Research Article
An Update on Of Hgsoc’s Management Emphasizing on Homologous Recombination Deficiency& Intra Tumor Heterogeneity-A Narrative Review &Inferring from A Case Report to Highlight Problems in Clinical Translation
1Centre For Human Reproduction721, G.T.B. Nagar, Jalandhar, Punjab, India.
2Centre for Human Reproduction 672, Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra, Mumbai, India.
*Corresponding Author: Kulvinder Kochar Kaur, Centre For Human Reproduction721, G.T.B. Nagar, Jalandhar, Punjab, India.
Citation: Kaur K K, Allahbadia G N K. (2024). ’An Update on Of Hgsoc’s Management Emphasizing on Homologous Recombination Deficiency& Intra Tumor Heterogeneity A- Narrative Review &Inferring from A Case Report to Highlight Problems in Clinical Translation. Journal of Women Health Care and Gynecology, BioRes Scientia Publishers. 3(4):1-17. DOI: 10.59657/2993-0871.brs.24.041
Copyright: © 2024 Kulvinder Kochar Kaur, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 17, 2024 | Accepted: May 10, 2024 | Published: May 20, 2024
Abstract
Ovarian Cancer incorporates a group of tumors which originate from germinal tissue and display unique, clinical, pathological and molecular characteristics. of these Epithelial ovarian cancers (EOC) portray the most frequently encountered, whose constituents are 5 unique tumor histokinds. Noticeably, high grade serous ovarian cancers (HGSOC)mirror the maximum, being implicated in 70% of the EOC subjects. In view of their silent and asymptomatic behavior, diagnosis of HGSOC’s is usually done in advancement of disease stages that evolved and complicated genomic state having the characteristics of considerable intratumor heterogeneity (ITH) in view of chromosomal instability (CIN) that aids in discriminating HGSOC’s. Histologically such cancers illustrate significant morphological variations within and amongst tumors. The histological paradigm correlated with solid, psudoendometrioid and transitional (SET) and canonical subtypes of HGSOC’s provide the prognostic understanding and might point particular molecular profiles. The evolution of HGSOC’s from the primary to metastasis is canonically characterized by clonal ITH, implicating shared or variations of the mutations in the neoplastic subclones amongst primary and metastatic regions. Propagation of disease, drug resistance is further impacted by nonclonal ITH, associated with crosstalk with tumor microenvironment and further genomic alterations. Noticeably, significant changes take place in nonmalignant cells, inclusive of cancer associated fibroblasts (CAF), and immune cells while tumor propagation. Having previously reviewed how origination of tumor from the fallopian tubes comprises one of the HGSOC’s, here we present a case report (not fully in view of permission not granted by patient ) of a totally asymptomatic patient whose presentation was in the form of an acute TO mass with differential diagnosis of hydrosalpinx with ?twisted TO mass ;however turned out to be an advanced stage III HGSOC apparently from fimbrial end and how in emergency settings maltreatment ensued with no preop PET Scan etc., and incomplete debulking primary surgery, complicating therapy.
Keywords: high grade serous ovarian cancers; intratumor heterogeneity; solid; psudoendometrioid and transitional; clonalevolution; chromosomal instability
Introduction
Ovarian Cancer is comprised of variable group of tumors having the properties of clinicopathological in addition to molecular characteristics, every one possessing different prognosis. According to the present classification system ovarian tumors classification is done dependent on the anatomy of their origination-for instance) Epithelial ovarian cancer (EOC) which originates from the ovarian surface epithelium (OSE) or extra ovarian epithelia, ii) sex cord- stromal ovary tumors (SC-SOC) which originate from the gonadal stromal cells along with iii) germ cells ovary tumors (GOC) which are generated from the primordial germ cells. Usually there is considerable overlap which result in inthe development of the mixed tumors [1].
Amongst the Ovarian Cancers,EOC possess the maximum prevalence. EOC’s display variety of the histopathological, immunohistochemical, in addition to genomic characteristics out of which 5 primary kinds have been isolated: i) High-grade Serous Ovarian Cancer (HGSOC) (70%), ii) endometrioid, carcinomas (10%) iii) clear cell carcinomas (10%) iv) mucinous carcinomas (3%) in addition to v) low grade serous Ovarian Cancer (LGSOC) (<5>
Here we deal with various facets of the ITH in case of HGSOC’s comprehensively taking into account the morphological, clonal along with non-clonal ITH [9], after earlier reviewing the advances in the therapy of advanced ovarian cancer with special emphasis on the PD1/PDL1pathway and reviewed comprehensively, an update on high grade serous ovarian carcinoma – with emphasis on how origination of tumor from thein situ surface epithelium of fallopian tubes comprises one of the HGSOC’s [10,11]. Further assessment of its repercussions with regard to metastatic spreading is done.
Methods
A narrative review was conducted utilizing search engine PubMed, google scholar ;web of science; embase; Cochrane review library utilizing the MeSH terms like; Epithelial ovarian cancer (EOC); High-grade Serous Ovarian Cancer (HGSOC); intra tumor heterogeneity (ITH); homologous recombination repair (HRR) pathway ; homologous recombination deficiency (HRD);primary debulking surgery; adjuvant chemotherapy; neoadjuvant chemotherapy (NACT) poly ADP ribose polymerase 1 (PARPi), hampering agent; clonal non clonal ITH from 2000 till date in 2024 march.
Results
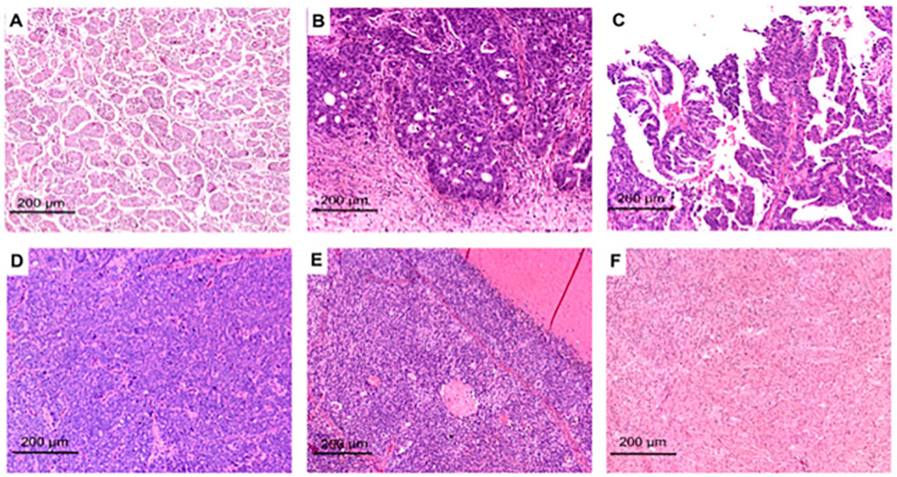
We found a total of 900 articles out of which we selected 84 articles for this review. No meta-analysis was done. Morphological- histological ITH in case of HGSOC’. High grade serous ovarian carcinoma displays considerable morphological variations both at the macroscopic in addition to the microscopic stages. with regard to microscale level, wide variety of the histomorphological changes might be experienced in view of the tumor’s metaplastic modifications as well as differentiation modes behind. Till date the morphology of the tumor has been classified by the Hematoxylin-and-eosin (H&E) morphologies lone or in addition to the molecular signatures. Classification of the HGSOC’s was attempted by Soslow et al. [12], dependent on the H&E -stained visualization into such growth fashions: i) transitional ii) solid iii) pseudo endometrioid iv) papillary v) infiltrative v) micro papillae as well as vi) papillary infiltrative. These designs get further categorized into groups which share canonical characteristics portrayed by the SET group (solid, pseudo endometrioid as well as transitional) [12] (figure 1).
