrown into the Bengal Sea has increased recently. The Chattogram region is impacted by a multitude of heavy metals due to its extensive exposure along the Bay of Bengal coast. Because heavy metal concentrations tend to bioaccumulate rather than disintegrate during food preparation, they are especially harmful to human health. Consuming meals high in heavy metals over an extended period of time might have detrimental effects on one's health. The cumulative effect of these heavy metals on consumers can cause concerns even in the case of their presence in modest concentrations. Because the edible portion of fish, or muscle, is not severely contaminated with metal and most of the concentration is below the standard limits set by the WHO and FAO, eating fish from the Chattogram coast of the southern Bay of Bengal may not be detrimental, according to the study's findings. In contrast, the concentration of chromium measured in all the tissues of the species under study was generally lower than the levels published by WHO/FAO. Arsenic, on the other hand, was found to be higher in all fish tissues than the certified level, raising concerns for fish accumulation. The results of this investigation led to the conclusion that Herpodon nehereus, Pampus chinensis, and Hilsa ilisha had the largest accumulation of heavy metals (As and Pb) in their fish kidneys. The three fish species under investigation had the maximum accumulation of Cr in their gills, which is less than the normal amount of Cr. Current studies have already shown that lead concentrations are approaching safety thresholds and that arsenic concentrations are well above them. Therefore, both government agencies and responsible non-governmental individuals and groups must take immediate safety measures and enforce stringent regulations on heavy metal discharge that is environmentally beneficial. Considering the significance of fish in the human diet, regular biological monitoring of fish and water is required to guarantee the safety of consuming fish and seafood.
Recommendations
The following suggestions should be taken into account even if all of the heavy metal concentration study results indicated that eating the three fish species on a regular basis did not have a particularly negative impact on human health:
To better characterize the heavy metal quality of fish species, more thorough sampling and analysis may be conducted, including sampling of marine fish species of various ages and heights from various Chattogram district regions.
A thorough evaluation program should be carried out throughout the Chattogram coast in order to determine the main causes of pollution in the fishing harbor and marine waters.
Similar research might be conducted to track the presence of other harmful heavy metals in commercially significant fish species, coastal water, and sediment, such as cadmium, mercury, cobalt, zinc, copper, and manganese.
Given that Herpodon nehereus from the Chattogram coastal area had the greatest percentage of arsenic, it is imperative to assess the environmental quality of that area and avoid the untreated discharge of waste water into the sea.
Monitoring for commercial fish in Chattogram strip markets is advised on a regular basis to make sure that metal concentrations stay within the acceptable global limits.
To ascertain the bio-accumulation of heavy metals in fish from the study area, further thorough research is required.
An essential and fundamental component of regulatory procedures is the research on the health risks connected to consumers' dietary consumption of excess heavy metals. To fully comprehend the harmful consequences of heavy metals on the human body, research of this kind must be done.
In order to maintain the unspoiled condition of the Chattogram fishing harbor, it is crucial to constantly analyze the amounts of heavy metals, hydrocarbons, and pesticides.
References
- Abouel-Naga EH, El-Moselhy KH and Hamid MA (2005). Toxicity of cadmium and copper and their effect on some biochemical parameters of marine fish Mugil seheli. Egyptian Journal of Aquatic Research, 31:60-71.
Publisher | Google Scholor - Adepoju-Bello AA and Alabi OM (2005). Heavy metals: A review. The Nigerian Journal of Pharmaceuticals, 37:41-45.
Publisher | Google Scholor - Agency for Toxic Substances and Disease Registry (ATSDR) (2000). Toxicological Profile for Arsenic TP-92/09. Georgia: Center for Disease Control, Atlanta.
Publisher | Google Scholor - Ajani OI and Magiera Y (2007). Determinants of an effective solid waste management in Ibadan Metropolis, Oyo state, Nigeria. Journal of Food Agriculture and Environment, 6:152-157.
Publisher | Google Scholor - Alcorlo P, Otero M, Crehuet M, Baltana` SA and Montes C (2006). The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailibility of heavy metals in environmental monitoring in the river Guadiamar (SW, Spain). Science of the Total Environment, 366:380-390.
