Research Article
An Update on the Generation of the Gastrointestinal Tract in Neonates with Specific Emphasis on the Hurdles Encountered in Feeding Preterm Infants with Associated Complications like Gut, Brain &G-B Axis Generation: A Narrative Review
1Director, Kulvinder Kaur Centre for Human Reproduction, India.
2Director, Ex-Rotunda-A Centre for Human Reproduction, India.
3Department of Neurology, Swami Satyanand Hospital, India.
*Corresponding Author: Kulvinder Kochar Kaur, Director, Kulvinder Kaur Centre for Human Reproduction, India.
Citation: Kulvinder K Kaur; GNK Allahbadia, Mandeep S. (2023). An Update on the Generation of the Gastrointestinal Tract in Neonates with Specific Emphasis on the Hurdles Encountered in Feeding Preterm Infants with Associated Complications like Gut, Brain &G-B Axis Generation: A Narrative Review. Journal of BioMed Research and Reports. 2(1); DOI: 10.59657/2837-4681.brs.23.005
Copyright: © 2023 Kulvinder Kochar Kaur, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 15, 2022 | Accepted: December 20, 2022 | Published: January 02, 2023
Abstract
The 2nd as well as 3rd trimester of pregnancy is key regarding the anatomical along with functional generation of the GIT as well as the correlated digestive functions. Subsequent to premature birth, there is the presence of immaturity of both digestive along with absorptive events as well as that of the GI motility poses considerable hurdles. Information regarding the major generational stages of the events implicated in the digestion along with absorption of protein, carbohydrate as well as lipids and modes of the maturation phases beneath the generation of the gastrointestinal tract (GIT), might help clinical paediatricians regarding maximization of the nutritional management of preterm infants. The immaturity of these GIT systems along with function might possess a negative impact on the designs of gut colonization that biases towards the generation of aberrant microbiome. Hence in turn further aids in the alteration of functional, immune along with neural generation of the gastrointestinal tract (GIT), as well as in particular in preterm infants has been correlated with an escalated risk of GIT complications like Necrotizing enterocolitis(NEC).A greater insight regarding the physiological colonization designs in case of term along with preterm infants might aid in the facilitation of these designs as well as prevention of the microbial disturbances correlated with the generation of the various diseases all through life. Here we have tried to provide full detail over the generational characteristics associated with the maturational properties of the major GIT functions along with their impact subsequent to preterm birth. We have concentrated on the generational variations in case of intestinal digestive along with absorptive events, motility, gut-brain axis(G-B-A) besides gut microbes in preterm besides term infants. Further we have highlighted the pathogenesis of Hirschsprung disease.
Keywords: GIT generation; preterm infants; digestion; absorption; NEC; Enteric nervous system; interstitial cells of cajal; GBA
Introduction
The maturation of the gastrointestinal (GI) system in full term as well as preterm infants represents a field of considerable attention regarding the nutritional along with medical scenario point of view. Specifically in case of preterm infants under 28 gestational weeks, delivery comprises of a nutritional emergency where the infant possesses a greater along with problematic for meeting the nutritional requirements [1]. A factor that aids in the generation of the nutritional emergency is the comparatively lesser formed GI system in the preterm neonates that restricts their capacity of utilization of enteral nutrition. The GI system of the preterm infants illustrates reduction in absorption besides digestion abilities, delayed gastric emptying duration, along with restricted intestinal motility in contrast to full term infants [2]. The akin nutritional factors that result in a critical nutritional status change the reaction of the preterm infant to the orally delivered therapeutic substances [3].
The abnormal GI development in addition to other factors like early postnatal stress, changes in microbiota secondary to infections or in case of early utilization of antimicrobial agents in the neonatal intensive care unit (NICU) lead to a dysfunctional activation of the intestinal peristalsis along with of the gut- brain axis [4]. Interference with the physiological inflammatory reaction further aid in the generation of this dysfunction. Aberrant generation of the bowel function is the major deciding factorthe formation of food tolerance, a main hurdle in the NICU.
Structural in addition to functional maturation of the GI tract (GIT)is the requirement of the new born regarding digestion along with absorption of the nutrients from colostrum besides breast milk. Furthermore, their requirement is the total generation of the intestinal motor function that is inclusive of the coordination of the suck-swallow, continence along with the tone of the gastroesophageal sphincter, enough gastric emptying as well as intestinal peristalsis. Regarding facilitation of fast growth that take place immediately subsequent to birth full term infants can get enough quantities of nutrients. Nevertheless, 50% of the preterm infants have postponement of acquisition of enteral feeding volumes along with possess a gastroesophageal reflux, residual gastric constituents as well as constipation in view of postponed gastric emptying, protracted bowel transit, abdominal distension, as well as postponed meconium expulsion, all of these constituting the functions of immaturity [5]. Occasional studies are present regarding fetal development in addition to early adaptability of the neonates to motility along with barrier functions of the human intestinal mucosa [6].The formation of the functional constituents of the human GIT do not take place concomitantly; Actually the anatomical differentiation of the intestine in general takes place within 20wks GA, functional maturation gets postponed ,needing organized peristalsis in addition to coordination of suckling as well as swallowing ,that do not get marked till 20-30 weeks along with 32-34 weeks respectively [7].
Earlier we reviewed the generation of Hirschsprung Disease, where ENS is not properly developed generation of variable Gut Microbiota (GM) along with environmental factors in particular in early diet patterns in generation of T1D inclusive of TEDDY and other detailed studies, complications associated with preterm birth, role of Gut -Brain axis, GM in the generation of neuropsychiatric diseases like depression ,autistic spectral disorders, schizophrenia, besides impact of altered GM in adult with NPD, along with NDD(Neurodevelopmental diseases) [8-13].Thus here we conducted a narrative review regarding the full view of basic intestinal functions existent in the GIT in full term infants as well as preterm infants .Specifically generational variation in intestinal digestion along with absorption working, motility, Gut-Brain axis cross along with microbiome is detailed.
Methods
Here we conducted a review utilizing search engine PubMed, google scholar; web of science; embase; Cochrane review library utilizing the MeSH terms like GIT development; preterm infants; full term infants; feeding patterns; intestinal electrical activity; gastric emptying; NICU admission; antibiotic use; gut microbiome, necrotizing enterocolitis; n Neurodevelopmental aberrations from 1940’s till date in 2022.
Results
We found a total of 250 articles out of which we selected 95 articles for this review. No meta-analysis was done.
Generation of lipid digestion along with absorption in the neonate
The human GIT represents an organ possessing one of the maximum surface areas. The intestinal size illustrated a calculated 1000-fold enhancement from 5 to 40GW [14]. Autopsy outcomes have demonstrated these intestinal prenatal lengths as follows; 125cm by 20 GW, 200 cm by 30 GW along with 275cm at full term. The continuation of growth leads to it finally reaching 575cm by 20yrs of life [15]. On inclusion of the villus along with micro villus structure, the surface area in case of adults is calculated to be 200m2. This surface area continues to be in intricate contact with food, microbes as well as other probable antigenic constituents that possess a critical part in digestive along with absorptive events [16]. The large intestine is around 60cm in full term infants that escalates to150cm by adulthood [14].
