Research Article
Antiplasmodial Activity of Aqueous Leaf Extracts of Mangifera Indica Against Plasmodium Berghei Infected Albino Rat
- Adamu Adamu Ahmed 1
- Ismail Muhammad 2*
- Muhammad Fatima Yakubu 3
- Hauwa’u Abdulkadir Usman 4
- Bala Abubakar 5
- Gilbert Eshun 6,7
- Olalekan John Okesanya 8,9
1Department of Biological Science, Federal University of Kashere, Nigeria.
2Department of Zoology, Gombe State University, Gombe, Nigeria.
3Federal College of Education (Tech) Gombe Department of Biology Education, Nigeria.
4Federal College of Horticultural Technology Dadin-kowa, Department of Science Laboratory Technology, Nigeria.
5School of Health Science and Technology Kaltingo, Gombe State, Department of Community Health, Nigeria.
6Seventh Day Adventist Hospital, Agona-Asamang, Ghana.
7The Royal (Dick) School of Veterinary Studies, University of Edinburgh, United Kingdom.
8Department of Medical Laboratory Science, Neuropsychiatric Hospital, Aro, Abeokuta, Nigeria.
9Department of Public Health and Maritime Transport, University of Thessaly, Volos, Greece.
*Corresponding Author: Ismail Muhammad, Department of Zoology, Gombe State University, Gombe, Nigeria.
Citation: Ahmed A.A., Muhammad I., Yakubu M.F., Usman H.A., et al. (2024). Antiplasmodial Activity of Aqueous Leaf Extracts of Mangifera Indica Against Plasmodium Berghei Infected Albino Rat. Journal of BioMed Research and Reports, BioRes Scientia Publishers. 5(6):1-9. DOI: 10.59657/2837-4681.brs.24.117
Copyright: © 2024 Ismail Muhammad, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 11, 2024 | Accepted: December 02, 2024 | Published: December 12, 2024
Abstract
Introduction: For years, malaria parasites, specifically Plasmodium falciparum, have developed resistance to all recommended drugs, including the most recently recommended artemisin. Hence, there is a need to explore other alternatives. Therefore, this study aimed to evaluate the anti-plasmodial activity of aqueous leaf extracts of Mangifera indica (Mango) against Plasmodium berghei-infected albino rats.
Methodology: The identified leaves were washed with distilled water and dried under shade at optimal ventilation for one week, after which it was crushed into labelled powder using electric blender. One hundred and fifty grams (150g) of the sample was socked in 2 liters of distilled water for the extraction of the phytochemical components of the leaves and the doses of the extract to be administered were determined according to the body weight of the experimental animals. For the oral acute toxicity test, five (5) albino rats were treated with 2000mg/kg of the extract. Twenty-four (24) albino rats were inoculated with the prepared inoculum of Plasmodium berghei 0.2ml (1 × 107 parasitized RBC). On the fourth day, the rats were randomly divided into 6 groups of four (4) rats each and labelled A-F. Group served as positive control and treated with 10mg/kg/day of Coartem, group B served as negative control received 10ml/kg/day of distilled water and group C, D, E and F were treated with 100mg/kg, 200mg/kg, 400 mg/kg and 800mg/kg body weight of the aqueous extract respectively.
Results: Alkaloids, Tannins, Caponins, Cardiac glycosides, Steroids and Terpenoids and Phenols were active phytochemicals recorded, while Flavonoids and reducing sugars were absent. The parasitaemia density for the negative control group progressively increased from 26.01±0.1 on the 4th day post inoculation to 67.7 ± 3.3 on 7th day post treatment. The mean percentage parasitaemia of the groups treated with different plant extract concentrations exhibited significant anti-malarial activities when compared with negative control group. The highest parasitaemia reduction was seen in Coatem followed by 800mg/kg of the aqueous extract on the 7th day. Changes of the body weight of all the infected rats in all groups were observed between day 1 and 4, and later improved from day 5 to day 7 in a dose-dependent manner.
Conclusion: Leaf extracts of Mangifera indica are effective for the treatment of malaria infection. It should use in the of malaria infection in a dose-regulated manner.