Publisher | Google Scholor - Allen T, Singhal R and Rana SVS (2004). Resistance to oxidative stress in a freshwater fish Channa punctatus after exposure to inorganic arsenic. Biological Trace Element Research, 98:63-72.
Publisher | Google Scholor - Al-Mohanna MM (1994). Residues of some heavy metals in fishes collected from (Red Sea Coast) Jisan, Saudi Arabia. Journal of Environmental Biology, 15(1):149-157.
Publisher | Google Scholor - Amundsen PA, Staldvik FJ, Lukin AA, Kashulin NA et.al. (1997). Heavy metal contamination in freshwater fish from the border region between Norway and Russia. The Science of the Total Environment, 201(1):211-224.
Publisher | Google Scholor - Annabi A, Said K, Messaoudi I (2013). Cadmium: bioaccumulation, histopathology and detoxifying mechanisms in fish. American Journal of Research Communication, 1(1):60-79.
Publisher | Google Scholor - Anwar MA, Elbekai HR and El-Kadi AO (2009). Regulation of CYP1A1 by heavy metals and consequences for drug metabolism. Expert Opinion on Drug Metabolism and Toxicology, 5(1):501-521.
Publisher | Google Scholor - Astawan M, Ikan Y (2004). Sedap dan bergizi. Penerbit Tiga Serangkai. Solo, 1-7.
Publisher | Google Scholor - Authman MMN, Zaki MS, Khallaf EA, Abbas HH (2015). Use of Fish as Bio-indicator of theEffects of Heavy Metals Pollution. Journal of Aquatic Resource Development, 6:328.
Publisher | Google Scholor - Barbier O, Jacquillet G, Tauc M, Cougnon M and Poujeol P (2005). Effect of heavy metals on, and handling by, the kidney. Nephron Physiology, 99(4):105-110.
Publisher | Google Scholor - Bashir FA and Alhemmali EM (2015). In The Second Symposium on Theories and Applications of Basic and Biosciences. September Misurata, Libya, 5:21-33.
Publisher | Google Scholor - Bhattacharya B, Sarkar SK and Maji PK. (1994). Bioaccumulation of heavy metals in flora and fauna of Hooglily Estuary, East coast of India. Toxicological and Environmental Chemistry, 42:123-130.
Publisher | Google Scholor - Bhupander KS, Sajwan DP and Mukherjee S (2012). Distribution of Heavy Metals in Valuable Coastal Fishes from North East Coast of India. Turkish Journal of Fisheries and Aquatic Sciences, 12:81-88.
Publisher | Google Scholor - Bolis CL, Cambria A and Fama M (1984). Effects of Acid Stress on Fish Gills. In: Toxins, Drugs and Pollutants in Marine Mammals. Springer Verlag, Berlin,122-129.
Publisher | Google Scholor - Bryan GW and Langston WJ (1992). Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environmental Pollution, 76:89-131.
Publisher | Google Scholor - Bukola D, Zaid A, Olalekan EI and Falilu A (2015). Consequences of Anthropogenic Activities on Fish and the Aquatic Environment. Poultry, Fisheries and Wildlife Science, 3(1):138-145.
Publisher | Google Scholor - Burton DT, Jones AH and Cairns J (1972). Acute zinc toxicity of rainbow trout (salmo gairdneri). Confirmation of the hypothesis that death is related to tissue hypoxia. Journal of Fisheries Research Board of Canada, 29:143-146.
Publisher | Google Scholor - Camusso M, Vigano L and Baitstrini R (1995). Bioaccumulation of trace metals in rainbow trout. Ecotoxicology and Environmental Safety, 31:133-141.
Publisher | Google Scholor - Canli M and Furness RW (1993). Heavy metals in tissues of the Norway lobster Nephrops norvegicus: effects of sex, size and season. Chemistry and Ecology, 8:19-32.
Publisher | Google Scholor - Carpene E and Vasak M (1989). Hepatic metallothioneins from oldfish (Carassius auratus L.) Comparative Biochemistry and Physiology, 92:463-468.
