Research Article
An Observational, Retrospective Study to Examine Safety of Off-Label Medicines by Analyzing ADR
- Vivek Shantilal Zade *
- Shivani Shamrao Kekare
Masters of science in Clinical Research, India.
*Corresponding Author: Vivek Shantilal Zade, Masters of science in Clinical Research, India.
Citation: Vivek S. Zade, Shivani S. Kekare. (2024). An Observational, Retrospective Study to Examine Safety of Off-Label Medicines by Analysing Adr. Journal of Clinical Medicine and Practice, BioRes Scientia Publishers. 1(1):1-6. DOI: 10.59657/jcmp.brs.24.008
Copyright: © 2024 Vivek Shantilal Zade, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: June 24, 2024 | Accepted: July 15, 2024 | Published: July 22, 2024
Abstract
The use of medicines for off-label purposes, where medications are utilized for indications outside of their approved label, is a common practice in clinical settings. This observational, cross-sectional study aims to examine the safety profile of medicines used for off-label purposes, focusing on adverse drug reactions (ADRs) and associated factors. Using a retrospective approach, data will be collected from old prescriptions of patients who received medications off-label. A structured data collection form will be used to gather information on patient demographics, medical history, concomitant medications, off-label use details, and ADRs. Statistical analysis, including descriptive statistics, will be employed to assess the ADRs, identify factors associated with ADR occurrence, and explore patterns of off-label medication use. This study seeks to provide valuable insights into the safety considerations of off-label medication use, informing clinicians, regulators, and policymakers about the potential risks and benefits associated with this practice.
Keywords: off-label use; adr; observational study; risk factors; clinical practice; patient safety
Introduction
The use of medications for purposes other than their approved indications, commonly known as off-label use, is a widespread practice in clinical medicine [1,2]. Off-label prescribing is often based on sound clinical judgment and supported by scientific evidence, but it also poses potential risks to patients due to the lack of regulatory oversight and comprehensive safety data for such uses. This observational, cross-sectional study aims to explore the safety profile of medicines used off-label, focusing on the occurrence of adverse drug reactions (ADRs). Off-label use occurs for various reasons, including the lack of approved treatment options for certain conditions, the emergence of new evidence supporting alternative uses, or individual patient characteristics that may warrant deviation from standard prescribing practices. In the pre-approval clinical experience with a new medicinal product or its new usages, particularly as the therapeutic dose(s) may not be established: all noxious and unintended responses to a medicinal product related to any dose should be considered Adverse Drug Reactions (ADRs) [2,3]. The safety of off-label medication use is a complex issue influenced by various factors, including the specific medication and indication, patient characteristics, dosing regimens, and concurrent use of other medications. ADRs associated with off-label use can range from mild and manageable to severe and life-threatening, highlighting the importance of assessing the safety profile of these practices.
The primary objective of this study is to evaluate the safety profile of commonly used off-label medicines. The focus will be on five categories of medicines:
Antihypertensive, Antidiabetic, Anti-depressant, Antacid, Anti-cancer.
Each category will include a detailed examination of five representative drugs, with a thorough analysis of their associated ADRs. By systematically reviewing these adverse reactions, the study aims to identify patterns and commonalities that may inform safer prescribing practices and guide future research. This research adopts a retrospective, observational, cross-sectional study design, leveraging existing medical records and databases to collect data on off-label medicine use and related ADRs. This approach allows for the comprehensive analysis of real-world data, reflecting the actual experiences of patients and healthcare providers. The retrospective nature of the study facilitates the identification of long-term outcomes and rare adverse events that may not be apparent in smaller, controlled clinical trials. The significance of this study lies in its potential to contribute to the broader understanding of off-label medicine use. By highlighting safety concerns and providing empirical evidence, the findings can support the development of guidelines and regulations aimed at minimizing risks and optimizing patient outcomes.