Figure 1: Courtesy ref no-13-Representative H&E images of HGSOCs with SET or classic features: micro-papillary (A), pseudo-endometrioid (B), papillary (C), solid (D), transitional-like (E), and healthy peritoneal fibrous tissue (F). This image has been already published by Azzalini et al. [14].
One more classification of the HGSOC’s that was given by Murukami et al. [15], is dependenton the histologic- morphologies, that are deduced from HGSOC’s molecular subtypes which leads to 4 kinds partly overlapping with those of Soslow et al. [12], i) mesenchymal transition (MT) ii) immune reactive (IR), iii) solid and proliferative (SP) iv) papilloglandular (PG) kind [15].
Irrespective of the morphological classification HGSOC’s histologic- morphologies are coexistent in a sample, usually with greater than 2 architectures. in view of the ITH canonically just the morphology which possesses the maximum prevalence inthe tumor slide is taken into account along with thereby, there is variation in the documented frequency of every tumor design documented inthe publication. Nevertheless, papillary characteristics are the generally maximum spread that gets followed by other kinds of architectures. Particular morphologies might simulate other kinds of histocytes for instance endometrioid as well as low grade serous which leads to escalation of the difficulty of the differential diagnosis [16]. Thereby recognition of this morphological heterogeneity dependent ontumor histologic- morphologies or SET/ canonical characteristics is clinically germane in case of HGSOC’s with the stratification in addition to the selecting the correct treatment. Utilization of morphological traits in the form of the proxy strategies for the determination of the HRD as well as isolation of the subject for whom the PARP hampering agent treatment would prove to be maximum advantageous.SET properties in addition to the ones with greater mitotic index along with tumor infiltrating lymphocytes (TIL) displayed a significant correlation with BRCA aberrations [12]. As per Fujiwara et al. [17], tumors which possessed the germline BRCA1 mutations (gBRCAm)could be isolated by a combination of the serous or the ‘’undifferentiated ‘’ histology, greater mitotic index, nuclei that are giant, atypical as well as lymphocytic infiltration which stands out [17]. Akin to that various kinds of the metastatic invasion designs (infiltrative vis a vis pushing border metastasis )were further anticipative of the BRCA deficiency [18] in addition to the survival of the patients [19]. Tumor along with architecture SET/ canonical groups that are correlated with particular clinical paradigms in chemotherapy naive scenario have been illustrated to portray independent prognostic factors with regards to survival of the patients [20]. Canonical characteristics, particularly inthe infiltrative designs have been isolated in the form of the accelerating/ kinds in the variety of the HGSOC’s. Their properties are sub ideal cytoreduction, lesser mitotic index, having BRCA proficiency (alias were homologous recombination proficient [HRP]), omental placement in addition to poorer prognosis. Conversely SET properties documenting ideal debulking surgery, greater mitotic index, greater quantities of TIL, ovarian placement displayed a considerably good prognosis. With regards to neoadjuvant scenario, despite chemotherapy might hamper with the histological evaluation in view of the cytological actions, the heterogeneity of the tumor growth designs determined inthe form of the Shannon index, has led to the availability of a prognostic biomarker in case of patients having a neoadjuvant chemotherapy (NACT) [4,21].
According to the historical perspectives, the assessment of histocytological heterogeneity of HGSOC’s has been performed with the utilization of visual examination taking into account just the selective areas in the tumor slide. Nevertheless, practically it is not feasible to attempt the tracking of every single tumor cell in addition to their crosstalk with the surroundings microenvironment with regards to the whole visually. Digital imaging evaluation possesses the capacity of conducting complete morphological scrutiny of the ITH in case of HGSOC’s with the provision of noticeable outcomes. It has been documented that whole slide imaging evaluation of the different morphological along with textural alone or in combination with multiomics results possess the capacity of anticipating the molecular changes for instance BRCA deficiency in addition to microsatellite instability along with the prognosis of the HGSOC’s subjects. What is of further significance is the existence of the morphological areas that are correlated with both areas of poorer prognosis as well as the ones having considerably good prognosis existing concurrently in the tissues slide underpinning the idea of ITH [22]. Origination of the heterogeneity might take place in view of active shaping of the of the morphological cancer cell plasticity by the tumor microenvironment. Earlier research illustrated that the format as well as crosstalk amongst TIL in addition to the cancer cells are associated with the survival of the patients as well as propagation [23]. Thereby samples of the HGSOC’s might be considered to a combination of the tumor facilitating in addition to the tumor hampering surroundings possessing the properties of different quantities of immune cells (lymphocytes) along with stromal cells, which might be visualized respectivelyin the form of the hampering/ resources for the growth of the tumor. Nawaz et al. [24], invented that the switch in the ecological balance to the resource enrichment of the tumor microenvironment possess the capacity of resulting in greater aggravating behavior of theHGSOC’s in addition to bad total rates of survival. Furthermore, various surroundings amongst the tissue samples possess the capacity of stimulating the generation of the clone via point mutations or aneuploidy [24].
Tumor chromosomal instability has been correlated with aneuploidy in addition to the diversity inthe nuclear characteristics across variable kinds of cancers [25]. A mean of 20 spatial zones might be displayed in case of a single HGSOC’s tumor slide where cancer nuclei reveal morphological variations. Incorporation of the spatial evaluation with the omics in addition to the clinical outcomes, it becomes feasible to isolate the correlation amongst the zones of the morphological variations, BRCA1expression, elimination of nuclear inherent ness, as well as survival of the patients [26]. The germaneness of ITH in case of HGSOC’s samples corroborates the molecular changes lying behind them. Numerous studies have been concentrating over the growth designs for acquisition of insight with regards to the correlation amongst the genotype, clinical results in addition to the molecular phenotype. At the level of the immunohistochemistry, there is not enough corroboration for generating a correlation amongst the growth designs of HGSOC’s as well as expression of a biomarker, plausibly in view of the restricted assessment of the quantities of the antibodies till now [27]. Moreover, in view of intra tumor differentiation is commonly patchy as well as not all the time an overlap is present amongst morphological heterogeneity in addition to the clonal heterogeneity [28], just rare spatial communication amongst the immunohistochemical protein expression as well as HGSOC’s designs.