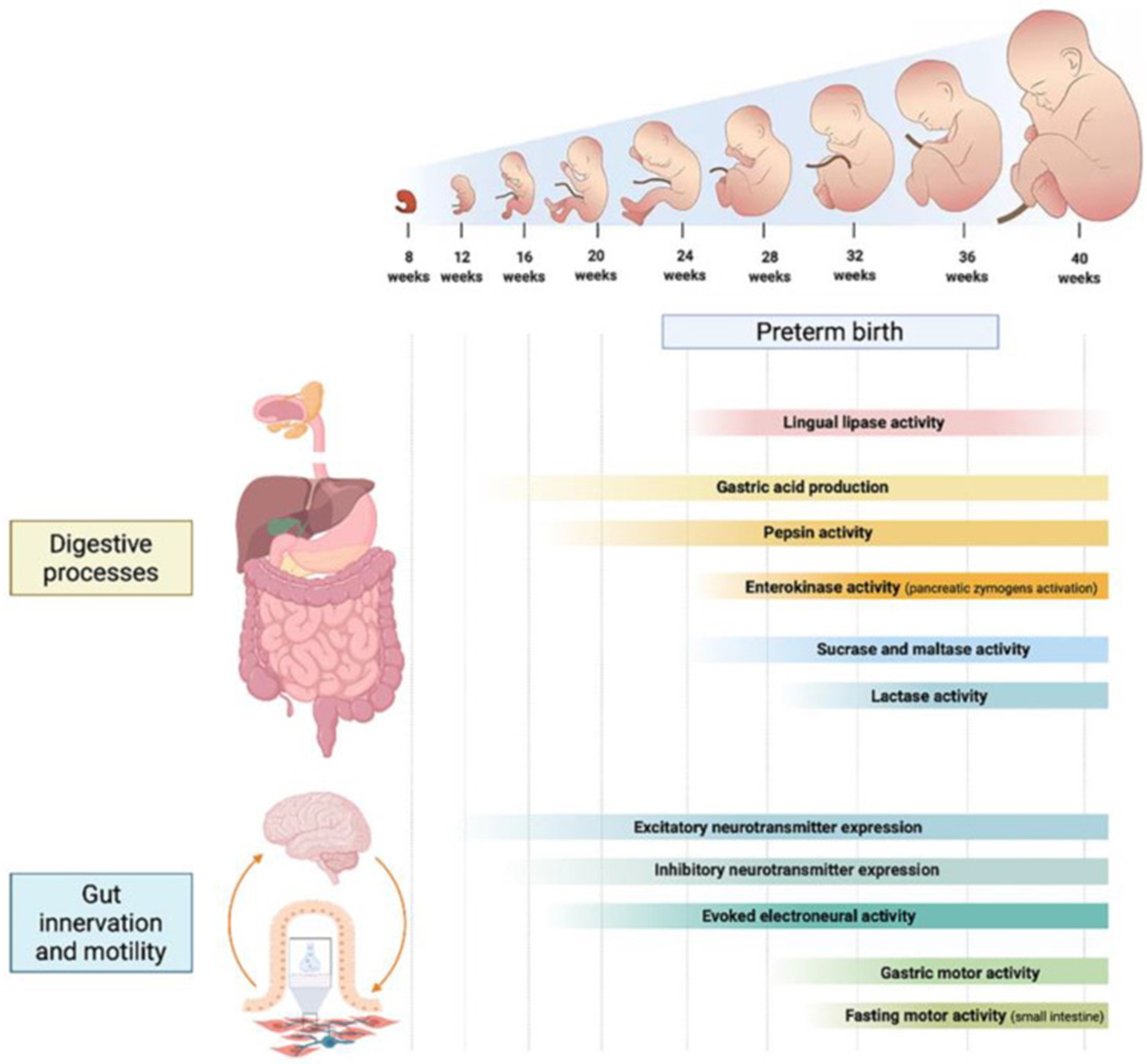
The GIT is made up of various structures inclusive of oropharynx, oesophagus, stomach, duodenum, jejunum, ileum along with colon. Every one of these possesses a unique function that is key for health. Believed to be an organ meant primarily regarding digestion, absorption, along with waste excretion it aids in total health in a considerably wider functionality [17]. Besides digestion along with absorption the intestinal tract possesses neural, endocrine, exocrine along with immunological functions [18]. The major generational characteristics regarding protein, carbohydrate as well as lipids is detailed later with representation in fig 1.
Figure 1: Courtesy ref no-19-Developmental phases of the main digestive processes, gastrointestinal innervation and motility patterns throughout different gestational weeks.
Proteins
The suppliers of proteins regarding term along with preterm infants are constituted by 2 main constituents; namely whey as well as casein. The digestion of proteins starts in the stomach, where hydrochloric acid gets liberated by the parietal cells combined with proenzyme pepsinogen, breakdown certain of these proteins. By the latter part of first trimester of pregnancy active liberation of gastric acid takes place along with by the 2nd trimester [20]. At the time of the initial 2mths subsequent to birth this gastric acid generation doubles [21]. The proenzyme pepsinogen can be discerned in the fatal stomach by the17- 18th GW. Subsequent to birth pepsin action parallels the infant’s maturity status [22].
Other main enzymes get generated by the pancreas at this particular time that are inclusive of trypsinogen, chymotrypsin along with carboxypeptidase, that are all zymogens whose requirement is to get activated by enterokinase, whose formation takes place in the upper intestinal epithelial cells. At the time of 24 GW enterokinase activity is observed in older infants [23]. These enzymes degrade larger protein molecules into oligopeptides, dipeptides as well as single amino acids. Once these molecules move towards lower portions the absorptive event in the small intestinal cells take place. This is associated with various transporter modes. Restriction of these event occur in case of preterm infants [24].
Hence the query arises is the tolerance of hydrolysed protein formulas better thus provision of escalated nutrition is feasible for these preterm infants? As per a study it was illustrated that in contrast to the infants that were in receipt of standard formulas, better tolerance provision took place in infants receiving hydrolysed protein formulas along with lesser time for attaining total feeds [24].
Carbohydrates
Salivary in addition to pancreatic amylases are implicated in the luminal digestion of complex sugars. Digestion subsequently into oligosaccharides takes place by absorptive hydrolysis into mono saccharides at the epithelial brush border by enzymes that are inclusive of lactase, sucrase, maltase, isomaltose along with glucoamylase. The activation of sucrase, maltase as well as isomaltose have been illustrated to get totally present in preterm infants. The activity of lactase that result in hydrolysis of lactose is the maximum enriched of disaccharides into glucose as well as galactose taking place is lesser in case of preterm infants [23].
The digestion of complicated carbohydrates take place through salivary amylase, digesting them of 18-29 glucose units starts the carbohydrates digestion [26]. The liberation of pancreatic amylases is restricted, whose liberation does not attain adult quantities till 2 years of age is implicated, for the maximum starting of early carbohydrates hydrolysis [27]. In view of lack of suck-swallow coordination, enteral feeding in various infants makes it essential to utilize feeding tubes. By bypassing the oral cavity by this they bypass the salivary amylase as well.
The carbohydrates that remain undigested move towards the distal intestine. Microbial fermentation occurs here that leads to the formation of short chain fatty acids (SCFA), whose absorption takes place following that. Utilization of these can take place in the form of energy primary resources, however butyrate has been illustrated to act in the form of considerably important fuel for the colonocytes, besides possessing the characteristicof changing proliferation, differentiation along with turnover of colonic cells [28].
From this angle lactose is in particular Intriguing from this point of view. In view of them being the primary carbohydrate resources in milk, lactose intolerance occurs just occasionally in the preterm infants. The transformation of unabsorbed lactose to SCFA gives provision of an effective pathway for salvaging. In a study it was pointed that the induction of lactase activity is feasible in preterm infants with enteral feedings of human milk [29].
Maximum lactase activity observation takes place at the middle of the top of the villus, whereas sucrase, maltase along with glucoamylase activity are seen in the mid-villus [30]. At the time of intestinal mucosal damage, the cells possessing high lactase are the ones that get damaged first, besides get total restoration at the end via the events of cell migration from the crypt to the tip of the villus.
Insulin possesses a significant part in intestinal maturation along with might aid in escalation of lactase activity subsequent to preterm birth. Amniotic fluid possesses protein carbohydrate lipids, electrolytes, immunoglobulins, along with growth factors. Up till 20μU/mL Insulin exists in amniotic fluid besides subsequent to the finishing of complete keratinization (about26wksGA), amniotic fluid represents the major Insulin resource. These significant fatal events get blocked in an abrupt fashion illustrated with the local insulin exposure of the GIT that possess deleterious sequelae [31].
Oral Insulin delivery in preterm infants up till 28days subsequent to delivery has been found to be efficacious regarding doubling of the lactase activity.
Lipids
Triglycerides (TG) comprise 50% of the non-protein constituents of human milk along with formula [18]. TG digestion gets started by bile Acid micellar emulsification which generates smaller droplet of TGs. This leads to larger surface area for crosstalk with lipases that result in hydrolysis of long chain triglycerides into monoglycerides along with free atty acids (FFAs), that in turn get absorbed into the small intestine epithelial cells. Of the lipases, pancreatic lipases is maximum significant of all the lipases.
The FFAs as well as 2 monoglycerides undergo re-esterification amongst the epithelial cells that get followed by transformation into the chylomicrons whose transportation take place in the basal area of the epithelial cells through the thoracic duct from there they reach the blood circulation.
