Review Article
Analyzing the Cytotoxicity of Chitosan Nanovehicles in Breast Tumor Therapy: A Critical Review of Key Insights
1Department of Chemistry, Amirkabir University of Technology, Tehran, Iran.
2Nanotechnology and Advanced Materials Department, Materials and Energy Research Center, Karaj, Iran.
*Corresponding Author: Mohammad Hossein Karami, Majid Abdouss, Department of Chemistry, Amirkabir University of Technology, Tehran, Iran.
Citation: Mohammad H. Karami, Majid Abdouss M, Behzad Aghabarari B. (2024). Analyzing the Cytotoxicity of Chitosan Nanovehicles in Breast Tumor Therapy: A Critical Review of Key Insights. Journal of Women Health Care and Gynecology, BioRes Scientia Publishers. 3(5):1-9. DOI: 10.59657/2993-0871.brs.24.046
Copyright: © 2024 Mohammad Hossein Karami, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 13, 2024 | Accepted: May 31, 2024 | Published: June 08, 2024
Abstract
Nanotechnology-driven drug release mechanisms minimize adverse reactions, prolong drug efficacy, and regulate drug administration. Polymeric nanoparticles act as effective carriers, enhancing drug solubility and durability, thus addressing concerns regarding the safety of chemical drugs and additives. Monitoring drug concentration is vital in assessing cytotoxic effects, impacting cell viability and proliferation. Efficient drug delivery via nanocarriers resolves challenges linked to poor solubility and instability within cells. Purpose-built nanocarriers enable prolonged drug release in tumor environments, thereby heightening cytotoxicity. Recent investigations predominantly utilize the double emulsion technique to fabricate nanocarriers, ensuring consistent and stable nanoparticle morphology and preventing premature release during drug delivery. This study focuses on assessing the cytotoxic potential of newly developed nanocarriers in breast cancer cell lines.
Keywords: cytotoxicity; breast cancer; nanocarrier; nanoparticles; drug release
Introduction
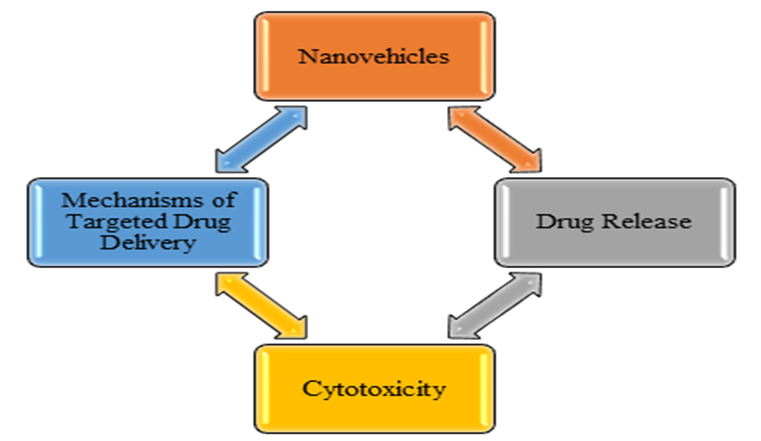
Cancer poses significant challenges in medical treatment, driving ongoing research for therapies with fewer side effects. While chemotherapy, targeted therapy, radiation therapy, and immunotherapy are common treatments, they often have limitations like reduced effectiveness and toxicity to healthy tissues. Nanoparticles have gained popularity for their versatility in various fields due to their size, typically under 100 nanometers [1]. They can deliver a wide range of drugs, including water-soluble and water-insoluble ones, vaccines, and biological molecules, with applications in disease treatment and diagnosis. Nanoparticles come in various forms like nanoliposomes, carbon nanotubes, nanofibers, and nanospheres, serving as drug carriers and cellular scaffolds [2-4]. Challenges like bloodstream entry and phagocytosis can be addressed through surface modification. Drug delivery systems improve drug efficacy and properties, acting as reservoirs for targeted drug release, thus reducing side effects and drug consumption. These systems, classified into organic and inorganic groups, encompass various nanoparticle types [5]. Synthesis focuses on nanoparticle carriers and drug encapsulation, using active and passive methods. Polymer nanoparticles, a common choice, offer advantages like increased drug solubility and stability. This study explores the importance of nanoparticles in drug delivery, recent advances in nanocarriers, their mechanisms, and their cellular toxicity assessment in breast cancer cell lines [6]. This research investigates the significance of chitosan-based nano systems and analyzes key points regarding their application [7]. Specifically, it delves into the mechanisms underlying drug release and cytotoxicity of pharmaceutical nanocomposites developed in recent years (Figure.1) [8].
Figure 1: Key Insights on Breast Tumor Therapy
Mechanisms of Targeted Drug Delivery
Different methods, ranging from simple to complex, can be utilized to implement targeted drug delivery mechanisms. In general, these mechanisms can be categorized into three groups: physical targeting, passive targeting, and active targeting [9].