With regards to transcriptional level, HGSOC’s histologic- morphologies associate with differing gene expression signatures that possess the capacity of anticipating the prognosis [15]. The preoperative clinical characteristics are further correlated with HGSOC’s histologic- morphologies [29]. Specifically, the MT kind, having the properties of infiltration along with the breakdown of the stromal partition was greater in the peritoneal regions inthe subject’s possessing disease inthe late stages having inimical prognosis. This kind had signs of aggravation inclusive of abundance of the epithelial –mesenchymal transition (EMT) gene sets, sub ideal cytoreduction, in addition to the greater quantities of ascites. On the other hand, the IR kind, which illustrated thick infiltration with thelymphocytes, was associated with gene sets correlated with immune reactions, younger aged women, ideal cytoreduction, as well as prognostically were much better. Miyagawa et al [30], validated these observations via Digital imaging evaluation [30]. Other workers have tried to isolate specific molecular characteristics for every kind of HGSOC’s histologic- morphology. Noticeably, a correlation amongst the architectural designs of HGSOC’sas well as variable molecular along with the biophysical characteristics inclusive of the tumor stiffness [14], in addition to the differential AKT isoforms transcripts expression [31], have been illustrated. Although there is existence of an intriguing correlation amongst the architectural designs of HGSOC’s along with the variable molecular biophysical characteristics, it is noticeable the manner gene expression signatures derived from tissue evaluation in bulky, might be impacted substantially by how much input is there from the stromal in addition to the immune cells [32]. Thereby MT kind of the morphology plausibly simulates stromal cells gene expression, whereas IR kind of tumors are plausibly SP or PG kind having a considerable immune reaction.
Alterations in morphology have been associated with spatial as well as temporal evolution in case of HGSOC’s. Lahtinen et al. [33],interpreted that temporal evolution of the HGSOC’s subsequent the recording of the clonal complicated nature in addition to the variations in case of 55 HGSOC subjects via multisampling whole genomic sequencing (WGS).They isolated 3 cancer evolutionary directions referred to in the form of evolving (alias earlier state), sustaining (intermediate state), in addition to the adapting (last state), that were associated with particular histologic- morphologies as well as for instance fibroblasts quantities along with the other characteristics for instance genomic along with transcriptomic characteristics. Tumor’s existent in the adapting state having minimal fibroblasts quantities were usually in the Set-in addition to had enrichment of wingless -related integration site (WNT) as well as NOTCH signaling, whereas tumors in the sustaining state usually infiltrating were maximum micropapillary in addition to had enrichment of protein kinase B (AKT) signaling. On the other hand, tumors in the evolving state having greater fibroblasts quantities were usually infiltrating kinds along with enrichment of ERBB2, (1 Of the4epidermal growth factorreceptor (EGFR) tyrosinekinase family members) in addition to the mitogen activated protein kinase (MAPK) pathways. Noticeably, as per Lahtinen et al. [33], HGSOC’s possess the capacity of further evolving from an evolving state towards an adapting state, directly or via sustaining state.
Despite assessment of morphological ITH has basically been evaluated at the microscopic level, recently, classification has been done with the utilization of laparoscopic images on gross visualization preoperatively into 2 sub kinds: type1- possess the properties of deep in addition to invasive look along with the disfigurement in addition to surrounding tissues getting retracted as well as type2- possessing the superficial exophytic (growing from the outer surface epithelium having a nodular look, canonically outlined by normal tissues. The multiomics evaluation performed, on these 2 sub kinds illustrated unique surgical results as well as molecular signatures. Particularly type1- possessed enrichment of angiogenesis, sonic hedgehog, in addition to the EMT signaling, while type2- revealed a changed lipid signatures along with possessed the enrichment of cell cycle as well as MYC signaling. Intriguingly, histological evaluation did not display any significant microscopic designs in case of the 2 sub kinds despite the existence of a papillary architecture in case of 50% of type1 in addition to just 5%of type2 tumors [34]. Corroborating these outcomes Foster etal [35], illustrated in a recently performed study were further correlated with unique radiographic characteristics on CT [37]. There is existence of the requirement of greater studies with regard to the isolation of an agreement- over a set of in addition to the morphological characteristics which might aid in the clear-cut differentiation of the phenotypic sub kinds despite assessing the morphological heterogeneity of HGSOC’s might aid in a clinical trial pattern in addition to with regard to the clinical scenario. Furthermore, the existence of different growth architectures in the same slide restricts the plausibility of the morphological evaluation, making it less replicable.
Clonal ITH in case of HGSOC’s
Genetic clonal evolution of cancer is associated with genomic instability in the form of a main characteristic of the carcinogenetic events [9]. HGSOC’s constitute a ‘’genetically unstable ‘’ cancer regarding this possessing complicated genomes having the properties of common chromosomal/ gene copy number change as well as structural alterations [6]. Each patient basically possesses the properties of variable genomic differences corroborating HGSOC’s in the form of a substantially heterogenous disease whose definitions might not be done with ease by a particular mutational alterations. The lack of significant changes amongst the anatomic region amongst a single subject points to the biological events behind genomic instability apparently get determined earlier at the time of the propagation of the disease [36]. At the level of the genome HGSOC’s possesses the properties of being practically omnipresent TP53 loss-of-function mutations inthe form of an early guiding process [38], in addition to the commonest etiology of chromosomal instability (CIN) [39]. HGSOC’s illustrate CIN in case of 100% of the solid tumors irrespective of the age, stage, chemotherapy or BRCA state, however, in 91
Conclusions
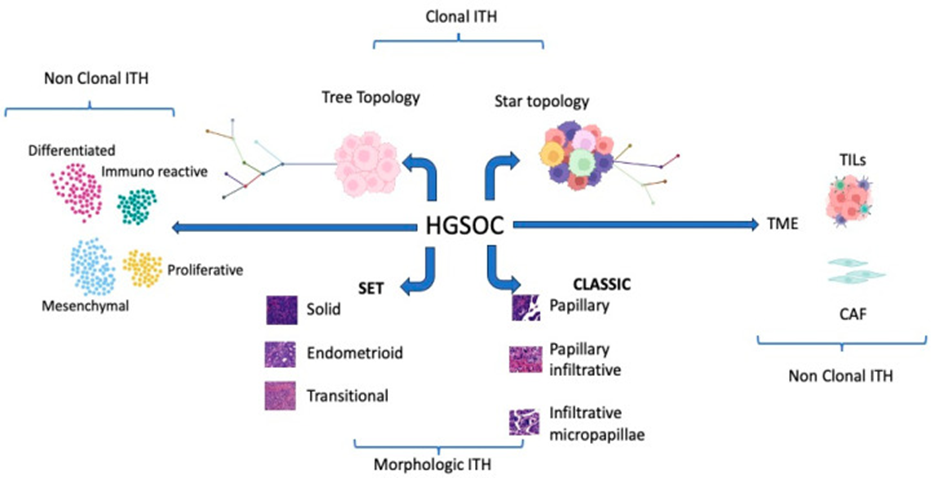
Thereby HGSOC portrays an extremely complex tumor kind as illustrated in Figure 2.