Medium chain triglycerides (MCT) digestion along with absorption is less complicated in contrast to long chain triglycerides. Bile Acid micellar emulsification is not needed by them, get absorbed into the enterocytes without any re-esterification, besides travelling directly into the portal venous system. MCT’s are usually advocated for patients with lymphatic obstruction [33].
Variable resources of lipase are inclusive of the lingual, gastric, pancreatic as well as epithelial cells. Human milk possesses a lipase known as Bile salt stimulated lipase. Subsequent to activation in the small intestine in the existence of Bile acids it promotes the digestion of long chain triglycerides. Liberation of lingual lipase takes place from the glands present at the base of tongue whose activity is lesser atbirth – infants at 26 GW, attain peak quantities at 30-32 GW, followed by reduction around term [34]. Deficiency of pancreatic lipase is commoner in case of preterm infants in contrast to older ones. Acquisition of adult quantities are in general not attained till 6mthsubsequent to birth [35].
The other restriction of fat digestion is associated with BA, whose formation along with excretion take place from the liver through the biliary system at comparatively lesser quantities in very low birth weight (VLBW) preterm infants in contrast to full term infants. Furthermore, bile reabsorption is lesser in preterm infants, that is implicated in lesser efficacious digestion of lipids in contrast to full term infants [36].
Essential fatty acids cannot be generated by the body, thus become deficient if enough amounts are not given in the diet. Hence for avoidance of deficiency enough dietary maximum consumption of linoleic acid as well as linolenic acid fatty acids can avoid deficiency. Moreover, linoleic acid as well as linolenic acid fatty acids (18 -carbons length of chain) go via breakdown along with elongation enzymatic events into long chain polyunsaturated chain fatty acids(LCPUFA’s), with greater than 20 carbons. They are needed for the formation of eicosanoids along with central nervous system(CNS) structure[37],it becomes problematic in preterm infants not possessing the enzymatic capacity of going via these transformations. Numerous preterm infants have the provision of formulas where supplementation of these LCPUFA’s is not done, besides even those in in receipt of human milk might not get enough amounts of these significant lipids [38].
Generation of intestinal motility
Intestinal motility, that is inclusive of coordinated motion of the GIT like mixing, progression of motor actions in addition to relaxation that is receptive. Controlling of these movements take place by numerous regulatory systems inclusive of extrinsic neurons, intrinsic neurons, the enteric nervous system (ENS), Interstitial cells of Cajal (ICC’s), cells that express platelet-derived growth factor receptor alpha (PDGFRα) as well as myogenic modes, which all possess the capacity of working simultaneously [39]. The main maturational properties of Intestinal motility are described subsequently with highlighting in figure1.
Anatomyalong with Physiology of motility of Gastrointestinal Tract
For every area the movements of Gastrointestinal Tract (GIT) are variable. Furthermore, they display considerable variations in association with prematurity [40].
Numerous studies are listed in the literature regarding GI motility in the preterm new-born infants in contrast to full term infants, possessing the characteristics of dysfunctional gastric electrical activity besides gastric emptying. Poor organization of gastric motility takes place in preterm infants along with fundal as well as antral pressure waves escalate with escalating gestation age (GA) [7,41]. Specifically, this is applicable to preterm new-borns amongst 28-32 weeks GA, whereas preterm infants amongst 32-36wks GA illustrate gastric electrical activity besides gastric emptying much more intricately in contrast to full term new-born infants.
Myoelectrical activity dictates the gastric motility which is constituted by rhythmically taking place transmembrane potential variability known as slow waves, which are independent of motor activity [42]. These waves passing slowly via the GIT by definitions are called motor migratory complex (MMC), taking place in each 2-4h with the idea of clearance of the GIT of undigested material, mucus besides debris [43]. MMC, to start with take place in humans just in view of vagal supply along with liberation of motilin in the duodenum. Conversely the coordination of their progression takes place via enteric neurons. MMCstake place in humans just at the time of fasting along with in small intestines in contrast to other species. Phasic contraction is correlated with a separate type of electrical activity known as electrical responses activity possessing the properties of spikes which are superimposed over the slow gastric waves. The initiation of electrical activity is observed on the greater curve, around the proximal corpus; the area from which the slow gastric waves progress towards the pylorus [43].
Variable motor patterns take place in the proximal as well as distal stomach. In case of proximal stomach receptive relaxation along with accommodation take place, both of which are modulated by neurons in brainstem through vagal reflexes. The lower stomach illustrates separate motor patterns just at the time of feeding along with fasting. During fasting, the lower stomach performs grinding along with mixing. Extrinsic neurons are not necessary regarding this contraction action; however, it can get manipulated via vagal pathways.
Intestinal motor activity is further associated with gestation age as well as motor generation, that gets divided in phases I, II, III of the migration of the motor complexes amongst 37wks GW besides term age [44]. Specifically, the frequentness of duodenal contractions, their number/impulse along with peak intraluminal pressure of duodenal motility in preterm in contrast to full term infants are variable [41]. Phasic contractions that are clustered are commoner, however are less frequent as well as possess intraduodenal coordination having greater immaturity in preterm infants [45].
A part of insulin on gastrointestinal motility has been corroborated by Shulman et al. [32]. who illustrated considerably significant reduction in gastric residuals at the time of first month on receipt of treatment with oral insulin as opposed to their untreated numbers [32].
Enteric Nervous system (ENS)
On reaching 12GW, the fetal colon possesses a rich neuronal network in the myenteric plexus along with expression of excitatory neurotransmitters as well as synaptic markers take place. Rather the markers of hampering neurotransmitters make their appearance as late as 14 GW. Nevertheless, electrical train stimulation of internodal strains did not elicit activity in the12-14-week GW tissues [46].
Initiation of the elicited electrical activity in the human fatal ENS make appearance at about 16 GW. This activity is apparently concordant with escalation of gene expression of different ion channels regarding which we possess knowledge of possessing the capacity of manipulation of enteric action potential. The generation temporally of different neural subtypes in addition to enteric glial cells (EGC) take place amongst 12 as well as 16 GW [47]. The ENS comprises of 3 parts namely i) enteric neurons ii) enteric glial cells as well as iii) Interstitial cells of Cajal (ICC’s). They generate 2main ganglionated structures in addition to functional subunits;i) submucosal plexus (alias Meissner’s plexus) along with Myenteric plexus (alias Auerbach’s plexus). Meissner’s plexus resides in the connective tissues of submucosa, besides providing innervation to the muscularis mucosae, intestinal neuroendocrine cells, glandular epithelium along with submucosal blood vessels, whereas Auerbach’s plexus placement is amongst circular along with longitudinal muscle layers besides being correlated with the contractile actions of circular along with longitudinal muscles [48].
Evaluation of the intestinal samples of the mice illustrated that ENS takes its origin from the cells of the vagal neural crest known as neural crest precursor cells (NCC) which make entry into the foregut at embryonic day 9 followed by rostro caudal migration along with colonization of the full intestine by embryonic day 14 [49].
At the time of sacral NCC migration to the hindgut 19 is directly targeted by Sox10 for generating Sox10 cadherin19-cadherin complexes, that crosstalk with the cytoskeleton in cells that are migrating [50]. Subsequent to migration phase proliferation of NCCs take place that result in formation of millions of ENS cells. Subsequently NCCs congregate into groups (Myenteric ganglia or submucosal ganglia) followed by differentiation into a wide kind of enteric neurons as well as glial cells besides generating complicated neuronal networks essential for regulated intestinal activity [51].
Variable population of fibroblast like Interstitial cells exist in the adult gut. Elimination or impairment of these cells have been associated with a broad kinds of GIT conditions. This wide cell kinds constitute different subpopulations of Kit positive or fibroblast like cells with expression of PDGFRα. ICCs reside place at the myenteric plexus level (ICC-MY), modulate small waves, the electrical processes that time the taking place of phasic contraction [52]. Proof accrual is going on regarding cells that illustrate expression of ICCs along with PDGFRα residing within as well as surrounding GI muscle bundles acting as intermediate excitatory as well as hampering neuromuscular transmission.
Converse to enteric neurons along with glial cells, ICCs, rather than originating from neural crest, possess an embryonic generation even before the NCCs reach the intestine.