Physical Targeting
Physical targeting employs various forces like magnetic fields, ultrasound, light, heat, and electric fields to concentrate or disperse drugs as needed. Magnetic fields, light, and ultrasound waves are commonly used, with magnetic fields being particularly favored due to their affordability and ease of application. Magnetic nanoparticles, notably iron oxide nanoparticles, are widely utilized in magnetic field-based drug delivery methods [10]. Ultrasound-based drug delivery and light-triggered targeting are innovative methods. Ultrasound utilizes microbubbles for enhanced absorption, while light-based approaches offer precise control over release. Recent advancements include light-sensitive systems triggering drug release in response to specific wavelengths, with strategies for single or multiple releases via nanoparticles [11].
Passive Targeting
After reaching a volume of 2 cubic millimeters or more, tumors experience limited permeability, affecting nutrient and oxygen uptake and prompting angiogenesis [12]. Enhanced Permeation Retention (EPR) is a common passive targeting strategy in tumor therapy, capitalizing on irregular tumor endothelial cell arrangement and the absence of lymphatic drainage to retain drugs in the tumor bed [13].
Active Targeting
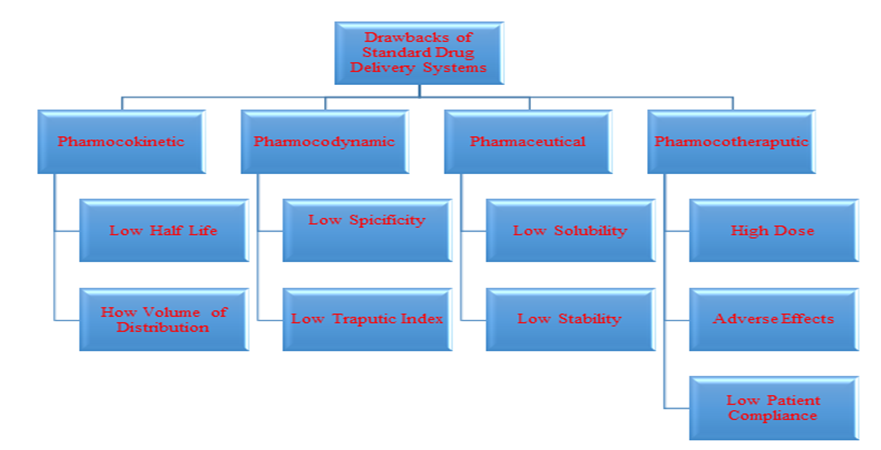
Active targeting represents the pinnacle of drug delivery targeting, achieved by attaching targeting receptor molecules to drug delivery systems. This method enables precise delivery of drugs to target tissues, intracellular organelles, or specific molecules [14]. In Figure 2, the defects of common drug delivery systems are depicted.
Figure 2: Defects in common drug delivery systems
Hydrogels in Drug Delivery
Hydrogel-based drug delivery systems are widely researched to enhance drug effectiveness and reduce side effects. Their soft and water-friendly nature makes them ideal for drug delivery. Hydrogels can incorporate drugs in various forms and release them at controlled rates due to their porous structure. These systems protect drugs from harsh environments and offer different release mechanisms [15]. With proper design, hydrogels are versatile for sustained, targeted, or biomolecule release. Table 1 outlines the types of hydrogel nanocomposites in drug release along with their characteristics and fabrication methods [16].
