Research article
Analytical Study of Heavy Metals and SNP Induced Oxidative Stress in Phospholpid Homogenate
1 Institute of Chemical Sciences, University of Peshawar, Peshawar 25120, Khyber Pakhtunkhwa, Pakistan.
2Department of Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan.
*Corresponding Author: Saddam Hussain, Institute of Chemical Sciences, University of Peshawar, Peshawar 25120, Khyber Pakhtunkhwa, Pakistan.
Citation: Hussain S, Akitsu T. (2023). Analytical Study of Heavy Metals and SNP Induced Oxidative Stress in Phospholpid Homogenate, Pollution and Community Health Effects, BioRes Scientia Publishers. 1(2):1-5. DOI: 10.59657/2993-5776.brs.23.012
Copyright: © 2023 Saddam Hussain, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 26, 2023 | Accepted: November 02, 2023 | Published: November 09, 2023
Abstract
Lipids are one of the main targets of oxidative burst. The phospholipids mixture and other unsaturated fatty acids found in egg yolks could provide a promising experimental model for investigating oxidative stress driven on by metals or radicals. ROS production and oxidative stress play a vital role in the toxicity and carcinogenicity of heavy metals, especially arsenic, lead, and mercury, which can lead to several health complications. The accumulation of inorganic arsenic in organs such as the kidneys, liver, spleen, lungs, and gastrointestinal tract can lead to heavy metal poisoning. The assimilation of herbicides, insecticides, pesticides, fungicides, or rodenticides, as well as occupational exposure during the manufacturing of paints, enamels, glass, and other materials, can lead to heavy metal exposure. The experimental results obtained at a concentration of 100uM have revealed significant information regarding the impact of specific environmental pollutants on oxidative stress levels. The tested compounds, including SNP, Hg, As, Fe, and Pb, exhibited varying degrees of influence on oxidative stress compared to the control group. However, SNP caused a significantly higher level of oxidative stress, measuring 3.39 times that of the control, followed by Hg was 2.89 times higher, As was 2.76 times higher, Fe was 2.49 times higher, and Pb was 1.09 times higher, respectively. These findings highlight the importance of taking measures to mitigate the adverse effects of heavy metal exposure on human health.
Keywords: heavy metals exposure; SNP; TBARs; oxidative stress
Introduction
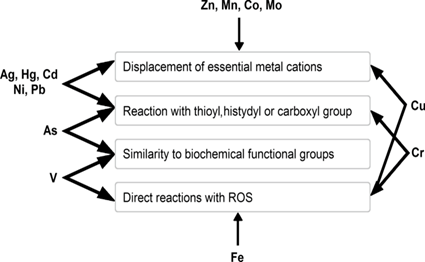
The deleterious effects of heavy metals on various cell organelles cannot be overstated. These toxic pollutants can disrupt the normal function of essential cell components such as cell membranes, mitochondria, lysosomes, endoplasmic reticulum, and nuclei, among others. Additionally, heavy metals are known to have a direct impact on critical cellular constituents such as DNA, lipids, and nuclear proteins, which can lead to molecular damage like lipid peroxidation, protein, and DNA damage [1].
This type of molecular damage can have serious consequences, inducing apoptosis or even carcinogenesis, as highlighted by Tchounwou et al. in their classical review. Reactive oxygen species (ROS) production and oxidative stress play a crucial role in the toxicity and carcinogenicity of heavy metals like arsenic, cadmium, chromium, lead, and mercury. The mechanisms by which these heavy metals cause cellular damage can be complex and multifactorial, often involving a cascade of events that ultimately lead to cellular dysfunction and death. For example, the accumulation of heavy metals in the body can activate transcription factors and signaling pathways that promote ROS production, further exacerbating oxidative stress [2,3].
Figure 1:Major mechanisms of toxicity of certain heavy metals.
Overall, the impact of heavy metals on human health is a critical public health concern, and the adverse effects of these pollutants on various cellular components, as well as their involvement in the development of serious diseases like cancer, should not be underestimated. Therefore, it is imperative to continue investigating the mechanisms by which heavy metals cause cellular damage and to develop effective strategies to monitor and mitigate their harmful effects on human health [4].
Heavy metals such as arsenic, lead, and mercury have profound and widespread effects on human health. Exposure to these toxic pollutants can result in a range of symptoms, including weakness, muscle aches, chills, fever, as well as more serious health issues. Inorganic arsenic, for example, accumulates in various organs like the liver, spleen, kidneys, lungs, and gastrointestinal tract, potentially causing significant damage to these vital organs. Adverse effects of arsenic exposure, well-documented in the literature, include nausea, vomiting, diarrhea, cardiomyopathy, renal tubular acidosis, hemolysis, ventricular arrhythmias, coma, and seizures [5,6].