Figure 2: Graphical summary of the different types of ITHS competing in HGSOC complexity. Some figure components were created with www.BioRender.com (accessed on 23 September 2023).
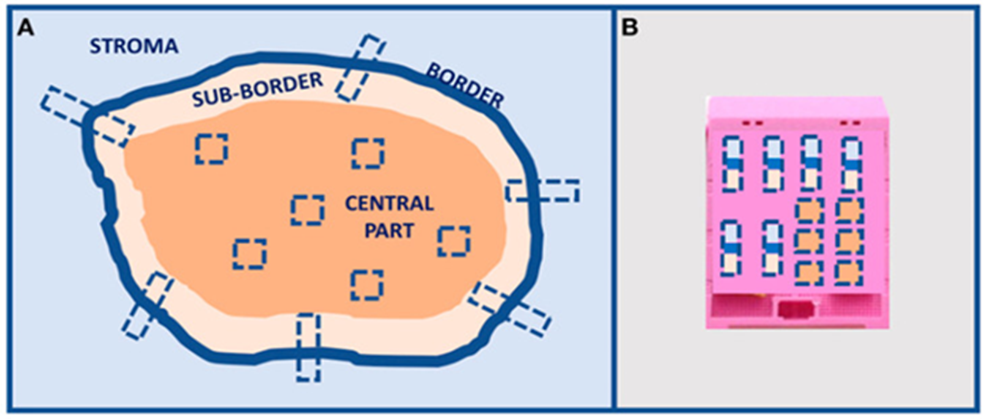
In the last 10 years considerable research acts have been performed with the idea of unveiling the further complicated nature of the HGSOC’s. Nevertheless, although enough work has been put in the translation of theresearch observations into practical clinical application in the context of patient’s care has continued to be restricted. At the present timePARP hampering agents, bevacizumab, in addition to the homologous recombination deficiency (HRD) have observed their position in the treatment protocols, however HRD continues to be a non-best anticipator of PARP HA reactions. The final so-called litmus test continues to be reactions to platinum dependent chemotherapy [80]. Without any uncertainties PARP HA’s have documented favorable improvements in the total survival of the patients of HGSOC’s [81]. Nevertheless, these advantages are basically observed in the case of genomic HR deficiency [7,81]. For diminishing the gap amongst research inventions detectable enhancements in the case of the patients results, it becomes imperative to revisit the research observations in addition to isolate biomarkers that are reliable prognostically along with the arrival at the final decision with regards to treatment. Acknowledged the profound complexities as well as the considerable outcomes accessibility in the case of HGSOC’s, incorporating Artificial Intelligence algorithms, the manner earlier illustrated in EMT prognostic stratification [82], might be significantly aiding in acquisition of insight. Furthermore, whatever knowledge we have gained subsequent to morphological evaluation underpins the significance of the exhaustive assessment of the properties of the HGSOC’s surgery specimens. This has the requirement of the inclusion of architectural fashion apart from the primary tumor regions, however further amongst the metastasis. Acknowledged the ITH, considerable multiple samples assessment [9], the manner previously posited as in Figure 2, is imperative in HGSOC’s for the discrimination amongst SET in addition to canonical HGSOC’s. Moreover, recognition of the key part of the tumor microenvironment in the propagation of the disease, Immunohistochemical evaluation of immune cells as well as stromal cells properties have to be conducted committedly is key [83]. This aids in isolating patients in whom immunotherapy therapy might be advantageous (Figure3).
Figure 3: Tumor multiple sampling proposal. (A) Multiple sampling locations and (B) organization in the inclusion block. Image already published in [9].
The close clonal evolution of the HGSOC from the primary to metastasis, usually highlighted by copy number changes, current botherations in generating dependable biomarkers for characterization. With regards tothis the, transcriptomic classes of the HGSOC correlated with fibroblast as well as immune cells characterizing apparently gives a greater direct pathway with the idea of the fast translation into the care of the patients. Appreciation of the clonal along with the non-clonal complicated nature in addition to heterogeneity of the HGSOC’s, spatial transcriptomic strategies or in situ multiplex evaluation of analytes determination might be the maximum appropriate methodology with regard to selection of the biomarker for the multicenter, corroboration studies. Nevertheless, the final clinical influence of any biomarker might be generated via meticulous corroboration in the bigger multicenter cohorts. Just via collaborative actions we will possess the capacity of carrying out the right evaluation of the biomarkers as candidates in addition to incorporate them in the clinical scenario for the prognostic as well as therapeutic approaches.
Here we are just providing a summary of a recently treated patient of HGSOC originating from the fallopian tube we found (full report not provided as pt refused to give consent) of using her tumor photos etc.