Publisher | Google Scholor - Censi P, Spoto SE, Saiano F, Sprovieri M, Mazzola S. et.al. (2006). Heavy metals in coastal water systems. A case study from the northwestern Gulf of Thailand. Chemosphere, 64:1167-1176.
Publisher | Google Scholor - Chakraborty S, Rudra T, Guha A, Ray A, Pal N and Mitra A (2016). Spatial variation of heavy metals in Tenualosa ilisha muscle: A case study from the lower Gangetic delta and coastal West Bengal. International Journal of Innovative Science, Engineering & Technology.
Publisher | Google Scholor - Chen BC and Liao CM (2004). Farmed tilapia Oreochromis mossambicus involved in transport and bio-uptake of arsenic in aquacultural ecosystems. Aquaculture, 242:365-380.
Publisher | Google Scholor - Cheung APL, Lam THJ and Chan KM (2004). Regulation of Tilapia metallothionein gene expression by heavy metal ions. Marine Environmental Research, 58:389-394.
Publisher | Google Scholor - Chilvers DC and Peterson PJ (2009). Global cycling of arsenic. In T.C. Hutchinson and K.M. Meema, eds., Lead, Mercury, Cadmium and Arsenic in the Environment. John Wiley & Sons, New York, NY, USA, 279-303.
Publisher | Google Scholor - Chowdhury KR, Alam SM, Shainsuddin SD, Shakhawat H, and Haque S (1994). A Preliminary Assessment of Water and Sediment Pollution Load Along the Coasts of Chittagong and Cox’s Bazar, The Journal of NOAMI, 11:2.
Publisher | Google Scholor - Csuros M and Csuros C (2002). Environmental Sampling and Analysis for Metals. Lewis Publishers, Boca Raton, FL, USA.
Publisher | Google Scholor - Cutter GA and Cutter LS (1995). Behavior of dissolved antimony, arsenic, and selenium in the Atlantic Ocean. Marine Chemistry, 49:295-306.
Publisher | Google Scholor - Denton GRW, Wood HR, Concepcion LP, Siegrist HG, Eflin VS, et.al. (1997). Analysis of in-place contaminants in marine Sediments from four harbor locations on Guam. A pilot study: water and environmental research institute of the western pacific, Technical Report No. 87. University of Guam, Mangilao, Guam.
Publisher | Google Scholor - Department of Water Affair and Forestry (DWAF) (1996). Water Quality Guidelines, Aquatic Ecosystem Use. DWAF, Pretoria, 7 (1).
Publisher | Google Scholor - DoE (1997). Survey on Ship Breaking Industries and Water Quality in Chittagong Region (unpublished), Dhaka, Bangladesh.
Publisher | Google Scholor - Dof (2018). Yearbook of Fisheries Statistics of Bangladesh, 2017-18. Fisheries Resources Survey System (FRSS), Department of Fisheries. Bangladesh: Ministry of Fisheries, 35:(129).
Publisher | Google Scholor - Duffus JH (2002). Heavy metals-a meaningless term? Pure Applied Chemistry, 74(5):793-807.
Publisher | Google Scholor - Edmonds JS and KA Francesconi (1993). Arsenic in seafoods: Human health aspects and regulations. Marine Pollution Bulletin, 26:665-674.
Publisher | Google Scholor - Eisler R (1998). Copper hazards to fish, wildlife and invertebrates: A synoptic review. Biological Science Report, 33(100).
Publisher | Google Scholor - ESCAP (1988). Coastal environment management plan for Bangladesh. Bangkok, Thailand, 2(149).
Publisher | Google Scholor - FAO (1999). World production of fish, crustaceans and mollusks by major fishing areas. Fisheries Information Data and Statistics Unit (FIDI), Fisheries Department, FAO, Rome, Italy, 33.
Publisher | Google Scholor - FAO (2016). The State of World Fisheries and Aquaculture. Contributing to food security and nutrition for all. Rome, 200.
Publisher | Google Scholor - FAO (2018). The State of World Fisheries and Aquaculture Meeting the sustainable development goals. Licence: CC BY-NC-SA 3.0 IGO. Rome.
Publisher | Google Scholor - FAO/WHO (2005). World Health Organization Provisional Tolerable Weekly Intake of Toxic Heavy Metals. (JECFA 1956-2003), (First Through Sixty First Meetings). ILSI Press International Life Sciences Institute.