Keywords: malaria; mangifera indica; plasmodium berghei; plasmodium falciparum; gombe
Introduction
Malaria is a life-threatening parasitic disease that remains endemic in most countries of the tropics [1]. The disease is caused by five Plasmodium species (P. vivax, P. malariae, P. ovale, P. knowlesi, and P. falcifarum) vectored by female anopheles’ mosquitoes, in which the parasites are transmitted during blood meals [2, 3]. The disease is one of the major causes of poverty in most developing countries due to disease treatment, as most of the commonly recommended drugs (artemisinin-based combination therapies) are quite expensive, thus negatively affecting economic development [4]. Pregnant women and children under the age of five are most vulnerable to the disease, mainly due to weak immunity. The symptoms of the disease range from high fever to headaches, drowsiness and confusion, which occur as the parasites spread and invade the erythrocytes of their host. In the case of severe malaria, some other vital organs like the brain and kidney may be affected, leading to their failure [5].
Almost half of the global population (3.4 billion) is at risk of the disease. Globally, a total of 249 million clinical cases of the disease were recorded in 2022 from 85 malaria countries, with Nigeria ranking third after Pakistan and Ethiopia, contributing 1.3 million cases (WHO, 2023); this translates to one-quarter of the total malaria burden in Africa. In terms of mortality, Nigeria contributed 23% of the total malaria deaths globally [6]. Effective and timely surveillance, early case management using recommended drugs, and vector control serve as the mainstays for the control and eradication of the disease [7]. One of the major setbacks to the control, prevention and elimination of the disease is the development of resistance by the parasite [8, 9] and the vector to all recommended synthetic drugs and insecticides, respectively. This is mainly due to the indiscriminate and long period of their usage. This has seriously led to a decrease in the therapeutic weapons for the prevention and treatment of the disease, hence the dire need for alternative drugs for the prevention and treatment of the disease.
For a long time, different plant part’s extracts have been effectively used in the treatment of so many infectious diseases [10], including malaria. Around 80% of the drugs are derived from plants [11] and specifically quite several plants are known to have antimalarial properties; in fact, the first antimalarial drug used in the occident was extracted from the bark of the Cinchona (Rubiaceae) species [12, 13] and artemisinin, obtained from Artemisia annua [14]. Different parts (root, stem bark, leaves) of Mangifera indica have been reported to possess not just antimicrobial potential but also other medicinal properties [15][16], as they contain most of the major known phytochemicals [17] such as alkaloids, phenols, flavonoids, saponins, and tannins in the leaf and stem bark of the plant. Additionally, by using GC-MS analysis, the presence of hydrocarbons, alcohols/ethers, aldehydes/ketones, carboxylic acids, fatty acids, phenols, and terpenes/sesquiterpenes was found in mango stem bark extracts [18]. Therefore, this research aimed to assess the antiplasmodial activity of aqueous leaf extracts of Mangifera indica in Plasmodium berghei-infected albino rats.
Methodology
Study Area
The research was conducted in Gombe State, Nigeria, which is located between latitudes 100 081 N and 110 241 E and longitudes 110 021 N and 110 181E, with a land mass area of about 45km2. The climate of Gombe is characterized by a dry season lasting six months, alternating with a six-month rainy season. The mean annual precipitation is 835 mm and the mean annual temperature is 26°C whereas relative humidity has the same pattern, being 94% in August and dropping to less than 10% during the harmattan period (Mbaya et al., 2012). The local government has 11 primary health care centres, one secondary health facility (Gombe State Specialist Hospital) and one tertiary hospital (Federal Teaching Hospital, Gombe), all of which provide different services concerning malaria diagnosis, prevention and treatment.
Plant Material Collection and Identification
The leaves of the mango (Mangifera indica) plant were collected from the premises near the local government secretariat, Dukku Local Government, Gombe State and identified in the herbarium unit of the Botany Department, Gombe State University, with voucher number (GSUH46).
Figure 1: The Map of the Study Area
Preparation of the plant material
Fresh leaves of the plant were gently washed with distilled water and dried under shade at optimal ventilation for one week. The dried leaves were crushed into powder using an electric blender, labelled and kept in a well-closed amber-coloured bottle at room temperature. One hundred and fifty grams (150g) of the sample was socked in 2 litres of distilled water and left at room temperature for seven days. The mixture was shaken thoroughly at six-hour intervals using an orbital shaker (KJ-201BS) daily to ensure that the solvent penetrated the sample. The mixture was then filtered using Whatman No. 1 filter paper, and then the filtrate was concentrated under vacuum in a rotatory evaporator at a temperature of 40oC, which yielded a gummy residue as extracts of the leaves. The extract was kept in a tightly labelled specimen bottle in a refrigerator at 40C until required for anti-malarial screening.