Study rational
Off-label use of medications, though common, presents a unique set of challenges and considerations in clinical practice. While off-label prescribing is often supported by clinical evidence and may provide valuable treatment options for patients, it also carries potential risks related to safety and efficacy. Assessing the safety profile of medications used off-label will provide valuable insights into the frequency and severity of adverse drug reactions (ADRs) associated with this practice. This information is crucial for clinicians to weigh the risks and benefits of off-label prescribing. Exploring the factors associated with ADR occurrence in patients receiving medications off-label can help identify high-risk populations and inform strategies to minimize potential harm. Understanding the patterns of off-label medication use which can provide valuable insights into prescribing practices in real- world clinical settings.
Aim and objectives
Aim: The aim of this study is to examine the safety profile of medicines used for off-label purposes, focusing on the occurrence of adverse drug reactions (ADRs) and associated factors.
Objectives
Primary Objective
To assess the safety profile of medicines used for off-label purposes, focusing on the occurrence of adverse drug reactions (ADRs).
Secondary Objectives
To identify the most commonly used medications for off-label purposes. To compare the safety profiles of medications used off-label with their approved indications. To provide recommendations for optimizing the safety of off-label medication use in clinical practice.
Hypothesis
Null Hypothesis [H0]
The safety profile of medicines used for off-label purposes does not significantly differ from the safety profile of these medicines when used for their approved purposes.
Alternative hypothesis [H1]:
The safety profile of medicines used for off-label purposes significantly differ from the safety profile of these medicines when used for their approved purposes.
Selection criteria
Inclusion Criteria
Patients of all ages. Patients who have received at least one medication off-label, defined as the use of a medication for an indication, dosage, or patient population that is not approved by regulatory authorities. Patients who have provided informed consent for the use of their medical data for research purposes. Patients who have received medications off-label for a minimum duration of time (e.g., at least one dose).
Exclusion Criteria
Patients with known allergies or contraindications to the off-label medication. Patients who have received medications off-label for investigational purposes in a clinical trial. Patients with a history of non-adherence to prescribed medication regimens. Patients who have received off-label medications as part of a compassionate use program or under special circumstances not reflective of standard clinical practice.
Participant’s discontinuation/ withdrawal criteria:
Participants can choose to withdraw from the study at any time without providing a reason. Participants experiencing severe or unexpected ADRs will be withdrawn to ensure their safety. Development of new, significant medical conditions or worsening of existing conditions that interfere with study participation will lead to withdrawal. Female participants who become pregnant during the study will be withdrawn due to potential risks to the fetus.
Methodology
Data Collection
Data collection involved the following steps:
Prescription Selection: 20 prescriptions, review article, published journals were randomly selected from the total prescriptions available in the healthcare facilities.
Classification of Medications: Each medication listed in the prescriptions was classified as either on-label or off-label based on the indications provided by healthcare professionals.
Data Recording: Information on the side effects experienced by patients was recorded for each medication. This included both on-label and off-label medications. The collected data was analysed to compare the of side effects between on-label and off-label medications. Statistical methods, such as descriptive statistics tests, were used to determine if there was a significant difference in the of side effects between the two categories. The analysis showed that on-label medications had a lower of side effects compared to off-label medications.
Retrospective study
A retrospective study is a type of observational research that looks back in time to analyze data that has already been collected [17]. The retrospective study design for this research project involves the systematic collection and analysis of historical data related to the use of medicines for off-label purposes. Data for the study will be collected from existing sources, such as health records, medical charts, pharmacy records, and adverse event reporting systems. These sources will provide information on patient demographics, medical history, prescribed medicines, dosages, duration of treatment, and reported ADRs. The collected data will be analysed to determine nature of ADRs associated with the off-label use of medicines. Statistical methods, such as descriptive statistics will be used to assess the relationship between off-label use and Adrs. One key advantage of a retrospective study is that it can be conducted relatively quickly and at a lower cost compared to prospective studies, which gather data going forward in time. This makes retrospective studies useful for investigating ADR that would be challenging to study in a prospective manner.