Publisher | Google Scholor - Farombi EO, Adelowo OA, Ajimoko YR (2007). Biomarkers of Oxidative Stress and Heavy Metal Levels as Indicators of Environmental Pollution in African Cat Fish (Clarias gariepinus) from Nigeria Ogun River. International Journal of Environmental Research on Public Health, 4(2):158-165.
Publisher | Google Scholor - Fei L, Zhang J, Zhu L and Chaoyang L (2010). Heavy metals and metalloid distribution in different organs and health risk assessment for edible tissues of fishes captured from Honghu Lake. Toxicology and Industrial Health, 26(1):649.
Publisher | Google Scholor - Fergusson JE (1990). editor. The Heavy Elements: Chemistry, Environmental Impact and Health Effects. Oxford: Pergamon Press.
Publisher | Google Scholor - FIDH (2002). Investigative Mission, where do the floating dustbins' end up? Labour rights in Shipbreaking Yards in South Asia: the cases of Chittagong (Bangladesh) and Alang (India), Report no. 348(2).
Publisher | Google Scholor - Fisheries Statistical Yearbook of Bangladesh (2013). Fisheries Resources Survey System (FRSS), Department of Fisheries, Bangladesh.
Publisher | Google Scholor - Flora SJS, Mittal M and Mehta A (2008). Heavy metal induced oxidative stress and its reversal by chelation therapy. Indonesian Journal of Medical Research,128:501-523.
Publisher | Google Scholor - Frenzilli G, Nigro M and Lyons BP (2009). The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mutation Research, 681(1):80-92.
Publisher | Google Scholor - Friedmann AS, Watzin MC, Brinck-Johnsen T, Leiter JC (1996). Low levels of dietary methylmercury inhibit growth and gonadal development in juvenile walleye (Stizostedion vitreum). Aquatic Toxicology, 35:265-278.
Publisher | Google Scholor - Gadzała-Kopciuch R, Berecka B, Bartoszewicz J and Buszewski B (2004). Some Considerations about Bioindicators in Environmental Monitoring. Journal of Environmental Studies, 13(5):453-462.
Publisher | Google Scholor - Giguere A, Campbell PGC, Hare L, mcdonald DG and Rasmussen JB (2004). Influence of lake chemistry and fish age on cadmium, copper, and zinc concentrations in various organs of indigenous yellow perch (Perca flavescens). Canadian Journal of Fisheries and Aquaic Science, 61:1702-1716.
Publisher | Google Scholor - Gjedrem T, Robinson N and Rye M (2012). The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture, 350:117-129.
Publisher | Google Scholor - Goel PK (1996). Water pollution: causes, effects and control, New Age International (P) Limited, New Delhi, India, 97-115.
Publisher | Google Scholor - Goyer RA and Clarkson TW (2001). Toxic effects of metals, Ch. Cassarett and Doull’s Toxicology, mcgraw-Hill Companie Inc, 23:811-867.
Publisher | Google Scholor - Hilles HA, Al HIA and Abu SAY (2014). Assessment of parasitic pollution in the coastal seawater of Gaza city. Journal of Environmental Health Science & Engineering, 12:26-28.
Publisher | Google Scholor - Hossain MM, Akanda MMR, Islam MR, Rashid MH, Iqbal MM (2010). Biodiversity of Fish Fauna and Fishing Operations of Katar Beel in Fulbaria Upazilla of Mymensingh District. Eco- Friendly Agriculture journal, 3(11):480-486.
Publisher | Google Scholor - Hossein Khezri P, Takhsha M, Aein Jamshid K And Aghshenas A (2014). Assessment level of heavy metals (Pb, Cd, Hg) in four fish species of Persian Gulf (bushehriran). International Journal of Advanced Technology & Engineering Research, 4(2):7-11.
Publisher | Google Scholor - International Agency for Research on Cancer (1987). Arsenic and Arsenic Compounds (Group 1). IARC Monograph. Lyon, France, 23(7):100-106.