Phytochemical Screening of Mangifera indica Leaf Extract
The prepared aqueous extract of Mangifera indica leaves was screened for the presence of active phytochemicals such as Saponins, Reducing sugars, Tannins, Cyanogenic glycosides, Phenols, Terpenoids, Flavonoids and Alkaloids, according to the method of Sofowora (1993).
Determination of Concentrations of Mangifera indica Leaf Extract for the In-Vivo Study
The volume or doses of the extract administered were calculated according to the dose and body weight of the experimental animals using the formula below.

Acute Oral Toxicity Test
Female non-infected, nulliparous and non-pregnant albino rats of age between 8-12 weeks were used for acute oral toxicity study. The oral toxicity study was conducted according to the internationally accepted protocol drawn up by the Organization for Economic Co-operation and Development (OECD) (2008). Ten (10) female rats were used for this study and they were observed to ensure that they were physically active and regularly consumed food and water. Thereafter, all the animals were fasted (food but not water) for 4 hours before and 2 hours after the administration of the extract. The rats were divided into two groups (the treatment group and the control group) of five rats each. A rat from the treatment was given a single dose of 2,000mg/kg of body weight of the extract by oral gavage using a suitable intubation cannula and then observed continuously for the first 30 minutes after administration of the extract, intermittently for 4 hours, and over 24 hours. When no death was observed in the first rat, the remaining four rats were used and administered the same dose (2000 mg/kg) of the extract, while distilled water was administered to the control group. All rats were observed after the treatment to monitor the signs of toxic manifestations like loss of appetite, hair erection, lacrimation, diarrhoea, tremors, seizures, mortality and other toxic effects for 14 days after administration of the extract.
Histopathological examination
The livers of two rats from each group were each cut to 0.5 mm thick on slabs using a microtome and fixed in 10% formalin at room temperature for 24–48 hours. Following fixation, they were transferred to 70% alcohol for dehydration. The tissues were each passed through 90% alcohol and chloroform for 10 minutes before being transferred into two changes of molten paraffin wax for 20 minutes each in an oven at 570C. Serial sections of 5μm thick were done using a microtome from solid block tissue. They were then stained separately with hematoxylin and eosin stains, after which they were passed through a mixture of equal concentrations of xylene and alcohol. Following clearance in xylene, the tissues were each oven-dried. Photomicrographs were taken with a coloured digital camera mounted on an Olympus light microscope.
Malarial parasite and Inoculum preparation
Five mature infected albino mice (with Plasmodium berghei) weighing between 20-25g, were obtained from the Institute of Medical Research, Yaba, Lagos State, Nigeria. They were stabilized for three days in the laboratory. Three of the five donor mice with a rising parasitemia level were sacrificed, and their blood was collected in a heparinized syringe. The percentage of parasitemia was determined by counting the number of parasitized red blood cells against the total number of red blood cells. Thereafter, 0.2ml of the blood samples from the infected mice were taken and subsequently diluted in 0.8ml of sterile normal saline, whose parasitemia level was approximately 30%. Therefore, the 0.2 ml of the final inoculum contained approximately 1 × 107 parasitized red blood cells, which is the standard inoculum for the infection of a single rat.
Experimental Animals
Another 35 Albino rats, comprising both sexes and free from infection, were also obtained from the animal house of the College of Medical Sciences of Gombe State University. All experimental rats were maintained in a well-ventilated room with an average temperature of 25 ± 1°C. They were fed with Excel feeds (Feed Masters) and watered daily throughout the experiment.
Antiplasmodial Activity (Curative Test)
Twenty-four (24) albino rats weighing between 70 and 120g were inoculated with the prepared inoculum of Plasmodium berghei by using a needle to pass 0.2ml of the inoculum via an intraperitoneal route. according to the method of Peter and Anatoli (1998) and left for 72 hours after infection for the development of optimum parasitemia. After which, they were screened for malarial parasites using a thin blood film fixed with absolute methanol and stained with a 10% Giemsa solution. The slides were allowed to air-dry at room temperature, after which five fields were randomly selected on each stained slide and examined under a microscope with an oil immersion and objective (×100 magnification power). The number of parasitized red blood cells (RBCs) was counted under a microscope, and the percentage of parasitemia was thereafter determined according to the method of [13].