Table 1: Types of Hydrogel Nanocomposites in Drug Release
| Type of Hydrogel Nanocomposite | Fabrication Method | Properties | References |
| Iron oxide-cellulose | Solution casting | Responsive to magnetic stimuli | [4] |
| Silver nanoparticles-acrylo-piperazine | In-situ polymerization | Temperature and pH responsive | [1] |
| Clay nanoparticles-polyvinyl alcohol | In-situ polymerization | Reduction in crystallinity and glass transition temperature | [5] |
| Silver nanoparticles-polyvinyl alcohol | Gamma irradiation | Increase in swelling behavior with nanoparticle increase | [13] |
| Modified acrylic amide-graphene | Free radical polymerization | Improved strength | [12] |
| Dendrimers-polyethylene glycol | Free radical polymerization | Self-healing capability | [10] |
| Hydroxyapatite-polyethylene glycol | Free radical polymerization | Enhanced mechanical properties | [11] |
| Silica-polyethylene glycol | Free radical polymerization | Improved cellular adhesion | [12] |
| Polyamidoamine-collagen | Physical cross-linking | Cross-linking associations | [16] |
| Graphene oxide-carboxymethyl cellulose | Cross-linking associations | Reduced drug release at pH=7.4 | [15] |
| Iron oxide-sodium carboxymethyl cellulose | Cross-linking associations | pH-responsive | [14] |
| Acrylate-6 amino-graphene oxide caproic acid | Solution casting | Mechanical strength improvement | [7] |
| Liposome-chitosan | In-situ polymerization | Improved drug release | [9] |
A Review of Designed Nanocarriers in Drug Delivery Systems
In the study, a chitosan (CS)-based nanocarrier incorporating reduced iron oxide nanoparticles and graphene oxide was examined for its ability to deliver curcumin (CUR) to breast cancer cells. The findings revealed a 33 Percentage cell death rate, suggesting potential enhancements in CUR release [17]. In a recent study, researchers tested a nano-carrier with Agarose and CS for targeted release of two anti-cancer drugs, CUR and 5-Fluorouracil (5-FU), in breast cancer cells. The carrier induced a 55% reduction in cell viability when loaded with both drugs. CS alone or with Agarose showed no significant deviation from the control. The CS -loaded carrier with Agarose had slightly lower cellular toxicity, indicating more viable cells. Also, the carrier with 5-FU had minimal deviation compared to the free drug [18]. A CS -based nanocarrier with aptamer and carbon quantum dots was studied to improve 5-FU release in breast cancer cells. Results showed CS with carbon quantum dots behaved like the control, indicating nanoparticle biocompatibility. The nanocarrier with the drug increased live cells by 67 Percentage, suggesting enhanced cellular toxicity. Addition of aptamer further increased toxicity. The nanocarrier exhibited slower, stable drug release, reducing wastage and side effects compared to the free drug [19]. The study examined a nanocarrier composed of polyvinylpyrrolidone, featuring CS and iron oxide nanoparticles, for the controlled release of doxorubicin (DOX) in breast cancer cell lines. The findings revealed that the drug encapsulated within this nanocarrier exhibited higher cellular toxicity against breast cancer cells compared to other samples. However, it demonstrated lower toxicity than the free drug, attributed to its dependency on release time. Additionally, it underscored the nanocarrier's stability in enhancing drug release [20].
In a study, a polyvinylpyrrolidone-based nanocarrier with agarose and hydroxyapatite was examined to enhance quercetin (QUE) release in breast cancer cell lines. Addition of hydroxyapatite increased cellular toxicity. The nanocarrier containing polyvinylpyrrolidone, agarose, and QUE showed higher cellular toxicity compared to samples with only polyvinylpyrrolidone, agarose, and hydroxyapatite, suggesting quercetin induced more toxicity. This nanocarrier is optimal for quercetin release, inhibiting tumor cell growth and providing stable drug release. Preparation using the double emulsion method results in stable, time-dependent drug release [21]. Investigators engineered a CS-based nano-delivery system featuring polyvinylpyrrolidone and gamma-alumina to administer quercetin and explore its impact on breast cancer cell lines. Findings revealed that initially, CS with polyvinylpyrrolidone displayed scant cellular toxicity, yielding over 80 Percentage cell viability. However, upon the integration of gamma-alumina nanoparticles, the proportion of viable cells dwindled due to the toxicological effects of alumina nanoparticles, resulting in a noteworthy surge in cellular demise. Introduction of quercetin to this nano-delivery system further precipitated a decline in live cells to a mere 9 Percentage, showcasing the potential of the CS -based nano-delivery system containing polyvinylpyrrolidone and alumina nanoparticles to significantly amplify cellular toxicity, thus manifesting a pronounced cytotoxic impact on breast cancer cells [22]. Scientists examined a CS-derived nano-transporter incorporating iron oxide and gamma-alumina nanoparticles to dispense 5-FU in breast cancer cell lines. Outcomes indicated that the mere presence of nanoparticles failed to heighten cellular toxicity, affirming the nanoparticles' biocompatibility. Furthermore, the formulated transporter impeded cancer cell growth and propagation. Preparation of this transporter entailed the double emulsion technique, ensuring nanoparticle shape uniformity and stability while hindering rapid nanoparticle diffusion during drug release within the body [23]. In a pioneering study, researchers investigated a CS-based nanocarrier containing iron oxide nanoparticles and nano clay for precise QUE release in breast cancer cell lines. While the nanocarrier showed minimal impact on tumor cells, it induced significant cell mortality upon drug loading, with 22% lower toxicity compared to free QUE. This formulation holds promise for enhancing drug release in breast cancer therapy [24].