List of Medicines (Approved VS Prescribed)
There are 5 categories we discuss here:
Anti-cancer
Anti-diabetic
Antihypertensive
Anti-Depressant
Antacid
Anti-cancer
Tamoxifen (Nolvadex):
Approved for: Breast cancer
Prescribed for: Ovarian cancer, endometrial cancer
Bevacizumab (Avastin):
Approved for: Colorectal cancer, lung cancer, kidney cancer, glioblastoma
Prescribed for: Breast cancer, ovarian cancer
Imatinib (Gleevec):
Approved for: Chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST)
Prescribed for: Dermatofibrosarcoma protuberans (DFSP), systemic mastocytosis, myelodysplastic syndrome (MDS)
Bortezomib (Velcade):
Approved for: Multiple myeloma, mantle cell lymphoma
Prescribed for: Non-Hodgkin's lymphoma, acute myeloid leukemia (AML).
Rituximab (Rituxan):
Approved for: Non-Hodgkin's lymphoma, chronic lymphocytic leukemia (CLL)
Prescribed for: Rheumatoid arthritis, autoimmune hemolytic anemia [18].
Anti-diabetic:
Metformin:
FDA-approved for: Type 2 Diabetes Mellitus (T2DM).
Prescribed off-label for: Type 1 Diabetes Mellitus (T1DM).
Liraglutide:
FDA-approved for T2DM and obesity.
Prescribed off-label for T1DM
Canagliflozin:
FDA-approved for T2DM.
Prescribed off-label for T1DM.
Dapagliflozin:
FDA-approved for T2DM.
Prescribed off-label for T1DM.
Sitagliptin:
FDA-approved for T2DM.
Prescribed off-label for T1DM [13,14].
Antihypertensive
Propranolol:
Approved for: Hypertension, angina, and tremors.
Prescribed for: Performance anxiety, migraine prophylaxis, and essential tremor.
Amlodipine:
Approved for: Hypertension and angina.
Prescribed for: Raynaud's phenomenon and migraine prophylaxis.
Clonidine:
Approved for: Hypertension and ADHD (Attention-Deficit/Hyperactivity Disorder).
Prescribed for: anxiety disorder, opioid withdrawal, and smoking cessation.
Losartan:
Approved for: Hypertension and diabetic nephropathy.
Prescribed for: Marfan syndrome and heart failure.
Methyldopa:
Approved for: Hypertension.
Prescribed for: Gestational hypertension and attention deficit disorder [9,10].
Anti-Depressant
Trazodone:
FDA-approved: depression.
Prescribed off-label: insomnia.
Amitriptyline:
FDA-approved for: depression.
Prescribed for: chronic pain, migraines, and insomnia.
Mirtazapine:
FDA-approved for: depression.
Prescribed off-label for: insomnia and anxiety.
Venlafaxine:
FDA-approved for: depression and anxiety disorders.
Prescribed off-label for: neuropathic pain and hot flashes.
Doxepin
FDA-approved for depression and anxiety.
Prescribed off-label for insomnia [15,16].
Antacid
Omeprazole (Prilosec):
FDA Approved for: Treatment of gastroesophageal reflux disease (GERD)
Prescribed for: Treatment of gastritis and peptic ulcer disease
Ranitidine (Zantac):
FDA Approved for: Treatment and prevention of ulcers in the stomach and intestines
Prescribed for: Heartburn and acid indigestion
Famotidine (Pepcid):
FDA Approved for: Treatment of ulcers, gastroesophageal reflux disease (GERD), and conditions where the stomach produces too much acid
Prescribed for: Prevention of gastrointestinal bleeding in critically ill patients
Esomeprazole (Nexium):
FDA Approved for: Treatment of GERD, ulcers, and conditions where the stomach makes too much acid
Prescribed for: Treatment of dyspepsia and peptic ulcer disease.
Pantoprazole (Protonix):
FDA Approved for: Treatment of erosive esophagitis, GERD, and Zollinger-Ellison syndrome
Prescribed for: Treatment of peptic ulcer disease [7,8].