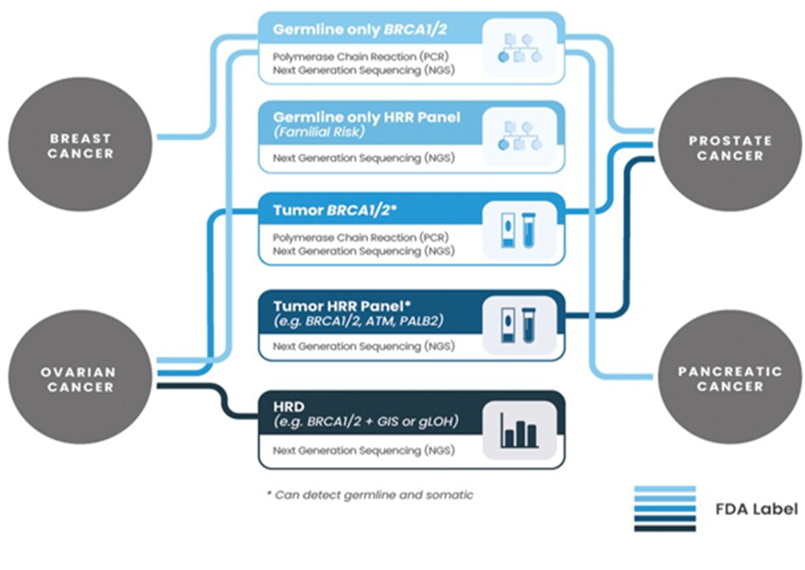
HPE-an irregular mass towards fimbria end 8x7cm, uterus & ovaries normal -tumor seen infiltrating serosal fat of adherent colonic mucosa &adjoining fasia-HPE-HGSC infiltrating the adherent serosal fat Lymph nodes 0/9& Pelvic peritoneum deposits noted, endocervix, cervix & momentum are uninvolved 2.WB PETSCAN-30-1-24-STATUS-post hysterectomy-mildly faintly FDG/AVIL Ill-defined soft tissue densities/ areas with adjacent fat stranding in this region &pelvis? post-surgical changes with inflammatory uptake minimal ascites FDG AVID subcentrimetric sized precaval lymph nodes - mildly FDG AVID mediastinal & hilar lymph nodes? inflammatory, rest normal study HPE & IHC-consistent with high grade serous carcinoma (HGSC)-IHC (report on 6-2 -24 was positive for CK7, PAX8, WT1, ER, p53 (mutant type -overexpression), p16.ki-67-70% Impression- high grade serous tubo-ovarian carcinoma (post-surgery? R2 Plan3# chemotherapy-NAB-paclitaxel 260mg/m2 &Carboplatin AUC-6 FU assessment Plan- peripheral Blood for BRCA1 germline mutations (inclusive of -MLPA), HRD testing over tissue blocks areas of adjacent Thus, administration of paclitaxel 260mg/m2 &Carboplatin with filgristine was done on 12th February with 3 weekly programs of paclitaxel. Despite insistence by the group of Azzalini E, etal. who have been working exclusively on HGSOC we did not have enough sections for getting surrogate HRD report to decide on PARP inhibitors to start with, even otherwise the oncologist was not willing to start PARP inhibitors with fixed idea of using paclitaxel& Carboplatin initially followed by PARP inhibitors in the form of the maintenance therapy. Patient had body aches &headaches on day 10 with hair fall and although we received the report of HRD from tissue blocks on 9-3-24 showing HRD positive with negative BRCA germline & somatic mutations Thus although recommended with HRR PARPi be started immediately even now the plan is to give Olaparib as maintenance therapy with fixed ideation of the treating oncologist. Now we await to follow up the prognosis of this most probably fallopian tube origin of this HGSC who had inadequate debulking surgery although the pathologist commented with WT1 + probably ovarian in origin though ovary did not reveal any tumor tissue. There were further opinions sought from 2-3 oncologists if we could add bevacizumab-although controversial opinion it was finally decided it might interfere with post op healing. HRD as explained earlier has gained importance recently to decide if initial PARP inhibitors might be used (see figures 4&5 on HRD significance.
Figure 4: Courtesy ref no-83-Present-day landscape of FDA-approved diagnostic tools for PARPi treatments. Not necessarily applicable for all PARPi and indications. Currently approved diagnostic tools can utilize different sample types: tissue, blood, or plasma (refer to Table 1 for details).
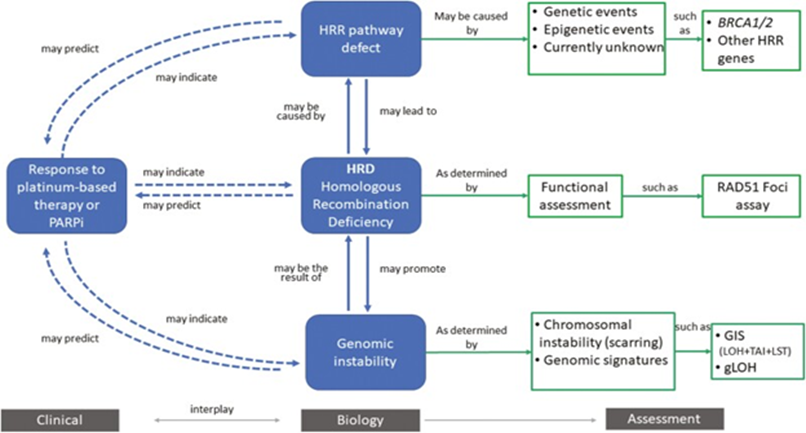
Figure 5: Courtesy ref no -83-Overview of homologous recombination deficiency (HRD). Homologous recombination deficiency is a phenotype that is characterized by the inability of a cell to effectively repair double-strand DNA breaks using the homologous recombination repair (HRR) pathway. Alterations in these genes have been deemed “causes” of HRD (e.g., genetic events and epigenetic events). This can result in an impaired HRR pathway, which can be deemed “consequences,” and assessed by probing the genome for evidence of genomic instability (e.g., chromosomal instability and other genomic signatures).
Thus, concluding despite all the work done in the last decade reluctance of treating clinicians to incorporate these innovative alterations is a big bone of contention in the improvement of prognosis of HGSOC’s.
References
- World Health Organization. (2020). International Agency for research on Cancer. Female Genital Tumors,5th ed. International Agency for research on Cancer: Lyon.
Publisher | Google Scholor - PratJ, D’Angelo E, Espinosa I. (2018). Ovarian carcinomas: At least 5 different diseases with distinct histological features and molecular genetics. Hum Pathol, 80:11-27.
Publisher | Google Scholor - Bowtell DD, Bohm S, Ahmed AA, Aspuria AJ,Bast RCJr, BeralV, etal. (2015). Rethinking ovarian cancerII: reducing mortality from high grade serous ovarian cancer. Nat Rev Cancer, 15:668-679.
Publisher | Google Scholor - Azzalini E, Barbazza R, Stanta G, Giorda G, Bortot L, Bartoletti M, etal. (2021). Histological patterns and intra tumor heterogeneity as prognostication tools in high grade serous ovarian cancers. Gynaecol Oncol, 163:498-505.
Publisher | Google Scholor - (2011). Cancer Genome Atlas Research Network: Integrated genomic analysis of ovarian carcinoma. 474:609.
Publisher | Google Scholor - Morden CR, Farrell AC, Sliwowski M, Lichtensztejn Z, Altman AD, Nachtigal MW, etal. (2021). Chromosomal instability is prevalent and dynamic in high grade serous ovarian cancer patients samples. Gynaecol Oncol, 161:769-778.
Publisher | Google Scholor - Takaya H, Nakai H, Takamatsu S, Mandai M, Matsumura N. (2020). Homologous recombination deficiency status-based classification of high grade serous ovarian carcinoma. Sci Rep, 10:2757.
Publisher | Google Scholor - Stewart MD, Merino Vegas D, Arend RC, Baden JF, Barbash O, Beaubier N, etal. (2022). Homologous recombination deficiency: concepts, definitions and assays. Oncologist, 27:167-174.