In a recent study, scientists investigated a CS -based nanocarrier incorporating agarose and nano clay for the controlled release of CUR in breast cancer cell lines. The CS nanocarrier demonstrated minimal changes in cellular toxicity without the drug, yet showed enhanced drug release capabilities. In contrast, free CUR increased cellular toxicity, while the nanocarrier containing the drug exhibited even higher toxicity levels, highlighting its effectiveness in delivering CUR and enhancing cellular toxicity in the tumor microenvironment [25]. In a study, scientists investigated a CS -based nanocarrier incorporating nano-halloysite and graphene carbon nitride for delivering QUE to breast cancer cell lines. Results indicated that CS alone, CS with nano-halloysite, and the nanocarrier containing CS with nano-halloysite and graphene carbon nitride showed cellular toxicity levels akin to the control sample, affirming the biocompatibility of these materials. In contrast, the free QUE drug heightened cellular toxicity. However, when combined with the nanocarrier containing CS with nano-halloysite and graphene carbon nitride, it exhibited the highest cellular toxicity among the samples, suggesting that this synthesized nanocarrier could significantly enhance cellular toxicity in breast cancer cell lines, reducing it by 35 Percentage [26]. Researchers investigated a CS-based nanocarrier containing halloysite nanoparticles and carbon nanotubes to enhance CUR release in breast cancer cell lines. The nanocarrier demonstrated excellent biocompatibility with 87 Percentage cell viability, and the inclusion of halloysite nanoparticles had minimal impact on cellular toxicity. Thus, the CS nanocarrier with halloysite nanoparticles proved effective for drug release. Comparative analysis with samples lacking the drug and free CUR showed increased cellular toxicity with the nanocarrier, highlighting its potent inhibitory effect on cancer cells [27].
Scientists studied a CS-based nanocarrier incorporating agarose and iron oxide nanoparticles for releasing CUR in both breast cancer and normal cells. Findings revealed that the engineered nanocarrier caused a 48 Percentage decrease in breast cancer cell viability, surpassing the efficacy of the free drug by 12 Percentage. Moreover, the CS nanocarrier containing agarose and iron oxide nanoparticles, devoid of the drug, demonstrated minimal cellular toxicity, highlighting the appropriateness and biocompatibility of this carrier system [28]. In their study, researchers explored a graphene-based nanocarrier incorporating graphene quantum dots and iron oxide nanoparticles, conjugated with folate acid, to enhance CUR release in breast cancer cell lines. Findings revealed that at lower concentrations of CUR loading (5μg/mL), the nanocarrier induced higher cellular death, suggesting a synergistic effect between the drug and the carrier [29].
Additionally, Mohammad et al. investigated a poly(methacrylamide) nanocarrier incorporating iron oxide nanoparticles for DOX release in lung cancer cell lines. Results indicated elevated cellular toxicity with the nanocarrier (at concentrations of 25 and 125μg/mL) compared to both the free drug and the drug-free nanocarrier. Researchers A CS-based nanocarrier incorporating serum albumin was developed to enhance the release of methotrexate, and its efficacy was investigated in breast cancer cell lines. Results from cellular toxicity assessments revealed that the addition of methotrexate at concentrations ranging from 0.5 to 10 micrograms per milliliter to this nanocarrier heightened cellular toxicity. The interplay of drug dosage and time within the concentration range of 0.5 to 5 micrograms per milliliter exhibited variations in cellular toxicity [30]. In a separate study, a nanocarrier comprising gold nanoparticles, iron oxide nanoparticles, and albumin was explored for podophyllotoxin release in breast cancer cell lines. Findings suggested that albumin functioned as a coating, encapsulating iron oxide and gold nanoparticles, thereby forming a core-shell structure. The final half-maximal inhibitory concentration (IC50) values for the free drug and the drug-loaded nanocarrier were 3085 nM and 1868 nM, respectively, indicating an increase in cellular toxicity induced by this nanocarrier. Atiroglu et al. delved into the simultaneous release of 5-FU and gemcitabine hydrochloride from a CS nanocarrier incorporating Metal Organic Framework nanoparticles of MIL-100. Their findings indicated an enhancement in drug release accompanied by an increase in cellular toxicity [31].
Balakumar et al. explored the dual release of 5-FU and hesperidin from a CS nanocarrier in breast cancer cell lines. Their results revealed that the cellular toxicity of free 5-FU was higher compared to the CS nanocarrier containing 5-FU alone and the CS nanocarrier containing both 5-FU and hesperidin, attributable to the slow and stable drug release kinetics of the nanocarrier [32]. Sil et al. investigated a silica nanocarrier incorporating polyacrylic acid and folic acid to enhance the release of chrysin. Their findings unveiled that the nanocarrier with folic acid exhibited heightened cellular toxicity compared to normal cells [33]. Kalindemirtas et al. devised a nanocarrier comprising rosin and polyethylene glycol to enhance the release of 5-FU and carmofur. This nanocarrier demonstrated heightened cellular toxicity for both drugs, suggesting a promising strategy for controlled and targeted drug delivery systems [34].