Result
We found following Adverse Drug Reactions (ADRs)of off-label and approved medications:
| Categories | On-Label Adrs | Off-Label Adrs |
| Anti-cancer | Hot flashes, Nausea, Fatigue, Mood swings, Cataracts, Increased risk of stroke, Hypertension, Wound healing complications, Diarrhea, Abdominal pain, Vomiting, Muscle cramps, Rash, Edema, Hepatotoxicity, Constipation, Anemia, Fever, Hepatitis B reactivation. | Hot flashes, vaginal discharge, irregular menstruation, weight loss, mood changes, High blood pressure, bleeding, slow wound healing, proteinuria, fatigue, Edema, muscle cramps, diarrhea, rash, fatigue, Nausea, peripheral neuropathy, flow platelet count, Infusion reactions, fever, chills, infections, low blood cell counts, Hepatitis B reactivation |
| Anti-diabetic | Gastrointestinal issue, diarrhea, abdominal pain, Lactic acidosis, Vitamin B12 deficiency, Taste disturbance, Nause, Vomiting, Constipation, Headache, Genital mycotic infections, Urinary tract infections, Bone fractures, Back pain, Nausea, Dizziness. | Gastrointestinal disturbances (e.g., diarrhea, nausea, abdominal pain), vitamin B12 deficiency, lactic acidosis (rare but serious), vomiting, pancreatitis (rare but serious), genital mycotic infections, urinary tract infections, volume depletion-related events (e.g., hypotension, syncope), genital mycotic infections, upper respiratory tract infection, nasopharyngitis, headache, hypoglycemia (especially when used in combination with insulin or sulfonylureas). |
| Anti- hypertensive | Fatigue, Dizziness, Bradycardia, Hypotension, Nausea, Vomiting, Diarrhea, Sleep disturbances, Edema, Headache, Back pain, Weakness | Fatigue, cold extremities, bradycardia, Peripheral edema, dizziness, flushing, Drowsiness, dry mouth, hypotension, Hyperkalemia, cough, Sedation |
| Anti-Depressant | Sedation, Drowsiness, Dizziness, Dry mouth, Nausea, Headache, Constipation, Weight gain, Increased heart rate, hypotension, Insomnia, Nervousness, | Headache, Drowsiness, dizziness, dry mouth, blurred vision, constipation, weight gain, increased appetite, nausea, insomnia, increased blood pressure. |
| Antacid | Headache, Abdominal pain, Nausea, Diarrhea, Vomiting, Dizziness, Constipation, Rash, Dry mouth, Joint pain | Headache, nausea, diarrhea, abdominal pain, vitamin B12 deficiency, dizziness, constipation, Hepatitis, pancreatitis, constipation, thrombocytopenia. |
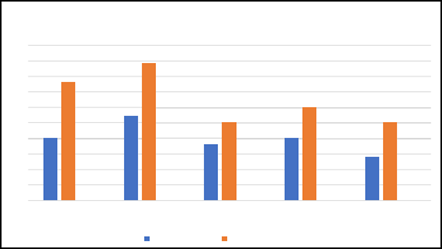
Figure 1: X –axis: Categories Y – axis: ADRs%
The pie chart illustrates the distribution of Adverse Drug Reactions (ADR) across above 5 different medication categories.
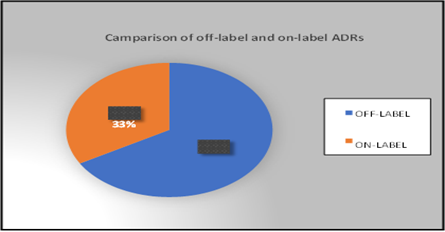
Figure 2
Hence, the pie-chart shows that off-label medications accounted for highest percentage of ADRs at67% than on-label at 33%.
Discussion
The study identified several medicines that are commonly repurposed for off-label uses. The efficacy of repurposed medicines in treating unconventional conditions varied. While some medicines showed promising results, others were found to be less effective. Adverse drug reactions were reported for some medicines, highlighting the importance of careful monitoring. The study concludes that while off-label use of medicines can offer innovative treatment options, careful consideration of efficacy and safety is essential. By improving the regulatory framework and promoting evidence-based off-label prescribing, the safe and effective use of repurposed medicines can be enhanced. Further research is warranted to better understand the risks associated with off-label use and to develop strategies to mitigate these risks.