Publisher | Google Scholor - Stanta G, Bonin S. (2018). Overview on clinical relevance of intra tumor heterogeneity. Front Med, 5:85.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Gautam Allahbadia, Mandeep Singh. (2016). Advances in the Therapy of Advanced Ovarian Cancer-Special Emphasis on the PD1/PDL1 Pathway. Curr Trends Biomedical Eng & Biosci, 1(2).
Publisher | Google Scholor - Kulvinder Kochar Kaur, Gautam Allahbadia, Mandeep Singh. (2019). “An Update on High Grade Serous Ovarian Carcinoma - A Comprehensive Review”. Acta Scientific Cancer Biology, 3(3):37-49.
Publisher | Google Scholor - Soslow RA, Han G, Park KJ, G K, Olvera N, Spriggs DR, etal. (2012). morphological patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol, 25:625-636.
Publisher | Google Scholor - Azzalini E, Stanta G, Canzonieri V, Bonin S. (2023). Overview of tumor heterogeneity in high grade serous ovarian cancers. Int J Mol Sci, 24:15077.
Publisher | Google Scholor - Azzalini E, Abdurakhmanova N, Parisse P, Bartoletti M, Canzonieri V, Stanta G, etal. (2021). Cell stiffness and morphological architectural patternsin clinical samples of high grade serous ovarian cancers. Nanomedicine, 37:102452.
Publisher | Google Scholor - Murukami R, Matsumura N, Mandai M, Yoshihara K, Tanabe H, Nakai H, etal. (2016). Establishment of a novel histopathological classification of ahigh grade serous ovarian carcinoma correlated with prognostically distinct gene expression subtypes. Am JPathol, 186:1103-1113.
Publisher | Google Scholor - SinghN, McCluggage WG, Gilks CB. (2017). High grade serous carcinoma of tubo- ovarian origin: recent developments. Histopathology, 71:339-356.
Publisher | Google Scholor - FujiwaraM, McGuire VA, Felberg A, Sieh W, Whittemore AS, Longcare TA. (2012). Prediction of germline BRCA1 mutation status in women with ovarian cancer using morphology based criteria: identification of a BRCA1 ovarian cancer phenotype. Am J Surg Pathol, 36:1170-1177.
Publisher | Google Scholor - Reyes MC, Arnold AG, Kauff ND, Levine DA, Soslow RA. (2014). Invasion patterns of metastatic high grade serous ovarian carcinoma of ovary or fallopian tube associated with BRCA1 deficiency. Mod Pathol, 27:1405-1411.
Publisher | Google Scholor - Hussein YR, Ducie JA, Arnold AG, Kauff ND, Vargas Alvarez HA, Sala E, etal. (2016). Invasion patterns of metastatic extra uterine high grade serous ovarian carcinoma with BRCA1 germline mutation and correlation with clinical outcomes. Am J Surg Pathol, 40:404-409.
Publisher | Google Scholor - Reid BM, Vyas S, ChenZ, ChenA, Kanetsky PA, Permuth JB, etal. (2021). Morphologic and molecular correlates of EZH2as a predictor of platinum resistance in high grade serous ovarian carcinoma. BMC Cancer, 21:714.
Publisher | Google Scholor - Uner H, Demir M, Goksuluk D, Kars A, Uner M, UsubutunA. (2022). Evidence for diverse prognosis in high grade serous ovarian carcinoma: solid, psudoendometrioid and transitional; so called ‘’SET morphology’ and’ progesterone status. Turk Patoloji Derg, 38:240-250.
Publisher | Google Scholor - ZengH, ChenL, ZhangM, LaoY, MaX. (2021). Integration of histopathological images and multi dimensional omics analysis predicts molecular features and prognosis of high grade serous ovarian cancers. Gynaecol Oncol, 163:171-180.
Publisher | Google Scholor - Azarianpour S, Corredor G, Bera K, Leo P, FuP, etal. (2022). Computational image features of immune architecture is associated with clinical benefit and survival in gynaecolical cancers across treatment modalities. J Immunother Cancer, 10:003833.
Publisher | Google Scholor - Nawaz S, Trahearn NA, Heindl A, Banerjee S, Maley CC, Sottoriva A, etal. (2019). Analysis of tumor ecological balance reveales resource dependent adaptive strategies of ovarian cancers. EBioMedicine, 48:224-235.
Publisher | Google Scholor - Abel J, Jain S, Rajan D, Padigela H, Leidal K, Prakash A, Conway J, etal. (2023). Cell type specific nuclear morphology’ predicts genomic instability and prognosis in multiplecancer type. bioRxiv.
Publisher | Google Scholor - Heindl A, KhanAM, RodriquesDN, Eason KN, Sadanandam A, Orbegoso C, etal. (2018). Microenvironmental niche divergence shapes BRCA1 dysregulated ovarian cancers morphological plasticity. NatCommun, 9:3917.
Publisher | Google Scholor - Khashaba M, Fawzy M, Abdel AzizA, Eladawei G, Nagib R. (2022). Subtyping of high grade serous ovarian carcinoma: histopathological and immunohistochemical approach. JEgypt Natl Cancer Inst, 34:6.
Publisher | Google Scholor - Khalique L, Ayhan A, Weale ME, Jacobs IJ, Ramus SJ, Gayther SJ. (2007). Genetic intra tumor heterogeneity in Epithelial ovarian cancer and its implications for molecular diagnosis of tumors. JPathol, 211:286-295.
Publisher | Google Scholor - Ohsuga T, Yamaguchi K, KidoA, Murukami R, Abiko K,Hamanishi J, etal. (2017). Distinct preoperative clinical features predict four histopathological subtypes of high grade serous ovarian carcinoma of the ovary or fallopian tube and peritoneum. BMC Cancer, 17:580.
Publisher | Google Scholor - Miyagawa C, Nakai H, Otani T, Murukami R, TakamuraS, Takaya T, etal. (2023). Histopathological subtyping of high grade serous ovarian cancer using whole slide imaging. JGynaecol Oncol, 34:e47.
Publisher | Google Scholor - Azzalini E, Tierno D, Bartoletti M, Barbazza R, GiordaG, Puglisi F, etal. (2022). AKT isoforms interplay in high grade serous ovarian cancer prognosis and characterization. Cancers, 14:304.
Publisher | Google Scholor - Hunt AL, Bateman NW, Barakat W, Makohon MooreS, Hood BL, Conrads KA, etal. (2021). Extensive three-dimensional intra tumor heterogeneity revealed by multi-region sampling in high grade serous ovarian tumor specimens. iScience, 24:102757.
Publisher | Google Scholor - Lahtinen A, Lavikka A, Virtanen A,LiY, Jamalzadeh S, Skorda A, etal. (2023). Evolutionary states and trajectories characterized by distinct pathways stratify patients with high grade serous ovarian carcinoma. Cancer Cell, 41: 1103-1117.e1112
Publisher | Google Scholor - Handley KF, Sims TT, Bateman NW, Glassman D, Foster KJ, LeeS, YJ, etal. (2022). Classification of high grade serous ovarian cancer using tumor morphological characteristics. JAMA Netw Open, 5:2236626.