Numerous studies pointed that smooth muscle cells along with ICCs are obtained from a shared mesenchymal precursor subsequent to epithelial –mesenchymal transition (EMT) event. ICCs along withCD4+ fibroblast like cells are obtained from the coelomic epithelium, most probably from a shared ancestor that has expression of chloride channel anoctamin 1 along with smooth muscle actin alpha. ICC phenotype differentiation at the time of embryogenesis is based on cells signalling through the receptor tyrosine kinase (Kit) [53] [see fig2&3 for the generation of c-kit positive Interstitial cells of Cajal along with pathogenesis of Hirsh sprung disease].
Recently conducted studies regarding generation of gastric electrical activity, myogenic regulation was observed to be immature at birth along with pointed that an event of generation from 1week -6months.
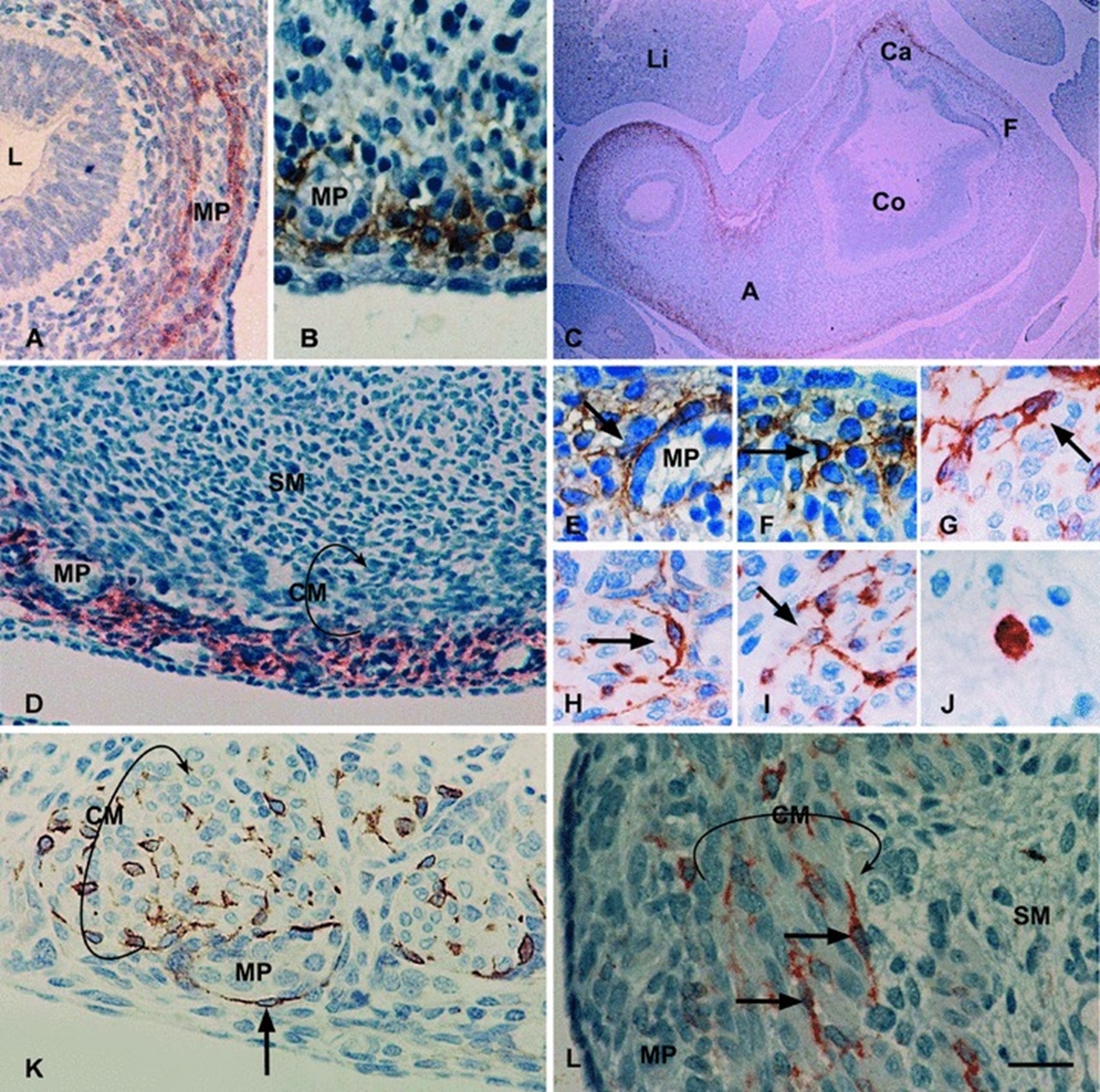
Figure 2: Courtesy ref no-94-c-kit immunohistochemistry. (A–L) Embryos and foetuses aged 7–14 weeks. (A) 7 weeks, antrum. C-kit-IR cells located in the external portion of the stomach wall, on both sides of a group of myenteric plexus elements. These cells can be considered ICC precursors. (B) 8 weeks, corpus. C-kit immune-positive cells are grouped at the myenteric plexus, enveloping a ganglion. These cells have small triangular body with three or more thin processes. (C) 8 weeks. The belt of c-kit-IR cells is present throughout the stomach circumference, except in the fundus, where it is very thin or absent. (D) 9 weeks, antrum. C-kit immune-positive cells are located external to the grouped myoblasts of the circular layer. (E) 8 weeks, corpus. C-kit-IR cell (arrow) with at least three processes. (F) 8 weeks, corpus. C-kit-IR cell (arrow) with at least three processes. (G) 11 weeks. An ICC-MP with three processes (arrow), one of which bifurcates. (H) 11 weeks, corpus. One spindle-shaped ICC-MP (arrow) with two primary processes that branch further into secondary processes. (I) 11 weeks, corpus. Four highly ramified ICC-MP, one of which has at least four processes (arrow). (J) 14 weeks, corpus. In the submucosa, isolated round c-kit-IR mast cell is seen. (K) 11 weeks, corpus. The arrow indicates ICC closely apposed around a ganglion. These cells can be considered ICC-MP. (L) 12 weeks, antrum. Two spindle-shaped ICC-CM (arrows) within the circular muscle layer. CM, circular muscle layer; MP, myenteric plexus; SM, submucosa; L, lumen; Li, liver; Ca, cardias; F, fundus; Co, corpus; A, antrum. Bar: A, D = 50 μm; B, K, L = 25 μm; C = 250 μm; E–J = 20 μm.
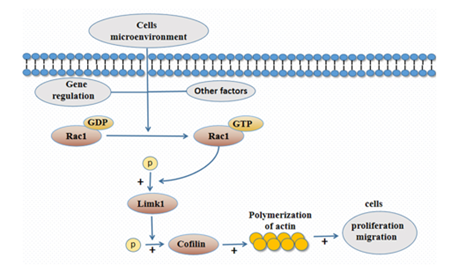
Figure 3: Regulation mode diagram of Rac1/LIMK1/ coffin signal pathway
The Gut Microbiota-Brain (GM-B)-Axis
At the time of early postnatal duration, infants go via congruous gut formation that is corresponding along with inter based despite not being concomitant with brain formation always. At the time of 1st years of life mammoth alterations takes place in the gut regardingmicrobiota that are resident along with considerable maturation of enterocytes taking place besides that of ENS. In the meantime, the generation of brain along with CNS undergo growth at a rapid pace, both regarding brain volumes as well as neural function.
The reason there is interdependency of the 2 generational events is in view of them getting modulated via the gut- brain axis that comprises of a bidirectional crosstalk amongst the gut along with brain, that are constituted of various intersected constituents [54]. In the recent decades part of microbiota in the gut- brain axis (GBA) has been evaluated in great details, thus the GBA is termed as microbiota-GBA (M GBA) [55]. Regarding this the gut microbiota (GM) changes have been directly correlated with physical as well as mental health via stepwise modes that still have to be determined totally [56].
The communication amongst gut along with brain at the time of early generation implicates both top-down along with bottom-up modes with signals from the brain manipulating the GI functions along with GI obtained molecules impacting brain events via variable pathways. Despite the characteristics of MGBA in health along with disease have been detailed considerably, the information of the precise modes via which the gut along with brain connect as well as crosstalk in young infants is still not clarified.
The modes implicated in the crosstalk amongst gut along with brain are inclusive of neural, humoral, endocrine, immune as well as metabolic mediators [57].