On the fourth day, the rats were randomly divided into 6 groups of four (4) rats each and labelled A-F. Group A received 10mg/kg/day of Coartem, a standard antimalarial drug (positive control group), group B also received 10ml/kg/day of distilled water (negative control group); and the remaining four treatment groups (C, D, E and F) were treated with 100mg/kg, 200mg/kg, 400 mg/kg and 800mg/kg body weight of the aqueous extract, respectively. The doses were administered orally once daily for three consecutive days. Thin blood films were prepared from the tail blood of each rat and stained with 10% Giemsa solution daily for 3 consecutive days to monitor the levels of parasitemia (Mulaw et al., 2019)].

The percentage activity (deparasitation) was determined by subtracting the percentage parasitemia from 100.
Percentage activity = 100 – (% Parasitaemia)
Determination of Body Weight in Albino Rats
The body weight of each rat was taken before infection (day 1), before treatment (day 4) and after completing the treatment (day 7). The average percentage change in body weight was compared with the control groups. The mean body weight was calculated using the following formula, adapted from the work of Gebreyohannes and Yalemtsehay (2018).

Data Analysis
All data generated were entered into SPSS software (Statistical Package for Social Sciences) version 26 (SPSS, IL, USA). The mean parasitemia and body weight were analyzed using descriptive statistics. The results were expressed as the mean ± standard deviation (SD).
Result
Phytochemical Analysis of Leaf Extract of Mangifera indica
The qualitative phytochemical study of the aqueous leaf extract of Mangifera indica was found to contain most of the active phytochemicals as presented in Table 1. Alkaloids, tannins, saponins, cardiac glycosides, steroids, terpenoids and phenols were present, while flavonoids and reducing sugars were absent.
Table 1: Result of Qualitative Phytochemical Analysis of Mangifera indica Leaf Extract
| Test | Inference |
| Alkaloids | Present |
| Saponins | Present |
| Flavonoids | Absent |
| Steroids | Present |
| Tannins | Present |
| Reducing sugars | Absent |
| Phenols | Present |
| Terpenoids | Present |
| Cardiac glycosides | Present |
Acute Toxicity Study
The acute oral toxicity test revealed that, in the first hour to 24 hours of the treatment, all extract-treated groups exhibited no gross physical and behavioural changes, like vomiting, loss of appetite, excitement, sleep, diarrhoea, abnormal secretion, or hair erection. In addition, all rats survived during the 14-day observational period. It was also recorded that altered feeding was observed, where food and water intake increased. There was a significant increase in the mean body weight of the extract-treated rats from 70.0±10g before the treatment to 80.5±7.5g after the period.
Histopathological analysis of Liver section of Albino rats
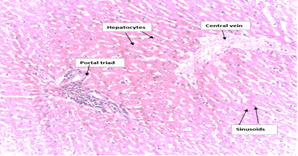
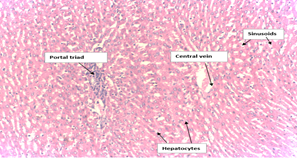
The histological examinations of the liver sections of the extract-treated rats showed normal morphology (Plate I), where hepatocyte plates, sinusoids, portal triads and congested central veins appeared normal when compared to the histology of the negative control (non-treated rats) (Plate II). Therefore, the results from the oral toxicity evaluation indicated that there was virtually no toxicity effect caused by the extract at the maximum single oral dose of 2000mg/kg body weight (acute toxicity).
Plate I: Histopathological Examination Control.
Plate II: Histopathological Examination of the Liver of the Treated Rat
Antiplasmodial activity (Curative test) of the leaf extract of Mangifera indica on Plasmodium berghei-Albino rats
The result of the treatment with different concentrations of Mangifera indica leaf extracts on the level of parasitemia in rats is presented in Table 2. The parasitaemia density for the negative control group progressively increased for the 3 days, showing the mean number of parasitized red cells as 26.01±0.1 on the 4th day post-inoculation to 67.7 ± 3.3 on the 7th day post-treatment. The mean percentage parasitaemia of the groups treated with different concentrations (100 mg/kg, 200 mg/kg, 400 mg/kg, and 800mg/kg) of the plant extract exhibited significant anti-malarial activities when compared with a negative control group. The highest parasitemia reduction was seen in Coatem (positive control group), followed by 800mg/kg of aqueous extract of Mangifera indica on the 7th day. The 10mg/kg dose of coartem showed a significant reduction of parasitemia from 28.01 ± 0.1 on the 4th day post-inoculation to 0.9 ± 0.2 on the 7th day post-treatment. The parasitemia density for the treated groups progressively decreased for the 3 days, showing the mean number of the percentage parasitized red cells as 26.0 ± 0.5, 27.0 ± 1.1, 26.02 ± 1.0, and 27.0 ± 1.1 on the 4th day post inoculation to 22.2 ± 1.1, 17.7 ± 0.9, 12.3 ± 0.5, and 9.9 ± 0.4 by the 7th day post-treatment, respectively. The comparison analysis revealed that all doses of the extract showed statistically significant differences (p less than 0.05) on the 7th day post-treatment compared to that of the negative control group.