Publisher | Google Scholor - Foster KJ, Handley KF, Glassman D, Sims TT, JavadiP, P SM, etal. (2023). Characterizing morphological subtypes of high grade serous ovarian cancer by CT:a retrospective cohort study. Int JGynaecol Oncol, 33:937-947.
Publisher | Google Scholor - Eckert MA, PanS, Hernandez KM, Loth RM, Andrade J, Volchenboum SL, etal. Genomics of ovarian cancer progression, reveales diverse metabolic trajectories including intraepithelial metastasis to fallopian tube. Cancer Discov, 6:1342-1351.
Publisher | Google Scholor - Cooke SL,Brenton JD. (2011). Evolution of platinum resistance in high grade serous ovarian cancer. Lancet Oncol, 12:1169-1174.
Publisher | Google Scholor - Donehower LA, Soussi T, Korkut A, LiuY, Schultz A, Cardenas M, LiX, etal. (2019). Integrated analysis of TP53 gene and pathway alterations in the Cancer Genome Atlas. Cell Rep, 28:1370-1384.
Publisher | Google Scholor - Cunnea P, CurryEW, Christie EL, Nixon K, Kwok CH, Pandey A, etal. (2023). Spatial and temporal intra tumor heterogeneity in advanced HGSOC’s: implications for surgical and clinical outcomes. Cell Rep Med, 4:101055.
Publisher | Google Scholor - Masoodi T, Siraj S, Siraj AK, Azam S, Qadri S, Parvatha reddy SK, etal. (2020). Genetic heterogeneity and evolutionary history of high grade serous ovarian carcinoma and matched distant metastasis. Br J Cancer, 122:1219-1230.
Publisher | Google Scholor - Yang SYC, Lheureux S, Karakasis K, Burnier JV, Bruce JP, Clouthier DL, etal. (2018). Landscape of genomic alterations in high grade serous ovarian cancer from exceptional long and short survivors. Genome Med, 10: 81.
Publisher | Google Scholor - Mittempergher L. Genomic characterization of high grade serous ovarian cancer dissecting its molecular heterogeneity as a road towards effective therapeutic strategies. Curr Oncol Rep 201618:44.
Publisher | Google Scholor - Filippova OT, Selenica P, Pareja F, Vahdantinia M, ZhuY, Pei X, etal. (2021). Molecular characterization of high grade serous ovarian cancers occurring in younger and older women. Gynaecol Oncol, 161:545-552.
Publisher | Google Scholor - Martins FC, Santiago I, TrinhA, XianJ, Guo A,Sayal K, etal. (2014). Combined image and genomic analysis of high grade serous ovarian cancer reveals PTEN loss as a common driver and prognostic classifier. Genome Biol, 15:526.
Publisher | Google Scholor - Stronach EA, Paul J, Timms KM, Hughe E, Brown K, Neff C, etal. (2018). Biomarker asssessment of HR deficiency, Tumor BRCA1/2 mutations and CCNE1 copy numberin ovarian cancer: association with clinical outcomes following platinum monotherapy. Mol Cancer Res, 16: 1103-1111.
Publisher | Google Scholor - Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, etal. (2015). Whole genome characterization of chemoresistant ovarian cancer. Nature, 521:489-494.
Publisher | Google Scholor - Martins FC, CouturierDL, Paterson A, KAN, ChowC, Nazeran TM, etal. (2020). Clinical and pathological associations of PTEN expressionin ovarian cancer: a multicenter, study from the Ovarian TissueTumor analysis Consortium. Br J Cancer, 123:793-802.
Publisher | Google Scholor - Kotnik EN, Mullen MM, Spies NC, LiT, Inkman M, ZhangJ, etal. (2023). Genetic characterization of primary and metastatic high grade serous ovarian cancer tumorsreveal distinct features associated with survival. Commun Biol, 6:688.
Publisher | Google Scholor - Van WagensveldL, vanBaal JOAM, Timmermans M, Gaillard D, Borghius L, Coffelt SB, etal. (2022). Homologous recombination deficiency and Cyclin E1 amplificationare correlated with immune cells infiltration and survival in high grade serous ovarian cancers. Cancers, 14:5965.
Publisher | Google Scholor - Martins FC, CouturierDL, de Santiago I, Sauer CM,Vias M, Anglelova M, etal. (2022). Clonal somatic copy number altered driver events inform drug sensitivity in high grade serous ovarian cancers. NatCommun, 13:6360.
Publisher | Google Scholor - SchwartzRF, NgCK, Cooke SL, Newman S, Temple J, Piskorz AM, etal. (2015). Spatial and temporal heterogeneityin high grade serous ovarian cancers:a phylogenetic analysis. PLoS Med, 12:e1001789.
Publisher | Google Scholor - VishwakarmaR, McManusKJ. (2020). Chromosomal instability: implications in cancer development, progression and clinical outcomes. Cancers, 2:824.
Publisher | Google Scholor - Blagden SP. (2015). Harnessing pandemomium: the clinical implications of tumor heterogeneity in ovarian cancers. FrontOncol, 5:149.
Publisher | Google Scholor - SunT, ZhangZ, TianL, ZhengY, WuL, GuoY, etal. (2023). Dualistic classification of high grade serous ovarian carcinoma has its root in spatial heterogeneity. JAdv Res, 48:213-225.
Publisher | Google Scholor - Geistlinger L, OhS, Ramos M, Schiffer L, LaRue RS, Henzler CM, etal. (2020). Multi omics analysis of subtypes evolution and heterogeneity in high grade serous ovarian carcinoma. Cancer Res, 80:4335-4345.
Publisher | Google Scholor - ReyesHD, DevorJ, Warrier A, Newtson AM, Mattson J, Wagner V, etal. (2019). Differential DNA methylation in high grade serous ovarian cancers (HGSOC’) is associated with tumor behaviour. Sci Rep, 9:17996.
Publisher | Google Scholor - Chan DW, Lam WY, ChenF, YungMMH, ChanYS, ChanWS, etal. (2021). DNA methylomeidentifies methylation associated with survival and drug resistance of ovarian cancers. Clin Epigenet, 13:142.
Publisher | Google Scholor - SilvaR, GlennonK, Metoudi M, Moran B, Salta S, Slattery K, etal. (2023). Unveiling the epigeneome mechanisms of the platinum resistance in high grade serous ovarian cancers. Int J Cancer, 153: 120-132.
Publisher | Google Scholor - Nishiyama A, Nakanishi M. (2021). Navigating the DNA methylation landscape of cancer. Trends Genet, 37:1012-1027.