Publisher | Google Scholor - Ishaq SE, shaíato R and Annune PA (2011). Bioaccumulation of heavy metals in fish (Tilapia Zilli and Clarias Gariepinus) organs from river Benue, North Central Nigeria. Pakistan Journal of Analytical and Environmental Chemistry, 12(1):225-31.
Publisher | Google Scholor - Jahan E, Nessa A, Hossain MF and Parveen ZP (2016). Characteristics of municipal landfill leachate and its impact on surrounding agricultural land. Bangladesh journal of Scientific Research, 29(1):31-39.
Publisher | Google Scholor - Jerry M (1997). Neff battelle ocean sciences laboratory, 397 washington street, duxbury, massachusetts 02332, USA ecotoxicology of arsenic in the marine environment. Environmental Toxicology and Chemistry, 16(5):917-927.
Publisher | Google Scholor - Jezierska B and Witeska M (2001). Metal Toxicity to Fish, Wydawnictwo Akademii Podlaskiej, Siedlce 318.
Publisher | Google Scholor - Jin-Ling L, Xiang-Rong X, Zhen-Hua D, Jia-Xi P, Ming-Hua J et.al. (2015). Heavy metals in wild marine fish from South China Sea: levels, tissue and species-specific accumulation and potential risk to humans. Ecotoxicology, 24(7):512-522.
Publisher | Google Scholor - Joseph B, Raj SJ, Edwin BT, Sankarganesh P, Jeevitha MV, Ajisha SU and Sheeja SR (2010). Toxic effect of heavy metals on aquatic environment. International Journal of Biological and Chemical Science, 4(1): 939-952.
Publisher | Google Scholor - Jothi JS, Anka IJ, Hashem S and Morshed S (2018). Assessment of heavy metal concentration in edible fish muscle and water sample collected from different location in Chittagong: a public health concern. Ukrainian Food Journal, 7(3).
Publisher | Google Scholor - Kakkar P and Jaffery FN (2005). Biological markers for metal toxicity. Journal of Environmental Toxicology and Pharmacology, 19:335-349.
Publisher | Google Scholor - Kakuschke A, Valentine-Thon E, Griesel S, Fonfara S and Prange A (2005). Immunological impact of metals in harbor seals (Phoca vitulina) of the North Sea. Environmental Science and Technology, 39:7568-7575.
Publisher | Google Scholor - Kalay M, Ay O, Canli M (1999). Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bulletin of Environmental Contamination and Toxicology, 63:673-681.
Publisher | Google Scholor - Kebede A and Wondimu T (2004). Distribution of trace elements in muscle and organs of Tilapia, Oreochromis niloticus from Lakes Awassa and Ziway. Bulletin of Environmental Contamination and Toxicology, 18:119-130.
Publisher | Google Scholor - Khalid AA, Abdelatty M, Abdelfattah L and Mahboob S (2015). Differential Uptake of Heavy Metals by Gill, Muscles and Liver of Four Selected Fish Species from Red Sea. Pakistan Journal of Zoology, 47(4):1031-1036.
Publisher | Google Scholor - Kock G, Triendl M and Hofer R (1998). Lead (Pb) in Arctic char (Salvelinus alpinus) from oligotrophic Alpine lakes: Gills versus digestive tract, Water Air Soil Pollution Journal, 102:303-312.
Publisher | Google Scholor - Köhler A (1991). Lysosomal perturbations in fish liver as indicators for toxic effects of environmental pollution. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 100(1):123-127.
Publisher | Google Scholor - Kris-Etherton P, Harris W and Appel L (2002). Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation, 106:2747-2757
Publisher | Google Scholor - Krishna PV, Rao KM, Swaruparani V and Rao DS (2014). Heavy metal concentrations in Fish Mugil cephalus from Machilipatnam Coast and possiible health risks to fish consumers. British Biotechnology Journal, 4(2):126-135.
Publisher | Google Scholor - Mahboob S, Kausar S, Jabeen F, Sultana S, et.al. (2016). Effect of Heavy Metals on Liver, Kidney, Gills and Muscles of Cyprinus carpio and Wallago attu inhabited in the Indus. Brazilian Archives of Biology and Technology, 59.