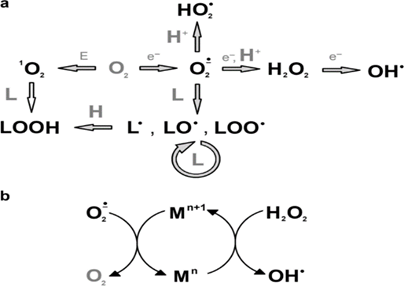
It is worth noting that heavy metal poisoning can result from various sources. These include the ingestion of herbicides, insecticides, pesticides, fungicides, or rodenticides, as well as occupational exposure to heavy metals during the manufacturing of paints, enamels, glass, and other related products. The manufacturing processes that pose the greatest risk for heavy metal exposure include galvanizing, soldering, etching, lead plating, smelting, wood preserving, and the manufacturing of thermometers, mirrors, incandescent lights, x-ray machines, and vacuum pumps [7].
Figure 2: Pathways of metal-induced oxidative stress
In addition to the immediate health consequences of heavy metal exposure, long-term exposure to these pollutants can have lasting effects on human health. For example, chronic exposure to lead has been linked to developmental delays, cognitive impairment, and behavioral disorders in children [9]. Furthermore, heavy metal exposure has been associated with an increased risk of cancer, especially in individuals with high levels of occupational exposure to heavy metals.
Given these serious health risks, it is crucial to continue raising awareness about the dangers of heavy metal exposure and take steps to minimize exposure to these toxic pollutants in workplaces and the environment [10]. This can include measures such as using protective equipment, implementing safe work practices, and promoting the use of alternative, less toxic substances in manufacturing processes.
Materials and Methods
Chemicals/Reagents
Tris–HCl, Phosphate Buffer (reagents), Hydrogen Peroxide (H2O2), n-Buthanol, Sodium Hydroxide (NaOH), Trichloroacetic acid (TCA), Sodium Nitropruside (SNP), Mercury (I) Chloride (HgCl2), Lead (II) Acetate (Pb (CH3COO)2), Thiobarbituric acid (TBA), Ferrous Sulphate (FeSO4), Arsenic (III) Oxide (AS2O3) and Deoxyribose were purchased from Sigma (St. Louis, MO, USA). All the other chemicals were commercial products of the highest purity grade available.
Phospholipids preparation
A simple method was used to extract the phospholipids from eggs. Briefly, 100 mL of 100 mM Tris-HCl (pH 7.4) was added to 1 g of egg yolk before being diluted. It was later used as a homogenate.
Lipid peroxidation or Thiobarbituric Acid-Reactive Species (TBARS) Assay
Lipid peroxidation was determined by measuring TBARS as previously described with slight modifications. In short, aliquots of the homogenate (100 µl) from phospholipids were incubated for 60 minutes in a medium containing 10 mM Tris–HCl, pH 7.4 in the presence of (control) and Sodium Nitropruside (SNP), Fe2+, As3+, Hg1+ and Pb2+ (Initial 1 mM and Final 100 µmol/L). The mixtures were incubated at 37°C for 60 min. The reaction was stopped by addition of 0.6 mL of acetic acid buffer and lipid peroxidation products were measured by the addition of 0.6 mL of TBA 0.6%. The color reaction was developed by incubating tubes in boiling water for 60 min. An aliquot of the supernatant was taken and the absorbance was read at 532 nm in a spectrophotometer. The data are expressed as % of inhibition in relation to respective control.
Statistical Analysis
The Data was statistically analyzed by one-way ANOVA, followed by Tukey’s multiple range tests when appropriate, and are presented as means ± SEM values average from 3 to 4 independent experiments performed in duplicate. Differences between groups were considered to be significant when P less then 0.05.
Results and Discussion
The replacement, reduction, and refinement (3Rs) ideas were put forward and introduced by Russell and Burch in 1959. In addition to advocating alternatives to animal experimentation and enhancing animal care, these concepts have a broader scope of use.