Jayakannan et al. explored a polycaprolactone nanocarrier incorporating carboxylic acid for the release of cisplatin in breast cancer cell lines. Their findings indicated that this nanocarrier displayed biocompatible behavior in the absence of drug presence, with low cellular toxicity. At a concentration of 1.0 microgram per milliliter, the nanocarrier showed greater cellular toxicity compared to the free drug, whereas at higher concentrations, the free drug displayed higher cellular toxicity than the nanocarrier. This pattern suggests that the slow release of the drug from the nanocarrier contributes to its potential effectiveness in breast cancer treatment [35]. The iron-based metal-organic framework Iron 3-benzene-3,1,5-tricarboxylate was investigated for the release of doxorubicin in breast cancer cell lines. This nanocarrier demonstrated dual-responsive behavior for drug release, sensitive to both ultrasound waves and pH fluctuations [36]. Pascual and collaborators examined a nano-composite composed of polyvinylpyrrolidone, polyethylene glycol, and titanium dioxide nanoparticles to enhance the release of quercetin in L929 healthy tissue cell lines. Their findings demonstrated that the positively charged surface can foster electrostatic attraction between nanoparticles, thereby improving interactions with cell membranes. The pH-responsive behavior of the designed nanocomposite proved crucial, as it resulted in less drug release in the neutral environment of L929 cells compared to the acidic environment of U87 cells, thereby mitigating potential side effects associated with nanocarriers [37]. The cellular toxicity of liposomes, liposome nanocarriers, iron particles, and iron-based metal-organic framework nanocarriers was assessed. Results revealed minimal cellular toxicity and high biocompatibility of liposomes. Additionally, liposome nanocarriers and iron particles showed comparable levels of cellular toxicity at concentrations ranging from 150 to 500 micrograms per milliliter. However, at higher concentrations (500 to 10,000 micrograms per milliliter), the cellular toxicity of liposome nanocarriers and iron particles escalated [38].
Yazdian et al. investigated a CS nanocarrier containing alumina nanoparticles and agarose for doxorubicin release in breast cancer cells. Results showed low cellular toxicity, meeting ISO biocompatibility standards. Additionally, nitrogen quantum dot nanoparticles heightened toxicity, and drug-loaded nanocarriers induced increased cellular toxicity compared to free drugs, suggesting enhanced apoptosis [39]. Pourmadadi et al. tested a nanocarrier with carboxymethyl cellulose, gelatin, and ZIF-8 nanoparticles to improve quercetin release in breast cancer cells. The nanocarrier increased cellular toxicity to 55 Percentage, compared to 70 Percentage for the free drug, enhancing cell death [40]. Pascual et al. investigated a nanocarrier containing carboxymethyl cellulose, starch, and reduced graphene oxide nanoparticles for CUR release in breast cancer cells. The nanocarrier showed higher cellular toxicity than the free drug, reducing side effects and enabling controlled release. Rashidi et al. investigated a CS-based nanocarrier with starch and molybdenum disulfide nanoparticles for CUR release. While CS and starch combination had no notable impact on cell viability, the designed nanocomposite showed toxicity. Free CUR displayed considerable toxicity, whereas the drug-loaded nanocarrier increased cellular toxicity to 54 Percentage, indicating enhanced anticancer activity [41].
Rahdar et al. utilized a polyacrylic acid nanocarrier with polyvinylpyrrolidone and carbon nanotubes for 5-FU release. It exhibited minimal toxicity and good biocompatibility without drugs. Pascual et al. examined a nano-composite of polyvinylpyrrolidone, polyethylene glycol, and titanium dioxide nanoparticles for quercetin release in L929 healthy tissue cell lines. They found that the positively charged surface improved cell membrane interactions, with pH-responsive behavior reducing drug release in neutral environments, potentially lowering side effects [42].
Yazdian and colleagues also explored a CS nanocarrier containing alumina nanoparticles and agarose to enhance the release of 5-FU in breast cancer cell lines. The cellular toxicity of the drug-loaded nanocarrier surpassed that of the free drug, resulting in live cell percentages of 28 Percentage and 36 Percentage, respectively. Additionally, the nanocarrier displayed minimal alterations in cellular toxicity when devoid of the drug, suggesting minimal inherent cellular toxicity in breast cancer cell lines [43].