Table 2: Mean percentage parasitaemia and deparasitization in Albino rats treated with different concentrations of leaf extract of Mangifera indica
| Group | Day 4 | Day 5 | Day 6 | Day 7 | % Change |
| Positive Control | 28.01 ± 0.1 | 17.1 ± 0.8 | 9.6 ± 0.4 | 0.9 ± 0.2 | - 27.1 ± 0.7 |
| Negative control | 26.01 ± 1.0 | 36.2 ± 0.6 | 46.8 ± 2.1 | 67.7 ± 3.3 | 40.7 ± 3.4 |
| 100mg/kg MI | 26.0 ± 0.5 | 26.3 ± 0.5 | 24.8 ± 0.7 | 22.2 ± 1.1 | - 3.8 ± 0.9 |
| 200mg/kg MI | 27.0 ± 1.1 | 24.1 ± 0.3 | 20.9 ± 0.9 | 17.7 ± 0.9 | - 9.3 ± 0.6 |
| 400mg/kg MI | 26.02 ± 1.0 | 22.4 ± 0.5 | 17.02 ± 0.9 | 12.3 ± 0.5 | -13.7 ± o.2 |
| 800mg/kg MI | 27.0 ± 1.1 | 20.9 ± 0.3 | 14.7 ± 0.7 | 9.9 ± 0.4 | -17.1 ± 0.4 |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Notes: Data are expressed as mean percentage ± SD. P less than 0.05; Key: Mangifera indica (MI), Standard Deviation (SD)
The effects of the Aqueous Extract of Mangifera indica on the body weight of Albino Rats
The changes in body weight of all the infected rats in all groups were observed between days 1 and 4. The highest degree of weight reduction from day 1 to day 4 in all dose levels were observed. The body weight of the rats in the treated groups began to improve from day 5 to day 7 in a dose-dependent manner. However, treatment with the aqueous leaf extract of Mangifera indica significantly prevented the loss of body weight from day 6 compared to the body weights on day 4 at all dose levels. The results also revealed that the positive control group and the extract-treated groups at all dose levels significantly prevented the loss of body weight when compared to that of the negative control group. On day 7, the percentage change in body weight increased in a dose-dependent manner (Table 3) when compared between the groups.
Table 3: Effect of Mangifera indica leaf extract on body weight (g) of the Albino rats
| Group | Day 1 | Day 4 | Day 7 | Change gain |
| Positive Control | 89.3 ± 13.4 | 79.7 ± 8.6 | 88.0 ± 5.3 | 8.3 ± 4.8 |
| Negative Control | 100.0 ± 7.2 | 93.5 ± 12.2 | 88.5 ± 13.9 | -5.0 ± 4.7 |
| 100mg/kg MI | 102.1 ± 8.2 | 95.8 ± 17.1 | 94.5 ± 17.1 | -1.3 ± 0.5 |
| 200mg/kg MI | 99.5 ± 14.2 | 88.7 ± 7.4 | 89.8 ± 6.7 | 1.0 ± 0.8 |
| 400mg/kg MI | 95.0 ± 6.7 | 89.5 ± 5.8 | 91.5 ± 5.6 | 2.0 ± 0.7 |
| 800mg/kg MI | 100. 2 ± 6.6 | 96.18 ± 6.2 | 99.17 ± 6.2 | 2.9 ± 0.3 |
| P-value | 0.001 | 0.001 | 0.002 | 0.000 |
percentage ± SD. P<0>Key: Mangifera indica (MI), Standard Deviation (SD).