Conclusion
In conclusion, the practice of off-label medication use is common and presents both opportunities and challenges in clinical practice. While off-label prescribing can provide valuable treatment options for patients, it also raises concerns regarding safety, efficacy, and regulatory oversight. By analysing data from old prescriptions, we seek to identify patterns of off-label medication use, assess the of ADRs, and explore factors influencing ADR occurrence. The findings of this study have the potential to inform clinical decision-making, regulatory policies, and guidelines related to off-label prescribing practices. By identifying high-risk populations and potential ADRs associated with off-label use, clinicians can make more informed decisions about prescribing off-label medications and monitoring patients for adverse effects. Ultimately, the goal of this study is to improve patient safety and outcomes in clinical practice. By providing evidence- based recommendations for off-label prescribing, this study aims to enhance the quality of care and optimize patient outcomes for individuals receiving medications off-label. We conclude that null hypothesis is rejected according to result, primary and secondary objectives are fulfilled.
References
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians.
Publisher | Google Scholor - Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population.
Publisher | Google Scholor - International council for harmonization (ICH-GCP) guideline E6R2.
Publisher | Google Scholor - Baraldi E, Girotto S, Dalla Pozza LV, et al. Off-label use of drugs in pediatric emergency medicine.
Publisher | Google Scholor - Gazarian, M., Kelly, M., McPhee, J.R., Graudins, L.V., Ward, R.L. and Campbell, T.J., (2006). Off-label use of medicines: consensus recommendations for evaluating appropriateness. Medical Journal of Australia.
Publisher | Google Scholor - Aronson, J.K. and Ferner, R.E., (2017). Unlicensed and off-label uses of medicines: definitions and clarification of terminology. British Journal of Clinical Pharmacology.
Publisher | Google Scholor - Lanthier M, Miller KL, Nardinelli C, et al. An improved approach to measuring drug innovation finds steady rates of first-in- class pharmaceuticals.
Publisher | Google Scholor - Bavdekar, S.B., (2013). Off-label drug use in children. The Indian Journal of Pediatrics.
Publisher | Google Scholor - Kimland, E. and Odlind, V., (2012). Off-label drug use in pediatric patients. Clinical Pharmacology & Therapeutics.
Publisher | Google Scholor - Arnaiz, J.A., Carne, X., Riba, N., Codina, C., Ribas, J., Trilla, A. and Laporte, J.R., (2001). The use of medicines in hospitals: off-label drug use. European Journal of Clinical Pharmacology.
Publisher | Google Scholor - Palmaro, A., Bissuel, R., Renaud, N., Dupouy, J., Durrieu, G., Montastruc, J.L. and Lapeyre-Mestre, M., (2015). Off-label prescribing in pediatric outpatients. Pediatrics.
Publisher | Google Scholor - Desai RJ, Sarpatwari A, Dejene S, et al. Comparative effectiveness of generic and brand-name medication use: a database study of US health insurance claims.
Publisher | Google Scholor - Friedman MA, Woodcock J, Lumpkin MM, et al. The safety of newly approved medicines.
Publisher | Google Scholor - Stafford RS. Regulating off-label drug use-rethinking the role of the FDA.
Publisher | Google Scholor - Bakalar N. Exploring the risks of off-label drug use.
Publisher | Google Scholor - Vlastarakos PV, Nikolopoulos TP, Maragoudakis P, Tzagaroulakis A, Ferekidis E. A comparative review of the adverse effects of antiepileptic drugs in children with epilepsy.
Publisher | Google Scholor - Lass J, Oselin K, Treufeldt T, et al. Off-label use of drugs in children in outpatient setting: a retrospective study.
Publisher | Google Scholor - Saiyed, M.M., Ong, P.S. and Chew, L., (2017). Off-label drug use in oncology: a systematic review of literature. Journal of Clinical Pharmacy and Therapeutics.
Publisher | Google Scholor