Pourmadadi et al. evaluated a nanocarrier comprising carboxymethyl cellulose, gelatin, and zeolitic imidazolate framework-8 (ZIF-8) nanoparticles to enhance the release of the anticancer drug quercetin in breast cancer cell lines. Results demonstrated that the cellular toxicity of both the drug and the drug-loaded nanocarrier was 70 Percentage and 55 Percentage, respectively. Therefore, the nanocarrier increased cellular toxicity compared to the free drug, leading to increased cell death. Additionally, Pascual et al. investigated a nanocarrier containing carboxymethyl cellulose, starch, and reduced graphene oxide nanoparticles for CUR drug release in breast cancer cell lines. The results showed that the nanocarrier exhibited higher cellular toxicity compared to the free drug, thus reducing side effects associated with anticancer drugs like CUR and facilitating controlled release [44]. Yazdian and colleagues investigated a CS nanocarrier comprising alumina nanoparticles and agarose to improve the release of 5-FU in breast cancer cell lines. The cellular toxicity of the drug-loaded nanocarrier exceeded that of the free drug, resulting in live cell percentages of 28 Percentage and 36 Percentage, respectively. Additionally, the nanocarrier displayed minimal changes in cellular toxicity when devoid of the drug, indicating negligible inherent cellular toxicity [45]. In another study by Tiwary et al., a nanocarrier containing ligand-modified multi-walled carbon nanotubes in the presence of lysine was explored for the release of doxorubicin in breast cancer cell lines. Results showed that at concentrations below 12.5 micrograms per milliliter, the cellular toxicity of the free drug surpassed that of the nanocarrier, potentially due to the slower release kinetics of the nanocarrier compared to the free drug. Furthermore, nanocarriers containing mannose- and galactose-modified carbon nanotubes exhibited increased cellular toxicity compared to the free drug [46].
Rashidi et al. examined a CS-based nanocarrier containing starch and molybdenum disulfide nanoparticles for CUR drug release. While the combination of CS and starch showed no significant changes in cell viability, indicating no adverse effects on cell survival, the designed nanocomposite demonstrated toxicity to cells. The results revealed that free CUR exhibited considerable toxicity, while the drug-loaded nanocarrier increased cellular toxicity to 54 Percentage, indicating enhanced anticancer activity [47]. Rashidi and colleagues also designed a CS-based nanocarrier containing iron nanoparticles and carbon quantum dots to improve CUR drug release. Results showed that drug release was faster in acidic conditions compared to neutral environments due to the pH sensitivity of the designed drug. Moreover, no burst or rapid release was observed, confirming controlled drug release [48-50].
Conclusion
In this study, we delved into the cellular toxicity of newly devised nanocarriers tailored for the precise delivery of anticancer medications in breast cancer cell lines. Over the recent years, a plethora of nanocarriers have emerged, each shedding light on the intricate relationship between drug concentration and cellular toxicity. While some investigations have revealed that nanocarriers don't exacerbate cellular toxicity when compared to free drugs, they do facilitate controlled and sustained drug release, underscoring the significance of timing in drug administration. Across the majority of these novel nanocarriers, the double emulsion method emerged as a predominant technique, ensuring both stability and uniformity in nanoparticle shape, while also averting any sudden burst or rapid release of nanoparticles during the drug delivery process.
Declarations
CRediT authorship contribution statement
Mohammad Hossein Karami: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Majid Abdouss: Supervision, Validation. Behzad Aghabarari: Supervision, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tan, L.F.; Wang, S.P.; Xu, K.; Liu, T.L.; Liang, P.; Niu, M.; Fu, C.H.; Shao, H.B.; Yu, J.; Ma, T.C.; et al. (2016). Layered MoS2 hollow spheres for highly-efficient photothermal therapy of rabbit liver orthotopic transplantation tumors. Small, 12:2046-2055.
Publisher | Google Scholor - Yuan, Z.; Tao, B.L.; He, Y.; Liu, J.; Lin, C.C.; Shen, X.K.; Ding, Y.; Yu, Y.L.; Mu, C.Y.; Liu, P.; et al. (2019). Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials, 217:119290.
Publisher | Google Scholor - Wu, H.H.; Yang, R.; Song, B.M.; Han, Q.S.; Li, J.Y.; Zhang, Y.; Fang, Y.; Tenne, R.; Wang, C. (2011). Biocompatible inorganic fullerene-like molybdenum disulfide nanoparticles produced by pulsed laser ablation in water. ACS Nano, 5:1276-1281.
Publisher | Google Scholor - Karami MH, Abdouss M, Rahdar A, Pandey, Graphene quantum dots: background, synthesis methods, and applications as nanocarrier in drug delivery and cancer treatment: an updated review. Inorg Chem Commun. 2024;161: 112032.
Publisher | Google Scholor - Li J, Li M, Tang J, Li X, Zhang H, Zhang Y. (2008). Resonance light-scattering spectrometric study of interaction between enzyme and MPA-modified CdTe nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc, 70(3):514-518.
Publisher | Google Scholor - Hami Z. (2021). A Brief Review on Advantages of Nano-based Drug Delivery Systems. Ann Mil Health Sci Res, 19(1):e112274.
Publisher | Google Scholor - Gelperina S, Kisich K, Iseman MD, Heifets L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med, 172(12):1487-1490.