Similarly, they contain unsaturated fatty acid residues, phospholipids—more specifically, mitochondrial phospholipids—are vulnerable to oxidative burst. Phosphatidylethanolamine (PE), phosphatidylcholine (PC), and cardiolipin (CL) content in mitochondrial phospholipids were examined in a recent study by Wiswedel et al. to determine the impact of iron-induced oxidative stress. HPLC was used to determine the contents of PE, PC, and CL. The objective of the current study is to investigate the toxic effects of various inducers, including radicals/SNP, Fe, Hg, As, and Pb. Through this project, we aim to gain a deeper understanding of the potential harm caused by these toxic pollutants and their impact on human health.
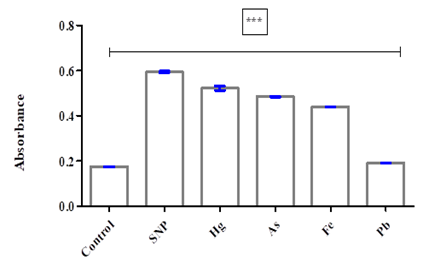
Our findings revealed that at a concentration of 100 uM, SNP, Hg, As, Fe, and Pb all had a significant impact in phospholipid degradation. Specifically, the degree of damage caused by these inducers followed the order of SNP > Hg > As > Fe > Pb. These results underscore the importance of further exploring the potential dangers of heavy metal exposure and the need for continued efforts to minimize exposure to these toxic pollutants.
Figure 3: Effect of different metals and SNP on lipid peroxidation in phospholipids obtained from egg yolk.
The experimental results obtained at a concentration of 100uM have revealed significant information regarding the impact of specific environmental pollutants on oxidative stress levels. The tested compounds, including SNP, Hg, As, Fe, and Pb, exhibited varying degrees of influence on oxidative stress compared to the control group. SNP caused a significantly higher level of oxidative stress, measuring 3.39 times that of the control, followed by Hg was 2.89 times higher, As was 2.76 times higher, Fe was 2.49 times higher, and Pb was 1.09 times higher, respectively.
Moreover, it was found that SNP had the highest potential for inducing oxidative stress, with a recorded increase of 67% compared to the control group. Mercury also exhibited a high potential for inducing oxidative stress, with a recorded increase of 65%. Arsenic and iron induced degradation in phospholipid homogenate at rates of 61% and 56%, respectively, compared to the control group. In contrast, lead showed the lowest potential for inducing oxidative stress, with only an 11% increase compared to the control. These findings provide valuable insights into the potential risks associated with exposure to these environmental pollutants and emphasize the importance of implementing measures to mitigate their adverse effects on human health.
Declarations
Author Contributions
All authors equally contribute.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul B Tchounwou, Clement G Yedjou, Anita K Patlolla, and Dwayne J Sutton. (2012). Heavy Metals Toxicity and the Environment. EXS, 101:133-164.
Publisher | Google Scholor - Jaishankar, M. Tseten, T. Anbalagan, N. Mathew, Beeregowda, et.al. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary toxicology, 7(2):60-72.
Publisher | Google Scholor - Ercal, N. Gurer-Orhan, H. & Aykin-Burns, N. (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Current topics in medicinal chemistry, 1(6):529-539.
Publisher | Google Scholor - Alengebawy, A. Abdelkhalek, S. T. Qureshi, S. R. & Wang, M. Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics, 9(3):42.
Publisher | Google Scholor - Ingrid Wiswedel, Andreas Gardemann, Andreas Storch, Daniela Peter & Lorenz Schild. (2010). Degradation of phospholipids by oxidative stress-Exceptional significance of cardiolipin. Free Radical Research, (44)2:135-145.
Publisher | Google Scholor - H. Jabeen, S. Saleemi, H. Razzaq, A. Yaqub, S. Shakoor R. (2018). Qureshi Investigating the scavenging of reactive oxygen species by antioxidants via theoretical and experimental methods. J. Photochem. Photobiol. B,180:268-275.
Publisher | Google Scholor - Jovanović, B. (2015). Critical review of public health regulations of titanium dioxide, a human food additive. Integrated environmental assessment and management, 11(1):10-20.
Publisher | Google Scholor - Hassan, A. S. (2019). Inorganic-based pesticides. a review article. Egypt Sci J Pestic, 5:39-52.
Publisher | Google Scholor - Kofman, O. (2002). The role of prenatal stress in the etiology of developmental behavioural disorders. Neuroscience & Biobehavioral Reviews, 26(4):457-470.
Publisher | Google Scholor - Charkiewicz, A. E. & Backstrand, J. R. (2020). Lead toxicity and pollution in Poland. International journal of environmental research and public health, 17(12):438.
Publisher | Google Scholor