The neural connections are inclusive of the CNS, ENS as well as the autonomic nervous system (ANS). Inputs are received by ENS from the brain that in return gives ascending knowledge via neural circuitry. The ANS is conventionally divided into sympathetic nervous system (SNS) as well as parasympathetic nervous system. The SNS possess a hampering impact on the gut, whereas the vagal nerves (VN) apparently possess mm the capacity of sensing hormones, cytokines along with metabolites from the GIT, sending afferent signals to the brain. The generation besides the myelination of VN is not finished at birth with continuation till adolescence, with peak of myelination rate seen in the 1st month of life, place at the time of maximum consumption of human milk along with human milk obtained bioactive substances [58]. The ANS communicates with the limbic system that is comprised of hippocampus, amygdala, as well as the limbic cortex, that are implicated in different brain events in health along with disease [57].
The humoral constituents of the MGBA are comprised of the hypothalamo-pituitary-adrenal (H-P-A) axis, the enteroendocrine system along with the immune system. Stress responses get modulated via the H-P-A through the liberation of corticosterone, adrenaline as well as nor adrenaline. The enteroendocrine cells (EEC) in the gut generate GIT hormones like ghrelin, glucagon like peptide 1(GLP-1), cholecystokinin, along with peptide YY (PYY), all hormones that control food consumption as well as satiety, besides being implicated in control of emotions along with mood [54].
The immune cells that are resident in the brain, namely microglia work in the form of neuroimmune constituents of the MGBA. They finetune neurogenesis along with synaptogenesis. The microglial maturation besides function is apparently associated with alterations in the GM, besides getting impacted by Short Chain Fatty Acids (SCFA) [59], that are end-products of bacterial fermentation of dietary fibres along with starch that is resistant in colon. Moreover, molecules regarding immune signalling whose formation is further guided by GM might play a part in the MGBA by binding to VN receptor by crossing blood brain barrier (BBB).
Regarding this recently conducted studies pointed the probability of bacterial peptidoglycans (PGN), that represent constituents of cell wall that get liberated besides from exogenous bacteria, are further liberated by the resident GM. It got posited that the PGN might get to the systemic circulation for arrival at the generating brain. There the expression of molecules that sense PGN occur in mammoth quantities at the time of particular windows of perinatal formation, thus the probability of a direct impact on brain function along with generation takes place [60].
Lastly a central part in the MGBA is performed by the metabolic mediators that is inclusive of tryptophan metabolites, serotonin [5-hydroxy tryptamine (5HT)], melatonin, SCFA’s, as well as other neurotransmitters. Serotonin [5HT] is implicated in maximum branches of MGBA, like it works in the form of a neurotransmitter both in CNS along with ENS as well as 5HT receptors possess a key function in the HPA [61]. The generation of host 5HT in the gut is controlled by microbiota in the form of native spore generating bacteria obtained from mouse along with human microbiota have been illustrated to facilitate 5HT bio generation from the enterochromaffin cells of colon, hence influencing the GIT motility along with homeostasis [62]. SCFA’s, inclusive of butyrate, acetate, propionate are believed to impact gut- brain connection in variable probable pathways, directly or indirectly. On generation by fermentation in the colon, SCFA’s can directly influence intestinal mucosal immunity along with modulation of intactness besides functioning of the gut- barrier. Moreover, by crosstalk with the EECs, SCFA’s can facilitate a direct gut- brain signalling via the liberation of gut hormones, like GLP-1 as well as PYY, besides other metabolic mediators like Gamma amino butyric acid (GABA)”; along with 5HT. Additionally, induction of systemic inflammation is feasible via differentiation, of T regulatory cells along with liberation of interleukins potentially by working centrally in the CNS by manipulating neuroinflammation [63].
Numerous factors possessing the capacity of impacting MGBA formation in particular in early life are present; of which the one possessing maximum importance may be prematurity, since preterm birth interferes with the physiological growth along with generation of GIT as well as nervous system besides result in some extent of microbial dysbiosis. In spite of these no studies are present for detail the distinct characteristics of the MGBA in preterm infants [64]. Additionally, nutrition at the time of sensitive time of generational window of sensitive time is believed to possess a main influence over the microbiota -gut-brain axis interaction [65], either by actions on single nutrient (like milk fat globule membrane [66], human milk oligosaccharides [67], or via the well appreciated advantages of just human milk feeding in contrast to other feeding resources [68].
The objective of maximum studies was detailing correlated properties of MGBA on the utilization of rodent models despite the gut as well as brain formation in rodents do not precisely replicate the human generational patterns [69]. In view of this the further pre-clinical investigations regarding MGBA research need the researchers to target animal models possessing akin anatomy along with physiology to humans (like piglets). These models would aid in acquisition of greater insight regarding MGBA at the time of early generation that would help in translating pre-clinical research into clinically meaningful clinical findings.
Besides the aiding of studies dependent on animal models, the feasibility of the microbiota -gut-brain crosstalk further at the time of early generation is corroborated by clinical findings; thus far restricted studies number have illustrated an association amongst particular microbial characteristics in the gut at the time of early post-natal duration along with neurogeneration in term or preterm infants [70,71].
The probability of correlation amongst microbiota as well as neurogeneration is specifically interesting for preterm infants. In view of the fact that prematurity per se results in microbial dysbiosis along with inherent risk of changed generation. It has been pointed that the interaction amongst early dysbiosis besides gut- brain axis might be implicated in laying the cornerstone for changed neurogeneration that is frequently correlated with preterm infants who undergo Necrotizing enterocolitis (NEC) [ 72]. Moreover, a probability of early changes of GMBA in latter phase of neurogeneration is posited regarding preterm infants not essentially undergoing via main neonatal complications like NEC. Like in the French EPIFLORE population study, the characteristics of GM in the 1st month of life in very preterm infants correlated with the 2year neurogenerational results despite subsequent to adjusting for confounders [70]. Akin to that a small cohort of very low birth weight infants, an association amongst particular microbial properties in the 1st month of life (like comparatively enrichment of Bifidobacterium) along with impairment of neurogeneration at24mths correction of age was pointed) [71].
Generation of the Gut Microbiome
In the human growth along with generation intestinal microbiota role is considerably significant. The human gut gives residence to trillions of bacterial, viral as well as fungal microorganisms which possess a symbiotic association with the host which possesses key part in health and disease [73,74].
There is a dynamic association amongst microbiome as well as host with numerous maternal factors (like genetic background, maternal diet before along with subsequent to pregnancy, maternal microbiome, delivery mode (like [lower segment caesarean section (LSCS), gestation age, perinatal stress, Infections at the time of pregnancy as well as delivery, Infections at the time of perinatal or neonatal duration, feed kind of neonates, in particular in preterm infants, besides other environmental factors like temperature, humidity, pH, along with oxygen amounts in the tissue kinds)result in the modulation of infants microbiome constituents along with its dynamic association with the host which can be converted from symbiotic to symbiotic along with can become life costing [75].
Noticeably, in a recently demonstrated narrative review in the past 10years numerous studies performed regarding meconium have pointed that the feta’s might get exposure to microbiota in utero, despite it was believed earlier that the amniotic fluid is sterile [76]. Placental colonization via a haematogenous route has been posited to be implicated in the fatal colonization prior to birth [77]. Advancements in high throughput nucleotide sequencing techniques have escalated GM results by evaluation how bacterial community generate over time, the factors impacting them, along with the method by which they influence health and disease [78].
Gut Microbiome along with Term Infants
The microbiota conducts foundational functions in human health in particular at the time of childhood. The initial year of life is believed to be a key window regarding any modulation of the intestinal microbiota for facilitation of the appropriate generation of the immune system along with human health as well [79]. The initial exposure of humans to these microorganisms take place at birth subsequent to the delivery of the baby, the entry of microorganisms takes place followed by their settlement in the intestine.
The environmental determinants of diabetes in the Young (TEDDY) [rev by using ref 1] that detailed the considerable significance of the intestinal microbiota in the initial year of life, illustrated that the formation of microbiota in a full-term infant gets impacted by breast feeding, kind of birth, geographical placement along with the sibling’s presence at home [80].
Conversely other studies, are concentrating on the influence maternal factors, antibiotic utilization, host genetics, ethnicity along with COVID-19 infections as well have on impacting microbiota generation [81]. Hence changes in the intestinal ecosystem alias dysbiosis at the time of key generation duration are usually correlated with different types of pathologies.