Publisher | Google Scholor - Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, et al. (2014). Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS nano, 8(10):9874-9883.
Publisher | Google Scholor - Karami MH, Abdouss M. (2024). Cutting-edge tumor nanotherapy: Advancements in 5-fluorouracil Drug-loaded chitosan nanoparticles, Inorganic Chemistry Communications, 164:112430.
Publisher | Google Scholor - Khan S, Rizvi SMD, Avaish M, Arshad M, Bagga P, Khan MS. (2015). A novel process for size controlled biosynthesis of gold nanoparticles using bromelain. Mater Lett, 159:373-376.
Publisher | Google Scholor - de Sousa IP, Cattoz B, Wilcox MD, Griffiths PC, Dalgliesh R, Rogers S, et al. (2015). Nanoparticles decorated with proteolytic enzymes, a promising strategy to overcome the mucus barrier. Eur J Pharm Biopharm, 97:257-264.
Publisher | Google Scholor - Roy, S.; Haloi, P.; Choudhary, R.; Chawla, S.; Kumari, M.; Konkimalla, V.B.; Jaiswal, A. (2023). Quaternary pullulan-functionalized 2D MoS2 glycosheets: A potent bactericidal nanoplatform for efficient wound disinfection and healing. ACS Appl. Mater. Interfaces, 15:24209-24227.
Publisher | Google Scholor - Ataide JA, Gérios EF, Cefali LC, Fernandes AR, Teixeira MdC, Ferreira NR, et al. (2019). Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain–Chitosan Nanoparticles. Polymers, 11(10):1681.
Publisher | Google Scholor - Kammona O, Kiparissides C. (2012). Recent advances in nanocarrier-based mucosal delivery of biomolecules. J Control Release, 161(3):781-794.
Publisher | Google Scholor - Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, et al. (2009). Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn, 9(4):325-341.
Publisher | Google Scholor - Nagpal K, Singh SK, Mishra DN. (2010). Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull, 58(11):1423-1430.
Publisher | Google Scholor - Karami MH, Pourmadadi M, Abdouss M, Kalaee MR, Moradi O, et al. (2023). Novel chitosan/γ-alumina/ carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int J Biol Macromol, 251:126280.
Publisher | Google Scholor - Karami M H, Abdouss M. (2024). Assessing Particle Size and Surface Charge in Drug Carrier Nanoparticles for Enhanced Cancer Treatment: A Comprehensive Review Utilizing DLS and Zeta Potential Characterization. PSPRJ, 5(3): 000615.
Publisher | Google Scholor - Brito AM, Oliveira V, Icimoto MY, Nantes-Cardoso IL. (2021). Collagenase activity of bromelain immobilized at gold nanoparticle interfaces for therapeutic applications. Pharmaceutics,13(8):1143.
Publisher | Google Scholor - Wang Y, Liu K, Huang K, Wei W, Huang Y, Dai H. (2024). Photothermal antibacterial MoS2 composited chitosan hydrogel for infectious wound healing, Biomaterials Advances, 156:213701,
Publisher | Google Scholor - Karami M H, Abdouss M. (2024). Recent advances of carbon quantum dots in tumor imaging. Nanomed J, 11(1): 13-35.
Publisher | Google Scholor - Karami M H, Abdouss M, Maleki B. The State of the Art Metal Nanoparticles in Drug Delivery Systems: A Comprehensive Review. Nanomed.
Publisher | Google Scholor - Kaushik R, Nandi S, Mandal M, Nath Gupta A. (2024). Biocompatible l-Cysteine-Capped MoS2 Nanoflowers for Antibacterial Applications: Mechanistic Insights, ACS Applied Nano Materials. 7 (7): 7753-7765.
Publisher | Google Scholor - Hirche C, Almeland SK, Dheansa B, Fuchs P, Governa M, Hoeksema H, et al. (2020). Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns, 46(4):782-796.
Publisher | Google Scholor - Li, F.; Cui, X.T.; Zheng, Y.L.; Wang, Q.; Zhou, Y.L.; Yin, H.S. (2022). Photoelectrochemical biosensor for DNA formylation based on WS2 nanosheets@polydopamine and MoS2 nanosheets. Biosens. Bioelectron, 10:100104.
Publisher | Google Scholor - Ghensi P, Cucchi A, Bonaccorso A, Ferroni L, Gardin C, Mortellaro C, et al. (2019). In vitro effect of bromelain on the regenerative properties of mesenchymal stem cells. J Craniofac Surg, 30(4):1064-1067.
Publisher | Google Scholor - Singer AJ, Taira BR, Anderson R, McClain SA, Rosenberg L. (2010). The effects of rapid enzymatic debridement of deep partialthickness burns with Debrase® on wound reepithelialization in swine. J Burn Care Res, 31(5):795-802.