Discussion
In the present study, the anti-plasmodial activity of aqueous leaf extracts of Mangifera indica against Plasmodium Berghei infected albino rats was evaluated. Phytochemical analysis of Mangifera indica leaf revealed the presence of alkaloids, saponins, steroids, tannins, phenols, terpenoids and cardiac glycosides. The phytochemical constituents of Manginefea indica reported in this study are similar to the findings of [27, 28], who reported the same constituent and some others that were not reported in this study. For example, reducing sugars and flavonoids was not reported in this study, which is in agreement with the findings of [29, 30]. The absence of these two phytochemicals (reducing sugars and flavonoids) could be partly attributed to the geographical location of the plant, the extraction method of the extract adopted, and also the solvent used, as explained by [31].
An acute oral toxicity test revealed that the experimental animals can withstand and effectively survive concentrations as high as 2000mg/kg of body weight of the extract. This finding is similar to the findings of [32], who reported LD50 greater than 5000 mg/kg and [33], who also reported oral acute toxicity in mice up to a dose of 5,000 mg/kg body weight. To further confirm the result of the acute oral toxicity test, histological examinations of the liver sections of rats treated with 2000mg/kg body weight of the extract were carried out, and both the treated and the control showed normal morphology. This demonstrated that the selected concentration can be regarded as practically non-toxic, as it did not cause any morphological or physiological changes in the experimental animal and also fell within the acceptable margin of safety
The anti-plasmodial activity of the leaf extract of Mangifera indica on Plasmodium berghei-albino rats demonstrated a high level of efficacy compared to the negative control. A significant decrease in parasitized red blood cells observed in all the treated groups (100 mg/kg, 200 mg/kg, 400 mg/kg, and 800mg/kg) in a dose-dependent manner could be attributed to the presence of the active component contained in the extract, which is known to possess anti-plasmodial activity [34][35]. This finding is similar to that of Iverson and. Dervan [36], who reported a similar scenario, as well as [37] and. In addition, the effectiveness of the extract is also influenced by the time/period of exposure. This is demonstrated by the progressive decrease in the number of parasitized red blood cells from day 4 of the treatment to the 7th day. This meant that the effectiveness of the extract is a result of the compounding effect of the longevity of the exposure period/time and the concentration of the extract. In addition, the exposure period makes the parasite take in and utilize the lethal dose of the extract, thereby producing the desired result.
The effect of Mangifera indica leaf extract on body weight (g) of the Albino rats in the negative control group revealed appreciable weight loss (from 100.0 ± 7.2 to 93.5 ± 12.2) four days after the experimental mice were infected. In addition, the same scenario was observed across all the treatments. This is similar to the finding of [38] who reported body weight loss in experimental infected-mice before treatment. The body weight loss observed could be attributed to the loss of appetite [39, 40] by the infected mice, due to parasitization and destruction of the infected red blood cell [41] during the erythrocytic phase of the parasite, thereby interfering with their normal feeding habits and thus leading to weight loss. On the other hand, the improved weight gain observed after the treatment with concentrations of 200mg/kg, 400mg/kg, and 800mg/kg MI could be attributed to the massive destruction of the parasite in a dose-dependent manner, thus, restoring their normal feeding habits. This finding is similar to the findings of [42], who reported body weight loss after extract administration.
Conclusion
The leaf extract of Mangifera indica has proved to be safe and non-toxic at concentrations as high as 1000mg/kg. In addition, the extract suppresses the level of parasitemia significantly at all selected concentrations; thus, the extract is effective for the treatment of malaria infection.
Recommendation
High evidence-based study should be conducted using different extraction method and at different concentration to further explore the anti-malarial potentials of the extract as established in the present study. In addition, further research should be carried out on some other plants to also explore their anti-malarial potential.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgement
We wish to acknowledge the management of Gombe State University, Gombe, for allowing us to use their facility (herbarium and laboratories) for the identification of the experimental plant (Mango leaves (Mangifera indica) and a bench space for conducting the experiment.
References
- Tarkang, P. A., Okalebo, F. A., Ayong, L. S., Agbor, G. A., & Guantai, A. N. (2014). In vitro antiplasmodial activities and synergistic combinations of differential solvent extracts of the polyherbal product, Nefang. Biomed Research International, 1-10.
Publisher | Google Scholor - Oseni, L. A., & Akwetey, G. (2012). An in-vivo evaluation of antiplasmodial activity of aqueous and ethanolic leaf extracts of Azadirachta indica in Plasmodium berghei infected BALB/c mice. International Journal of Pharmaceutical Sciences and Research, 3(5):1406-1410.