Gut Microbiome along with Preterm Infants
Prematurity at the time of birth has become a deciding factor in the generation of the intestinal microbiota [82]. The premature baby subsequent to birth gets admission in NICU, hence exposure takes place to hospital acquisition of bacteria in contrast to the bacteria we have a greater familiarity with in such babies [83]. Moreover, the clinical events to which such infant received exposure like ventilation, artificial feeding besides receipt of antibiotic, change the generation of the microbiota. Furthermore, Preterm infants possess immature GIT in addition to naïve immune system [84].
Numerous studies performed have illustrated the fashion in which this immaturity along with variations of the microbiota influence the generation of the intestinal function besides the anatomy of intestinal villi, crypts along with intestinal barrier [85], that result in robust disorders in the premature new-borns like late onset sepsis (LOC) along with NEC [86]. Considerable work has been done over the last 20-30 years for acquiring insight regarding the microbial properties of LOC along with NEC, besides forceful outcomes have correlated both of the disorders with intestinal dysbiosis.
Human milk is believed to confer protection against of LOC along with NEC since it provides immunological advantages to the baby, besides provision of microbes that are nutrient exclusive, which hence cause enrichments of the immune system of the new-born [87]. In particular provision of nutrients like oligosaccharides, occurs with the utilization of Human milk, that is necessary for the growth of Bifidobacterium kind of bacteria [88]. Bifidobacterium has been demonstrated to have immune-modulatory along with anti-inflammatory characteristics by provision of strength to the intestinal barrier along with reduction in the risk of NEC [88,89]. Premature new-born’s possess lesser quantities of these bacterial species, hence have greater risk of exposure to NEC. The effectiveness along with safety of probiotics delivery for manipulating early gut microbiome are under investigations [90] [earlier we have reviewed the role of probiotics &prebiotics for avoidance of obesity, Type 1 Diabetes Mellitus(T1DM), Non-alcoholic Fatty Liver Disease (NAFLD), Non-alcoholic steatohepatitis (NASH) [91-93]. Acquisition of greater insight of the microbiome with the utilization of appropriate strategies would aid in the evaluation innovative target therapeutic approaches in preterm disease besides in wider kinds of pathological conditions.
Conclusion
The intestine reflects a huge interface amongst the internal along with external environment. The 2nd as well as 3rdtrimester of pregnancy is key regarding the anatomical along with functional generation of the GIT as well as the correlated digestive functions. Subsequent to premature birth, there is the presence of immaturity of both digestive along with absorptive events as well as that of the GI motility poses considerable hurdles for the generation of enteral consumption in the population of preterm infants along with are usually implicated in the generation of preterm correlated complications that vary from food intolerance to NEC. Regarding gut immaturity, the ideal nutrition source for preterm infants is foundationally to keep remembering that mother’s milk works in the form of a magic bullet although further work needs to be continued in the context of any substitute for mother’s milk like donor milk along with formula. In particular the probable actions of donor milk treatment like freezing along with pasteurization on milk bioactive characteristics require future assessment, whereas the further research regarding infant formula needs to concentrateon generating a milk substitute that would prove to be nutritionally enough along with simultaneously have great tolerance as well as easy digestibility for such immature infants.
Furthermore, lot of corroboration is there regarding the part of resident microbiota in the intestinal tract in the immune system formation along with crosstalk with the gut as well as CNS. A double crosstalk exists amongst GI generation besides colonization with microbes; whereas the immature anatomical as well as functional properties of preterm gut bias to an aberrant intestinal colonization with the microbiota that would have functional implications ,besides the immune as well as CNS generation of the GIT, It has to be taken into account that the perinatal period most probably is equivalent to a key time period when the setpoints get influenced, hence it is essential to get greater insight regarding the normal along with healthy colonization patterns in infants for facilitation of these patterns along with avoidance of disturbances resulting in disease.
Moreover, we have highlighted the etiopathology of the generation of Hirsh sprung disease with emphasis on the generation of ENS, its association with ICC as well as a in turn with c-kit and how it might be important to get insight regarding early insight once no passage of stool occurs for early diagnosis.
References
- Hardinge JE, Cormack BE, Alexander T, Alsweiler JM, Bloomfield FH. (2017). Advancements in nutrition of the new-born’s infant. Lancet. 389:1660-1668.
Publisher | Google Scholor - Pouquet L, Wooster TJ. (2016). Infant digestion physiology and the in vitro biochemical models to test infant formula lipid digestion. Mol Nutr Food Res. 60:1876-1895.
Publisher | Google Scholor - Mooij MG, DeKoning BA, Huijsman ML, De Wildt SN. (2012). Ontogeny of oral drug absorption processes in children. Expert Opin Drug Metab Toxicol. 8:1293-1303.
Publisher | Google Scholor - Lin HC. (2004). Small intestinal bacterial overgrowth: a framework of irritable bowel syndrome. JAMA. 292:852-858.
Publisher | Google Scholor - Lucchini R, Bizzarri B, Giampietro S, DeCuris M. (2011). Feeding intolerance in preterm infants. How to understand warning signs. J Maternal -Fetal Neonatal Med. 24(S1):72-74.
Publisher | Google Scholor - Lebenthal A, Lebenthal E. (1999). The Ontogeny of the small intestinal epithelium. J Parent Enter Nutr. 23:S3-S6.
Publisher | Google Scholor - Indrio F, Riezzo G, Cavallo D, Di Maurro A, Francavilla R. (2011). Physiological basis of food intolerance in VLBW. J Maternal -Fetal Neonatal Med. 24:64-66.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2021). ’Are we any close to unraveling the mechanism of interactions among susceptibility genes towards Type 1 Diabetes, Gut Microbiota Along with Environmental factors, specifically early diet patterns –A Systematic Review’’. Endocrinology and Surgical Endocrinology. 2(1). DOI: http;//doi.org/03.2021/1.1005.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2020). The association of dietary fatty acids and gut microbiota alterations in the development of neuropsychiatric diseases: A systematic review. Obes Res Open J. 7(1):19-45. doi: 10.17140/OROJ-7-143.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2021). An Update on the Insight of Embryonic, Etiopathogenesis, Current Surgical Advances Besides the Advocated ERNICA Guidelines for the Management of Rectosigmoid Hirschsprung Disease, and Clinical Referral Score Model for Early Diagnosis with Future Use of Stem Cells - A Systematic Review. Acta Scientific Ophthalmology. 4(11):1-9.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2019). “A Review Prevention of Preterm Births and its Complications - An Update”. Acta Scientific Paediatrics. 2(12).
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2020). Intestinal Immune System in the Regulation of Obesity and Metabolic Syndrome-Therapeutic Implications-A Systematic Review
Publisher | Google Scholor - Kulvinder Kochar Kaur. (2021). Can Targeting Gut Microbiota in Perinatal Events Aid in Prevention of Type 1 Diabetes Development – Influence T1D Genes Crosstalk with Environmental Factors
Publisher | Google Scholor - Gray H, Warwick R, Williams PL. (1980). Gray’s Anatomy, 36thed; Saunders: Philadelphia, PA, USA.
Publisher | Google Scholor - Weaver LT, Austin S, Cole TJ. (1991). Small intestine length: a factor essential for gut adaptation. Gut. 32:1321-1323.
Publisher | Google Scholor - Mezoff EA, Shroyer NF. (2016). Anatomy and physiology of small and large intestines. In Paediatric Gastrointestinal and Liver disease.5 thed; Wyllie R, Hyams JS, Kay M, Eds; Elsevier: Amsterdam. The Netherlands.
Publisher | Google Scholor - Montgomery RK, Mullberg AE, Grand RJ. (1999). Development of the human Gastrointestinal tract: twenty years of progress. Gastroenterology. 116:702-731.
Publisher | Google Scholor - Neu J, Douglas-Escobar M, Fucile S. (2016). Gastrointestinal Development: implications for infant feeding. In Nutrition in Paediatrics, 5 thed; Duggan C, Watkins JB, Koletzko B, Walker WA Eds; People’s Medical Publishing House: Beijing, China. 1;387-398.
Publisher | Google Scholor - Indrio F, Neu J, Pettoello Mantovani M, Marchese F, Martini S, Salatto A, Aceti A. (2022). Development of the Gastrointestinal Tract in new-borns as a challenge for an appropriate nutrition:a narrative review. Nutrients. 14:1405.