Publisher | Google Scholor - Karami MH, Abdouss M, Karami M. (2023). Evaluation of in vitro and ex vivo models for studying the effectiveness of vaginal drug systems in controlling microbe infections: A systematic review. Clin J Obst Gynecol, 6: 201-215.
Publisher | Google Scholor - Miranda ÍKSPB, Santana FR, Camilloto GP, Detoni CB, Souza FVD, de Magalhães Cabral-Albuquerque EC, et al. (2021). Development of membranes based on carboxymethyl cellulose/acetylated arrowroot starch containing bromelain extract carried on nanoparticles and liposomes. J Pharm Sci, 110(6):2372-2378.
Publisher | Google Scholor - Bayat S, Zabihi AR, Farzad SA, Movaffagh J, Hashemi E, Arabzadeh S, et al. (2021). Evaluation of debridement effects of bromelain-loaded sodium alginate nanoparticles incorporated into chitosan hydrogel in animal models. Iran J Basic Med Sci, 24(10):1404.
Publisher | Google Scholor - Karami, M., Abdouss, M., Kalaee, M., Moradi, O. (2023). Investigating the Antibacterial Properties of Chitosan Nanocomposites Containing Metal Nanoparticles for Using in Wound Healings: A Review Study. Basparesh.
Publisher | Google Scholor - Karami, M. H., Abdouss, M., Kalaee, M., Moradi, O. (2023). The application of chitosan-based nanocarriers in improving the release of the anticancer drug quercetin: a review study. Nano World, 19(70): 21-11.
Publisher | Google Scholor - Dabiri G, Damstetter E, Phillips T. (2016). Choosing a Wound Dressing Based on Common Wound Characteristics. Adv Wound Care, 5:32-41.
Publisher | Google Scholor - Du X, Zhou J, Shi J, Xu B. (2015). Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem Rev, 115:13165-13307.
Publisher | Google Scholor - Bashir S, Hina M, Iqbal J, Rajpar AH, Mujtaba MA, Alghamdi NA, Wageh S, Ramesh K, Ramesh S. (2020). Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers, 12:2702.
Publisher | Google Scholor - Rebers L, Reichsöllner R, Regett S, Tovar G, Borchers K, Baudis S, Southan A. (2021). Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci Rep, 11:3256.
Publisher | Google Scholor - Morello G, Polini A, Scalera F, Rizzo R, Gigli G, Gervaso F. (2021). Preparation and Characterization of Salt-Mediated Injectable Thermosensitive Chitosan/Pectin Hydrogels for Cell Embedding and Culturing. Polymers. 13:2674.
Publisher | Google Scholor - O'Meara S, Martyn-St James M, Adderley UJ. (2015). Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev, CD010182.
Publisher | Google Scholor - Chang NS, Lin R, Sze CI, Aqeilan RI. (2019). Editorial: WWDomain Proteins in Signaling, Cancer Growth, Neural Diseases, and Metabolic Disorders. Front Oncol, 9:719.
Publisher | Google Scholor - Brown BN, Badylak SF. (2014). Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res, 163(4):268-285.
Publisher | Google Scholor - Zhang X, Tan B, Wu Y, Zhang M, Liao J. (2021). A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers, 13(12):2100.
Publisher | Google Scholor - Verhelst S, Bonger KM, Willems LI. (2020). Bioorthogonal Reactions in Activity-Based Protein Profiling. Molecules, 25(26):5994.
Publisher | Google Scholor - Gupta A, Briffa SM, Swingler S, Gibson H, Kannappan V, Adamus G, Kowalczuk M, Martin C, Radecka I. (2020). Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules, 21(5):1802-1811.
Publisher | Google Scholor - Nešović K, Janković A, Kojić V, Vukašinović-Sekulić M, Perić-Grujić A, Rhee KY, Mišković-Stanković V. (2018). Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels—Synthesis, biological and physicochemical properties and silver release kinetics. Compos Part B Eng, 154:175-185.
Publisher | Google Scholor - Sun A, He X, Li L, Li T, Liu Q, Zhou X, Ji X, Li W, Qian Z. (2020). An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater, 12:25.
Publisher | Google Scholor - Echalier C, Laurine V, Martinez J, Mehdi A, Gilles S. (2019). Chemical cross-linking methods for cell encapsulation in hydrogels. Materials Today Communications, 20:100536.
Publisher | Google Scholor - Xin H, Biswas N, Li P, Zhong C, Chan TC, Nudleman E, Ferrara N. (2021). Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders. Proc Natl Acad Sci U S A, 118(5):e1921252118.
Publisher | Google Scholor - Gelperina S, Kisich K, Iseman MD, Heifets L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med, 172(12):1487-1490.
Publisher | Google Scholor - Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, et al. (2014). Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS nano, 8(10):9874-9883.
Publisher | Google Scholor