Publisher | Google Scholor - Wang J, LiJ, ChenR, YueH, LiW, WuB, etal. (2021). DNA methylation-based profiling reveals distinct clusters with survival heterogeneity in high grade serous ovarian cancers. Clin Epigenet, 13:190.
Publisher | Google Scholor - BitlerBG, Bailey CA, Yamamoto TM, McMellen A, Kim H, Watson ZL. (2023). Targeting BRPF3 moderately reverses Olaparib resistance in high grade serous ovarian cancers. Mol Carcinogenesis, 62(11):1713-1730
Publisher | Google Scholor - Watson ZL, Yamamoto TM, McMellen A, Kim H, HughesCL, W heelerLJ etal. (2019). Histone MethyltransferasesEHMT1 and EHMT2(GLP/ G9A) maintain PARP inhibitor resistance in high grade serous ovarian carcinoma. Clin Epigenet, 11:165.
Publisher | Google Scholor - Kukita A, Sone K, Oda K, Hamamoto R, Kaneko S, Kamatsu M, etal. (2019). Histone Methyltransferases SMYD2 selective inhibitor LLY-507 in combination with poly ADP ribose polymerase inhibitor has therapeutic potential against high grade serous ovarian carcinoma. Biochim Biophys Res Commun, 513:340-346.
Publisher | Google Scholor - Talhouk A, GeorgeJ, WangC, Budden T, TanTZ, Chiu DS, etal. (2020). Developmental validation of the gene expression predictor of high grade serous ovarian carcinoma molecularsubTYPE(PrOTYPE). Clin Cancer Res, 26:5411-5423.
Publisher | Google Scholor - Sohn HM, KimSJ, ShinJY, KimHS, ChungHH, KimJW etal. (2021). Classification of high grade serous ovarian carcinomaby epithelial –mesenchymal transition and homologous recombination repair gene signatures. Genes, 12:1103.
Publisher | Google Scholor - Chirshev H, Hojo N, Bertucci A, Sanderman L, l Nguyen AWangH, etal. (2020). Epithelial /–mesenchymal heterogeneity of high grade serous ovarian carcinoma samples correlates withmiRNA let 7 levels and predicts tumor growth and metastasis. Mol Oncol, 14: 2796-2813.
Publisher | Google Scholor - FerriBorgognoS, ZhuY, ShengJ, Burkes JK, Gomez JA, Woong KK, etal. (2023). Spatial transcriptomicsdepict ligand receptor crosstalk heterogeneity at the tumor stroma interface in long term ovarian cancers survivors. Cancer Res, 83:1503-1516.
Publisher | Google Scholor - Nath A, Cosgrove PA, Mirsafian H, Christie EL, Fflieger L, Copeland B, etal. (2021). Evolution of core archetypal phenotypes in progressive high grade serous ovarian cancers. NatCommun, 12:3039.
Publisher | Google Scholor - IzarB, Tirosh I, Stover EH, Wakiro I, Cuoco MS, Alter I, etal. (2020). A single cell landscape of high grade serous ovarian cancers. NatMed, 26:1271-1279.
Publisher | Google Scholor - Stur E, Corgivno S, XuM, ChenK, TanY LeeS I, etal. (2022). Spatially resolved transcriptomics of high grade serous ovarian carcinoma. iScience, 25:103923.
Publisher | Google Scholor - SunJ, YanC, XuD, ZhangZ, LiK, LiS, etal. (2022). Immunogenomic characterization of high grade serous ovariancancer reveals immune evasion mechanisms and identifies an immunologic subtype with a favourable prognosis and improved therapeutic efficacy. Br J Cancer, 126:1570-1580.
Publisher | Google Scholor - XuJ, FangY, ChenK, LiS, TangS, RenY, etal. (2022). Single cell RNA sequencing reveals the negative tissue architecturein human high grade serous ovarian cancers. Clin Cancer Res, 28:3590-3602.
Publisher | Google Scholor - YangB, LiX, ZhangW, FanJ, ZhouY, LiW, etal. (2022). Spatial heterogeneity of infiltrating T cells in high grade serous ovarian cancers revealed by Multi omics analysis. Cell Rep Med, 3:100856
Publisher | Google Scholor - Vasquez- GarciaI, Uhlitz F, Ceglia N, Lim JLP, WuM, Mohibullah N, etal. (2022). Ovarian cancer mutational processes drive site specific immune evasion. Nature, 612:778-786.
Publisher | Google Scholor - Jiminez- Sanchez A, Cybulska P, Mager KL, Koplev S, Cast O, Couturier DL, etal. (2020). Unravelling tumor immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat Genet, 52:582-593.
Publisher | Google Scholor - Kelliher L, Lengyel E. (2023). Understanding long term survival of patients with ovarian cancers- the tumor microenvironment comes to the forefront. Cancer Res, 83:1383-1385.
Publisher | Google Scholor - Lecker LSM, Berlato C, Maniati E, Delaine Smith R, PearceOMT, Heath O, etal. (2021). TGFB1 production by macrophages contributes to an immunosuppressive microenvironment in ovarian cancer. Cancer Res, 81:5706-5719.
Publisher | Google Scholor - ElArabey AA, Denizli M, Kanliilicer P, Bayraktar R, Ivan C, Rashed M, etal. (2020). GATA3as a master regulator for interactions of tumor associated macrophages with high grade serous ovarian carcinoma. Cell Signal, 68:109539.
Publisher | Google Scholor - Pejovic T, Fitch K, Mills G. (2022). Ovarian cancer recurrence: is the definition of platinum resistance modified by PARP inhibitors and other intervening treatments? Cancer Drug Resist, 5:451-458.
Publisher | Google Scholor - DiSilvestro P, Banerjee S, Colombo N, Scambia G, KimBG, Oaknin A, etal. (2023). Overall survival with maintenance Olaparib at a 7 yr follow upin patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: The SOLO1/GOG 3004 Trial. J Clin Oncol, 41:609-617.
Publisher | Google Scholor - LiQ, XiaoX, FengJ, YanR, XiJ. (2023). Machine Learning-assisted analysis of epithelial – mesenchymal transition pathway for prognostic stratification and immune infiltration asssessment in ovarian cancers. Front Endocrinol, 14:1196094.
Publisher | Google Scholor - Montfort A, Barker Clarke RJ, Piskorz AM, Supernat A, Moore L, AlKhiladi S, etal. (2020). Combiningmeasures of immune infiltration shows additive effect on survival prediction in high grade serous ovarian carcinoma. Br J Cancer, 122:1803-1810.
Publisher | Google Scholor - Stewart MD, Vega DM, Arend RC, Baden JF, Barbash O, Beaubier N, etal. (2022). Homologous recombination deficiency:concepts, definitions, and assays. Oncologist, 27:167-174.
Publisher | Google Scholor