Publisher | Google Scholor - Mance G (1987). Pollution Threat of Heavy Metals in Aquatic Environments. Elsevier Applied Science Publishers Ltd, New York, USA.
Publisher | Google Scholor - Markert B (1994). Biomonitoring - Quo vadis. UWSF-Z. Umweltchem. Okotox, 6(3):145- 149.
Publisher | Google Scholor - Mazid MA (2005). Manual on culture of small and threatened indigenous fish species. Bangladesh Fisheries Research Institute, Department of Fisheries, Bangladesh Agricultural University, and Ministry of Fisheries and Livestock.
Publisher | Google Scholor - Minareci E, Oztrurk M and Ozozen G (2009). Determination of heavy metals in fish, water and sediments of avsar dam lake in turkey. Iranian Journal of Environmental and Health Science, 6:73-80.
Publisher | Google Scholor - Monwar M (2001). Flow and discharge characteristics in the Karnaphuli River Estuary. In- term Research Paper. Institute of Marine Sciences, Chittagong University, Bangladesh.
Publisher | Google Scholor - MSDS (2006). The MSDS hyperglossary heavy metal. Available online.
Publisher | Google Scholor - Mukherjee DP and Kumar B (2011). Assessment of arsenic, cadmium and mercury level in commonly consumed coastal fishes from Bay of Bengal, India. Food Science and Quality Management, 2224-6088
Publisher | Google Scholor - Myers MS, Rhodes LD, mccain BB (1987). Pathologic anatomy and patterns of occurrence of hepatic neoplasms, putative preneoplastic lesions, and other idiopathic hepatic conditions in English sole (Parophrys vetulus) from Puget Sound, Washington. Journal of the National Cancer Institute, 78(2):333-363.
Publisher | Google Scholor - Namminga HN and Wilhm J (1976). Effects of high discharge and an oil refinery cleanup operation bon heavy metals in water and sediments in Skeleton Creek. Proceedings of the Oklahoma Academy of Science, 56:133-138.
Publisher | Google Scholor - Needleman HL (1987). Low level lead exposure and children's intelligence: A quantitative and critical review of modern studies. Proc. 6th International Conference on heavy metals in the environment, New Orlean. CEP Consultants Ltd, Edinburg,1-8.
Publisher | Google Scholor - NIFES (2016). The National Institute of Nutrition and Seafood Research. Undesirable Substances in Seafood.
Publisher | Google Scholor - Nussey G, van Vuren JHJ, du Preez HH (2000). Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank dam, Mpumalanga. Water Science, 26:269-284.
Publisher | Google Scholor - Oza A and Muralidharan L (2018). Seasonal study on bioaccumulation of heavy metal, lead in various tissues of fish, harpodon nehereus collected from sassoon dock, mumbai coast of maharshtra, India. Internation Journal of Advance Research in Science and Engineering, 7(4):265-272.
Publisher | Google Scholor - Pempkowiak J, Sikora A, Biernacka E (1999). Speciation of heavy metals in marine sediments vs their bioaccumulation by mussels. Chemosphere, 39:313-321.
Publisher | Google Scholor - Quader O (2010). Coastal and marine biodiversity of Bangladesh (Bay of Bengal). Procedings of International Conference on Environmental Aspects of Bangladesh (ICEAB10), Japan.83-85.
Publisher | Google Scholor - Rahman A and Chowdhury N (1988). Chittagong: The endangered port city. The Weekly Bichitra,17(27).
Publisher | Google Scholor - Rahman MM, Rahman F, Afroz F, Yesmin F, Fatema KK et.al. (2012). Prevalence of pathogenic bacteria in shrimp amples collected from hatchery, local markets and the shrimp processing plant for export quality frozen shrimp. Bangladesh Journal of Microbiology (accepted).
Publisher | Google Scholor - Rashed MN (2004). Biomarkers as indicators as indicators for water pollution in rivers and oceans. Environment International, 27-33.
Publisher | Google Scholor - Reid SD and mcdonald DG (1991). Metal binding activity of the gills of the rainbow trout (Onchorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Science, 48:1061-1068.