Publisher | Google Scholor - Idowu, E. T., Ajaegbu, H. C. N., Omotayo, A. I., Aina, O. O., & Otubanjo, O. A. (2015). In vivo anti-plasmodial activities and toxic impacts of lime extract of a combination of Picralima nitida, Alstonia boonei, and Gongronema latifolium in mice infected with chloroquine-sensitive Plasmodium berghei. African Health Sciences, 15(4):1262-1270.
Publisher | Google Scholor - Kristhien, A. (2019). Antiplasmodial and toxicological effects of ethanolic extracts of mango (Mangifera indica) leaves and bitter cola (Garcinia kola) seeds in albino rats. International Journal of Research in Science and Innovation, 6(8i):91-97.
Publisher | Google Scholor - Olayode, A. A., Ofusori, D. A., Ogunniyi, T. A. B., & Saka, O. S. (2015). Histomorphological studies of the liver of Plasmodium-infected albino mice after administration of aqueous leaf extract of Mangifera indica (Linn.). Anatomy, 9(3):168-176.
Publisher | Google Scholor - Okpe, O., Joshua, P. E., Obi, B. C., & Nwodo, O. F. C. (2023). Vitex doniana, in vitro antioxidant, membrane stabilization potential and protective impact against Plasmodium berghei-passaged mice. Research Journal of Pharmacognosy, 10(3):15-23.
Publisher | Google Scholor - Omagha, R., Taiwo, E., Chibuisi, I., & Alimba, G. (2022). In vivo antiplasmodial activities and acute toxicity assessment of two plant cocktail extracts commonly used among Southwestern Nigerians. Journal of Parasitic Diseases, 46(2):343-353.
Publisher | Google Scholor - Omoya, F. O. (2016). The in vivo assessment of antiplasmodial activities of leaves and stem bark extracts of Mangifera indica (Linn) and Cola nitida (Linn). International Journal of Infectious Diseases, 45:373.
Publisher | Google Scholor - Angupale, J. R., Tusiimire, J., & Ngwuluka, N. C. (2023). A review of efficacy and safety of Ugandan anti-malarial plants with application of RITAM score. Malaria Journal, 22(1):1-19.
Publisher | Google Scholor - Alaiya, M. A., & Odeniyi, M. A. (2023). Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: A review. Futures Journal of Pharmaceutical Sciences, 9(29):1-19.
Publisher | Google Scholor - Olasehinde, G. I., Ogunleye, A. F., Ajayi, T. E., & Ogunleye, O. T. (2018). Phytochemical and antimicrobial properties of Mangifera indica leaf extracts. Covenant Journal of Physical and Life Sciences, 6(1):55-63.
Publisher | Google Scholor - S, P., & Others. (2023). West African medicinal plants: A review of their antimalarial activity. Open Access Journal of Science, 6(1):35-43.
Publisher | Google Scholor - Olajide, J. A., Simon-Oke, I. A., & Oladokun, O. (2021). Antiplasmodial activity of ethanolic extract of neem leaf (Azadirachta indica) in albino mice infected with Plasmodium berghei. International Archives of Clinical Pharmacology, 7(1):1-7.
Publisher | Google Scholor - Tajbakhsh, E., Kwenti, T. E., Kheyri, P., Nezaratizade, S., Lindsay, D. S., & Khamesipour, F. (2021). Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: A systematic review of literature. Malaria Journal, 20(1):1-50.
Publisher | Google Scholor - Airaodion, A. I., Megwas, A. U., Edom, C. V., Winifred, N., Njoku, O. C., & Oladosu, N. O. (2021). Antiplasmodial potential of mango (Mangifera indica) stem bark against Plasmodium berghei in infected Swiss albino mice. International Journal of Advanced Herbal and Alternative Medicine, 4(1):42-48.
Publisher | Google Scholor - Iyamah, P. C., & Idu, M. (2015). Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. Journal of Ethnopharmacology, 173:287-302.
Publisher | Google Scholor - Shah, K., Patel, M., Patel, R., & Parmar, P. (2010). Mangifera indica (Mango). Pharmacognosy Reviews, 4(7):42-48.
Publisher | Google Scholor - Sellés, A. J. N., Agüero, J. A., & Paz, L. N. (2021). GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects. Open Chemistry, 19(1):27-38.