Publisher | Google Scholor - Kelly EJ, Lagopoulos M, Primrose JN. (1993). Immunocytochemical localization of parietal cells and G cells in developing human stomach. Gut. 34:1057-1059.
Publisher | Google Scholor - Hyman PE, Clarke DD, Everett SL, Sonne B, Stewart D, Harada T, et al. (1985). Gastric acid secretory function in preterm infants. J Pediatr. 106:467-471.
Publisher | Google Scholor - Werner B. (1948). Peptic and tryptic capacity of the digestive glands in new-borns: a comparison between premature and full-term infants. Acta Paedia tr Jpn. 35(S5):1.
Publisher | Google Scholor - Antonowicz I, Lebentha lE. (1977). Developmental patterns of small intestinal enterokinase and disaccharidases activities in the human fetus. Gastroenterology. 72:1299-1303.
Publisher | Google Scholor - Demers Mathieu V, Underwood MA, Borghese R, Dallas DC. (2018). Premature infants have lower gastric digestion capacity for human milk proteins than term infants. J Pediatr Gastroenterol Nutr. 66:816-821.
Publisher | Google Scholor - Mihalsch WA, Franz AR, HobelJ, Pohlandt F. (2002). Hydrolyzed protein accelerates feeding advancements in very low birth weight infants. Pediatrics. 116:1199-1203.
Publisher | Google Scholor - Murray RD, Kurzner B, Sloan HR, McClung HJ, Gilbert M, Aliabouni A. (1986). The contributions of Salivary amylasesin glucose polymer hydrolysis in preterm infants. Pediatr Res. 20:186-191.
Publisher | Google Scholor - McCleanP Weaver LT. (1993). Ontogeny of human pancreatic exocrine function.Arch Dis Child. 68:62-65.
Publisher | Google Scholor - Arpaia N, Campbell C, Fan X, Dikly S, Vander Veeken J, De Roos P, et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T cells generation. Nature. 504:45145-45145.
Publisher | Google Scholor - Schulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. (1998). Early feeding, feeding tolerance, and lactase activity in preterm infants. J Pediatr. 133:645-649.
Publisher | Google Scholor - Boyle JT, Celano P, Koldovsky O. (1980). Demonstration of a difference in expression of maximal lactase and sucrase activity along the villus in the adult rat jejunum. Gastroenterology. 79:503-507.
Publisher | Google Scholor - Neu J, Li N. (2003). The neonatal Gastrointestinal Tract: Developmental Anatomy, Physiology and Clinical implications. Neoreviews. 4:e7-e13.
Publisher | Google Scholor - Schulman RJ. (2002). Effect of enteral administration of Insulin on intestinal development and feeding tolerancein preterm infants: a pilot study. Arch Dis Child Fetal Neonatal Ed. 86:F131-F133.
Publisher | Google Scholor - Klenoff Brumberg-HL, GenenLH. (2003). High versus low medium chain triglycerides content of formula for promoting short term growth of preterm neonates. Cochrane Database Syst Rev. 1:CD002777.
Publisher | Google Scholor - Freed LM, York CM, Homosh P, Mehta NR, Homosh M. (1987). Bile salt stimulated lipase Human milk: characteristics of milk of mothers of premature and full-term infants. J PediatrGastroenterol Nutr. 6:598-604.
Publisher | Google Scholor - Zoppi G, Andreotti G, Pajno Ferrara F, Njai DM, Gabrurro D. (1972). Exocrine pancreas function in premature and full-term neonates. Pediatr Res. 6:880-886.
Publisher | Google Scholor - Boehm G, Braun W, Moro G, Minoli I. (1997). Bile acids Concentrations in serum and duodenal aspirates of healthy preterm infants: effects of gestational and post natalage. Biol Neonate. 71:207-214.
Publisher | Google Scholor - Goorgieff MK, Innis SM. (2005). Controversial nutrients that potentially affect preterm neuro development: Essential fatty acids and iron. Pediatr Res. 57(2):99R-103R.
Publisher | Google Scholor - Lapillone A, Jensen CL. Re-evaluation of the DHA requirement for the preterm infant. Prostaglandins Leukot Essent Fat Acids. 81:143-150.
Publisher | Google Scholor - Sanders KM, Ward SM, Koh SD. (2014). Interstitial cells: regulators of smooth muscle function. Physiol Rev. 94:859-907.
Publisher | Google Scholor - Neal Kluever A, Fisher J, Grylack L. (2019). Physiology of the neonatal Gastrointestinal system relevant to the disposition of orally administered medications. Drug Metab Dispo. 47:296-313.
Publisher | Google Scholor - Bisset WM, Watt JB, Rivers RP, Milla PJ. (1988). Ontogeny of fasting small intestinal motor activity in the human infant. Gut. 29:483-488.
Publisher | Google Scholor - Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. (2000). Gastric electrical activity and gastric emptying in term and preterm infants. Neuro-gastroenterol Motil. 12:223-229.
Publisher | Google Scholor - Hassler WI. (2009). Motility of the small intestine and colon. In Text book of Gastroenterology; Yamada T, Ed; Wiley Blackwell: Philadelphia, PA, USA. 231-63.
Publisher | Google Scholor - Sarna S. (1988). In vivo myoelectrical activity: Methods, analysis and interpretation. In Hand book of Physiology. The Gastrointestinal system, Schultz S, Wood JD, Ed; Waverly Press: Baltimore, MD, USA. 817-863.
Publisher | Google Scholor - Berseth C. (1989). Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. 115:646-651.
Publisher | Google Scholor - Ittman PI, Amarnath R, Berseth CL. (1992). Maturation of antroduodenal motor activity in preterm and term infants. Am J Diges Dis. 37:14-19.
Publisher | Google Scholor - McCann C, Alves MM, Brosens E, Natarajan D, Perin C, Chapman C, et al. Neuronal development and onset of electrical activity the human Enteric Nervous system. Gastroenterology. 156:1483-1495.
Publisher | Google Scholor - Luo S, Zhu H, Zhang J, Wan D. The pivotal role of microbiota in modulating the neuronal-glial- epithelial unit. Infect Drug Resist. 14:5613-5628.
Publisher | Google Scholor - Wallace AS, Burns AJ. (2005). Development of the Enteric Nervous system, smooth muscle and Interstitial cells of Cajal in the human Gastrointestinal tract. Cell Tissue Res. 319:367-382.
Publisher | Google Scholor - Young HM, Hearn CJ, Ciampoli D, Southwell BR, Brunet JF, New green DF. (1998). A Single rostro caudal colonization of the rodent intestine by enteric neurons is revealed by the expression of Phox2b, Ret and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol. 202:67-84.
Publisher | Google Scholor - Huang T, H Y, Wang X, Wang L, YC Wang Cetal. Direct interaction of Sox10with cadherin19mediates early sacral neural crest cells migration: implications for Enteric Nervous system development defects. Gastroenterology. 162:179-192.
Publisher | Google Scholor - Young HM, Jones BR, McKeown SJ. (2002). The projections of early enteric neurons are influenced by the direction of neural crest cells migration. J Neurosci. 22:6005-6018.
Publisher | Google Scholor - Kluppel M, Hulzinga JD, Malysz J, Bernstein A. (1998). Developmental origin and kit dependent development of the Interstitial cells of Cajal in the mammalian small intestine. Dev Dyn. 211:60-71.
Publisher | Google Scholor - Jana A, Montaya CA, Mullaney JA, Dilger RN, Young W, McNabb WC, et al. (2020). Gut Brain Axis in the early postnatal years of life: a developmental perspective. Front Integr Neurosci. 14:44.
Publisher | Google Scholor - Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastianssen TFS, Boehme M, et al. (2019). The microbiota- Gut Brain Axis. Physiol Rev. 99:1877-2013.
Publisher | Google Scholor - Brett BE, De Weerth C. The microbiota- Gut Brain Axis: A promising avenue to foster healthy developmental outcomes. Dev Psychobiol. 61:7720-7782.
Publisher | Google Scholor - Collins SM, Surette M, Bercik P. (2012). The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 10:735-742.
Publisher | Google Scholor - Deoni S, Dean O, Joelson S, O’Regan J, Schneider N. (2018). Early nutrition influences developmental myelination and cognitionin infants and young children. Neuroimage. 178:649-659.