Publisher | Google Scholor - Salam MA, Paul SC, Noor SNBM, Siddiqua SA, Aka TD. et.al. (2019). Contamination profile of heavy metals in marine fish and shellfish. Global Journal of Environmental Science and Management, 5(2):225-236.
Publisher | Google Scholor - Sarker MN, NaserMN, Uddin MS, Das NN and Humayun M (2017). Population dynamics of bombay duck, harpodon nehereus of the bay of bengal along bangladesh coast. Bangladesh journal of Zoology, 45(2):101-110.
Publisher | Google Scholor - Sarker MN, NaserMN, Uddin MS, Das NN and Humayun M (2019). On the management of single fish species of hilsa shad (tenualosa ilisha) resources of bangladesh. Bangladesh journal of Zoology, 47(1):173-183.
Publisher | Google Scholor - Sioen I, Henauw S, Verdonck F, Thuyne N, Van Camp J (2007). Development of a nutrient database and distributions for use in a probabilistic risk-benefit analysis of human seafood consumption. Journal of Food Composition and Analysis, 20:66-670.
Publisher | Google Scholor - Smith DG (1986). Heavy Metals in the New Zealand Aquatic Environment: A Review, Water Quality Centre, Ministry of Works and Development, Wellington, New Zealand.
Publisher | Google Scholor - Spicer JI and Weber RE (1991). Respiratory impairment in crustaceans and molluscs due to exposure to heavy metals. Comparative Biochemistry and Physiology, part C, 100:339-342.
Publisher | Google Scholor - Storelli MM, Storelli A, d’ddabbo R, Marano C, Bruno R and Marcotrigiano GO (2005). Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: Overview and evaluation. Environmental Pollution, 135:163-170.
Publisher | Google Scholor - Sujatha K, Durairajsinghu V and Silambarasan K (2016). Determination of some heavy metals in fish, water and sediments from bay of bengal. Pollution research paper, 35(4):797-799.
Publisher | Google Scholor - Taghavi Jelodar H, Sharifzadeh Baei M, Najafpour SH and Fazli H (2011). The comparison of heavy metals concentrations in different organs of Liza aurata inhabiting in southern part of Caspian Sea. World Applied Sciences Journal, 14:96-100.
Publisher | Google Scholor - Tchounwou PB, Centeno JA, Patlolla AK (2004). Arsenic toxicity, mutagenesis and carcinogenesis - a health risk assessment and management approach. Molecular and Cellular Biochemistry, 255:47-55.
Publisher | Google Scholor - Tinwell H, Stephens SC and Ashley J (1991). Arsenite as the probable active species in the human carcinogenicity of arsenic: Mouse micronucleus assays on Na and K arsenite, orpiment, and Fowler’s solution. Environmental Health Perspectives, 95:205-210.
Publisher | Google Scholor - UNEP (1984). Sampling of selected marine organisms and sample preparation for trace metal analysis: Reference Method for Marine Pollution Studies.
Publisher | Google Scholor - United Nation Environment Programme and European Environment Agency (UNEP/ EEA), (1999). State and Pressures of the Marine and Coastal Mediterranean Environment. Environmental issues series.
Publisher | Google Scholor - Ventura-Lima J, de Castro MR, Acosta D, Fattorini D, Regoli F, de Carvalho LM et.al. (2009). Effects of arsenic (As) exposure on the antioxidant status of gills of the zebrafish Danio rerio (Cyprinidae). Comparative Biochemistry, Physiology, Toxicology and Pharmacology, 149:538-543.
Publisher | Google Scholor - Weis JS, Weis P (1989). Effects of environmental pollutants on early fish development. Reviews in Aquatic Sciences, 1:45-73
Publisher | Google Scholor - Wong CK, Wong PPK and Chu LM (2001). Heavy metal concentrations in marine fishes collected from fish culture sites in Hong Kong. Archives of Environmental Contamination and Toxicology, 40(1):60-69.
Publisher | Google Scholor - Younis A, Amin H, Alkaladi A, and Mosleh Y (2015). Bioaccumulation of heavy metals in fish, squids and crustaceans from the Red Sea, Jeddah Coast, Saudi Arabia. Open Journal of Marine Science, 5:369-378.
Publisher | Google Scholor