Publisher | Google Scholor - (2016). Antiplasmodial activity of three plant extracts used in local traditional medicine in Saloum (Senegal). European Scientific Journal, 12(12):157.
Publisher | Google Scholor - N., I. P., E. N., & A. B. A. (2019). Phytochemical and vitamin contents of Mangifera indica (Mango) fruits subjected to ripening by artificial methods. International Journal of Environmental Agriculture and Biotechnology, 4(3):677-684.
Publisher | Google Scholor - Mashinchian, O., Johari-Ahar, M., Ghaemi, B., Rashidi, M., Barar, J., & Omidi, Y. (2014). Impacts of quantum dots in molecular detection and bioimaging of cancer. BioImpacts, 4(3):149-166.
Publisher | Google Scholor - Omotayo, O. T., Oladipo, G. A., Adekunle, D. O., Akinola, A., & Pure, O. F. (2022). Phytochemical and antibacterial activity of Mangifera indica Linn (Mango) bark and leaf extracts on bacteria isolated from domestic wastewater samples. African Journal of Clinical and Experimental Microbiology, 23(1):73-82.
Publisher | Google Scholor - N., O. F., Amarachi, I. J., & Odoh, U. E. (2021). Pharmacognostic screening and antiemetic evaluation of the ethanol extract of the leaves of Morinda lucida Benth. (Rubiaceae). World Journal of Biological and Pharmaceutical Health Sciences, 7(3):1-14.
Publisher | Google Scholor - Ahmed, A. A., Muhammad, I., Muhammad, A., & Bapeto, A. S. (2023). Histopathological studies of liver of albino rat treated with Mangifera indica aqueous leaf extract. International Journal of Pharmacognosy and Pharmaceutical Research, 5(1):35-40.
Publisher | Google Scholor - Kingsley, O., Oseni, A. L., Olga, Q., Antwi, S., & Mavis, T. (2012). A comparative evaluation of in vivo antiplasmodial activity of aqueous leaf extracts of Carica papaya, Azadirachta indica, Mangifera indica, and the combination thereof using Plasmodium infected BALB/c mice. International Journal of Applied Biology and Pharmaceutical Technology, 3(3):372-378.
Publisher | Google Scholor - Tarkang, P. A., Okalebo, F. A., Ayong, L. S., Agbor, G. A., & Guantai, A. N. (2014). Anti-malarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models. Malaria Journal, 13(1):1-11.
Publisher | Google Scholor - Oseni, L. A., & Akwetey, G. M. (2012). An in-vivo evaluation of antiplasmodial activity of aqueous and ethanolic leaf extracts of Azadirachta indica in Plasmodium berghei infected BALB/c mice. International Journal of Pharmaceutical Sciences and Research,1406-1410.
Publisher | Google Scholor - Kigondu, E. V. M., & others. (2011). Antiplasmodial and cytotoxicity activities of some selected plants used by the Maasai community, Kenya. South African Journal of Botany, 77(3):725-729.
Publisher | Google Scholor - Iverson, B. L., & Dervan, P. B. (2014). Journal of Agricultural Science and Technology, 16(1):7823-7830.
Publisher | Google Scholor - Okolie, N. J. C., & Kristhien, A. (2019). Toxicological and antiplasmodial activities of ethanolic extracts of mango (Mangifera indica) leaves and bitter cola (Garcinia kola) seeds in albino rats. International Journal of Scientific Research, 8(5):1777-1781.
Publisher | Google Scholor - Daminabo, J. G. D., & V. M. (2017). Natural Science, 15(1):10-17.
Publisher | Google Scholor - Mulaw, T., Wubetu, M., Dessie, B., Demeke, G., & Molla, Y. (2019). Evaluation of antimalarial activity of the 80% methanolic stem bark extract of Combretum molle against Plasmodium berghei in mice. Journal of Evidence-Based Integrative Medicine, 24:1-9.
Publisher | Google Scholor - Ismail, M. A., Sale, P. M., & Muhammad, T. M. (2022). Molecular markers associated with drug resistance in PFMDR-1 gene of Nigeria. Science Forum (Journal of Pure and Applied Sciences), 22:456-463.
Publisher | Google Scholor - Nwibani, M., Michael, O., & Barine, I. (2008). Effects of aqueous extract of Mangifera indica L. (Mango) stem bark on haematological parameters of normal albino rats. Pakistan Journal of Nutrition, 7(5):663-666.
Publisher | Google Scholor