Publisher | Google Scholor - Erny D, Dokalis N, Mezo C, Castoldi A, Mossad OJ, Staszewski O, et al. (2021). Microbiota derived acetate enables the metabolic fitness of the brain- innate immune system during health and disease. Cell Metab. 33:2260-2276.
Publisher | Google Scholor - Tosoni G, Conti M, Heijtz RD. (2019). Bacterial peptidoglycans as novel signalling molecules from microbiota- to brain. Curr Opin Pharmacol. 48:107-113.
Publisher | Google Scholor - Layunta E, Buey B, Mesoniero JE, Latorre E. (2021). Crosstalk between intestinal serotonergic system and pattern recognition receptors on the microbiota -gut-brain Axis. Front Endocrinol. 12:1-24.
Publisher | Google Scholor - Yano JM, Yu K, Donaldson GP, Shastri GC, Ann P, Ma L, et al. (2015). Indigenous Bacteria from the gut microbiota regulate the host serotonin biosynthesis. Cell. 161:264-276. Erratum in Cell. 163:258.
Publisher | Google Scholor - Silva YP, Bernardi A, Frozza RL. (2020). The role of short chain fatty acids from gut microbiota in Gut-Brain communication. Front Endocrinol. 11:25.
Publisher | Google Scholor - Bresetti I, Salvatore S, Valetti G, Baj A, Giaroni C, Agoni M. (2022). The microbiota-gut axis in preterm infants: physio pathological implications. Cells. 11:379.
Publisher | Google Scholor - Ratsika A, Codagnone M, O’Mahogany S, Stanton C, Cryan J. (2021). Priming for life: Early life nutrition and the microbiota -gut-brain Axis. Nutrients. 13:423.
Publisher | Google Scholor - Brink LR, Lonnendral B. (2020). Milk fat globule membrane: The role of its various components in infant health and development. J Nutr Biochem. 85:108465.
Publisher | Google Scholor - Al-Khafaji AH, Jepsen SD, Christensen KR, Vigsnaes LK. (2020). The potential of milk oligosaccharides to impact the microbiota -gut-brain Axis through modulation of the gut microbiota. J Funct Foods. 74:104176.
Publisher | Google Scholor - Miller J, Tonkin E, Damarell RA, McPhee AJ, Suganuma M, Suganuma H, et al. (2018). Systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients. 10:707.
Publisher | Google Scholor - Lu L, Claud EC. (2019). Connection between gut microbiome and brain development in preterm infants. Dev Psychobiol. 61:739-751.
Publisher | Google Scholor - Roze JC, Ancel PY, Marchand Martin L, Rousseau C, Montassier E, Monot C. et al. (2020). Assessment of neonatal intensive care unit practices and preterm neonatal gut microbiota and 2year neurodevelopmental outcomes. JAMA Netw Open. 3:2018119.
Publisher | Google Scholor - Beghetti I, Barone M, Turroni S, Biagi E, Sansavini A, Brigidi P, et al. (2021). Early life gut microbiota and neurodevelopment in preterm infants: any role of Bifidobacterium? EurJ Pediatr.
Publisher | Google Scholor - Niemarkt HJ, DeMeij TG, Van Ganzewinkel CJ, DeBoer NKH, Anderiessen P, Hutten PC, et al. (2019). Necrotizing enterocolitis, gut microbiota and brain development: role of the Brain Gut. Axis. Neonatalogy. 115:423-431.
Publisher | Google Scholor - Ihekweazu FD, Versalovic J. (2018). Development of the Paediatric gut microbiome: impact on health and disease. Am J Med Sci. 356:413-23.
Publisher | Google Scholor - Human microbiome project Consortium, function and diversity of the healthy human microbiome. Nature. 486:207-214.
Publisher | Google Scholor - Dimitrakopoulou EI, Ppouliakis A, Falaina V, Xanthos T, Zoumpoulakis P, Tsiaka T, et al. (2022). The metagenomic and metabolomic profile of infantile gut: can they be predicted by the Feed type? Children. 9:154.
Publisher | Google Scholor - Willyyard C. (2018). Could baby’s first bacteria take root before birth? Nature. 553:264-266.
Publisher | Google Scholor - Aagaard K, MaJ, Antony KM, Ganu R, Petrosino J, Versalovic J. (2014). The placenta harbours a unique microbiome. Sci Trans. 6:237ra65.
Publisher | Google Scholor - Ranjan R, Rani A, Metwally A, Mc Gee HS, Perkins DL. (2016). Analysis of the microbiome: advantages of whole genome shotgun 16S amplicon sequencing. Biochim Biophys Res Commun. 469:967-977.
Publisher | Google Scholor - Ahearn Ford S, Berrington JE, Stewart CJ. (2022). Development of the gut microbiome in early life. Exp Physiol.
Publisher | Google Scholor - Chu DM, Antony KM, MaJ, Prince AL, Showalter L, Moller M, Aagaard K. (2016). The early infant gut microbiome varies in association with a maternal high fat diet. Genome Med. 8:1-12.
Publisher | Google Scholor - Romano Keeler J, Zhang J, Sun J. (2021). COVID-19 and the neonatal micro-biome: will the pandemic cost the infants their microbes? Gut Microbes. 13:1-7.
Publisher | Google Scholor - LaRosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, HallMoore CM, et al. (2014). Patterned progression of bacterial populations in the premature infant gut. Proc NatlAcad Sci USA. 111:12522-12527.
Publisher | Google Scholor - Jia J, Xun P, Wang X, He K, Tang Q, Zhang T, et al. (2020). Impact of post-natal antibiotics and parenteral nutrition on the gut microbiota in preterm infants during Early life.J JParenterEnter Nutr. 44:639-654.
Publisher | Google Scholor - Hendrickx JGE, Zwittink RD, VanLingem RA, KnolJ, Belzer C. (2019). The preterm gut microbiota: an inconspicuous challenge in nutritional neonatal care. Front Cell Infect Microbiol. 9:85.
Publisher | Google Scholor - Yu Y, Lu L, Sun J, Petrof EO, Claud EC. (2016). Preterm infant gut microbiota affects intestinal development in a humanized microbiome gnotobiotic mouse model. Am J Physiol Gastroenterol Liver Physiol. 311:G521-G532.
Publisher | Google Scholor - Battersby C, Santhalingam T, Costeloe K, Moodi N. (2018). Incidence of neonatal necrotizing enterocolitis in high income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 103:G521-G532.
Publisher | Google Scholor - Henrick BM, Rodriquez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. (2021). Bifidobacterium- mediated immune system imprinting early in life. Cell. 184:3884-3898.
Publisher | Google Scholor - Dong Y, Speer CP. (2018). Late onset neonatal sepsis: Recent Development. Arch Dis Child Fetal Neonatal Ed. 103:G521-G532.
Publisher | Google Scholor - Metcalf GA, et al. Temporal of the development gut microbiome in early childhood from the TEDDY study. Nature. 562: 583-588.
Publisher | Google Scholor - Kurath Koller S, Neumann C, Moisl Eichinger C, Kanduth C, Hopfer B, et al. (2020). Hospital regimens including probiotics guide the individual development of gut microbiome of very low birth weight infants in the first two weeks of life. Nutrients. 12:1256.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2019). Have Probiotics and Synbiotics passed the test of time to be implemented in management of obesity and related metabolic disorders-a comprehensive review. Adv Obes Weight Manag Control. 9(1):21-28. DOI: 10.15406/aowmc.2019.09.00269
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2020). Will Probiotics Provide the Answer for Therapy of Non-alcoholic Fatty Liver Disease (NAFLD)? – A Systematic Review. Biochem Physiol. 9:257.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. Are we any close to unraveling the mechanism of interactions among susceptibility genes towards Type 1 Diabetes, Gut Microbiota Along with Environmental factors, specifically early diet patterns-A Systematic Review’’
Publisher | Google Scholor - Radenkovic G, Savic V, Mitic D, Grahovac S, Bielakovic M, Krstic M. (2010). Development of c-kit positive Interstitial cells of Cajal in the human stomach. J Cell Mol Med. 14(5):1125-1134.
Publisher | Google Scholor - Zhou WK, Qu Y, Liu YM, Gao MJ, Tang CY, Huang L, et al. (2022). The abnormal phosphorylation of Rac1, Lim Kinase -1 and the Cofilin proteins in the pathogenesis of Hirsh sprung disease. Bioengineered. 13(4): 8548–8557.
Publisher | Google Scholor