Research Article
Advancements In Glipizide Drug Delivery: Analysing Fast and Prolonged Release Tablets
- Tushar Sonare
- Aman Kumar
- Kratika Khadsondni
- Krutika Mandloi
- Akash Yadav *
- Dinesh Kumar Jain
IPS Academy College of Pharmacy, Knowledge Village, Rajendra Nagar, A.B. Road, Indore-452012, India.
*Corresponding Author: Akash Yadav, IPS Academy College of Pharmacy, Knowledge Village, Rajendra Nagar, A.B. Road, Indore-452012, India.
Citation: Sonare T., Kumar A., Khadsondni K., Mandloi K., Yadav A., et al. (2025). Advancements In Glipizide Drug Delivery: Analysing Fast and Prolonged Release Tablets, Journal of Clinical Research and Clinical Trials, BioRes Scientia Publishers. 4(3):1-11. DOI: 10.59657/2837-7184.brs.25.049
Copyright: © 2025 Akash Yadav, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 07, 2025 | Accepted: March 05, 2025 | Published: March 11, 2025
Abstract
Diabetes mellitus is a long-term metabolic condition marked by ongoing high blood sugar levels, which can lead to serious complications in the liver, kidneys, and heart. The most common type, Type 2 diabetes, is caused by insulin resistance and/or reduced function of pancreatic β-cells, often requiring medication to manage blood sugar effectively. Glipizide, a second-generation sulfonylurea, is widely used to stimulate insulin secretion and improve sensitivity to insulin in peripheral tissues. This study offers a thorough comparison of fast-dissolving tablets (FDTs) and sustained-release (SR) tablets of Glipizide, focusing on their formulation features, drug release behaviors, and pharmacokinetic properties. FDTs dissolve quickly in the mouth, leading to faster systemic absorption and a quicker response. In contrast, SR tablets are formulated for extended drug release, which helps maintain stable plasma levels and reduces the need for frequent dosing, thus improving patient adherence to treatment. Key formulation factors-such as the choice of excipients, dissolution rates, bioavailability, and considerations for patients-are examined in detail. The goal of this study is to refine pharmaceutical formulation methods to enhance drug delivery effectiveness and ensure lasting therapeutic benefits in the ongoing management of Type 2 diabetes.
Keywords: diabetes mellitus; glipizide; pancreatic β-cells; fast-dissolving tablets; sustained-release tablets
Introduction
Diabetes mellitus is a chronic metabolic disorder marked by consistently elevated blood sugar levels, which can gradually damage vital organs like the liver, blood vessels, eyes, and kidneys, as well as other bodily systems. If not properly managed, diabetes can lead to significant health complications over time. There are primarily two varieties of diabetes: Type 1 and Type 2. Type 2 diabetes is the more common form and typically affects older adults, although it has increasingly been diagnosed in younger people due to lifestyle changes. This condition develops when the body resists insulin or the pancreas fails to produce enough insulin to keep blood sugar levels normal. Over the past three decades, the prevalence of type 2 diabetes has significantly increased worldwide across all income levels, mainly due to rising rates of obesity, unhealthy eating habits, and insufficient physical activity. Conversely, Type 1 diabetes, often called juvenile diabetes or insulin-dependent diabetes, is an autoimmune condition where the pancreas produces very little or no insulin. This type often arises in childhood or adolescence, but adults can also develop it. Individuals with diabetes—regardless of type—require continuous and affordable access to healthcare, especially for critical medications like insulin, to manage their condition and prevent serious complications. Diabetes constitutes a major global health crisis, leading to approximately 1.5 million deaths annually. The number of diagnosed diabetes cases is steadily rising around the world, posing significant challenges for healthcare systems. Addressing these growing epidemic calls for urgent global efforts. It is essential to implement comprehensive plans aimed at reducing diabetes and obesity rates by 2025. This includes public health campaigns, improved access to healthcare, and policies that promote healthier lifestyles. Effectively fighting diabetes necessitates a coordinated international effort to alleviate future health and economic burdens [1-4]. Glipizide, a sulfonylurea, is used to manage Type 2 diabetes mellitus by stimulating insulin production and improving insulin sensitivity. With a molecular weight of 445.536 g/mol and a half-life of 2–6 hours, it is available as sustained-release tablets and oral solutions, listed in IP, BP, and USP, ensuring effective blood sugar control [5,6].
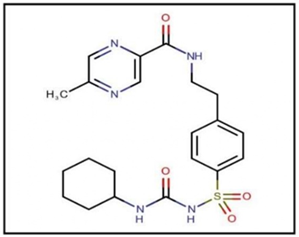
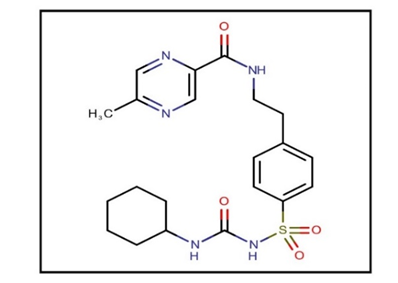
Figure 1: Glipizide.
Fast Dissolving Tablets
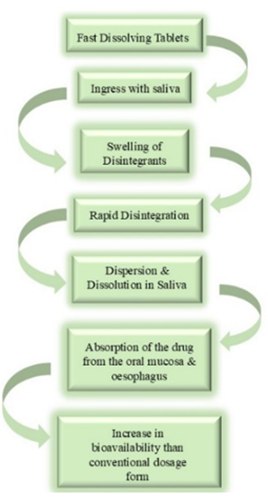
Fast-dissolving tablets are formulated to swiftly dissolve in liquids, serving as an alternative to conventional tablets. They are available in two varieties: coated and uncoated. These tablets form a consistent mixture, which facilitates easier intake, particularly for individuals who struggle with swallowing pills. They can be dissolved in water prior to consumption or taken directly with saliva for quicker absorption. This convenience is especially advantageous for those taking prescribed medications or dietary supplements. Fast-dissolving tablets excel in providing immediate relief, promoting faster effects. Their user-friendly nature simplifies the process of medication delivery, offering a faster and more effective method for achieving therapeutic results, making them a valuable option for both patients and healthcare professionals.
Figure 2: Action of Fast Dissolving Tablets.
Mechanism of Fast Dissolving Tablets
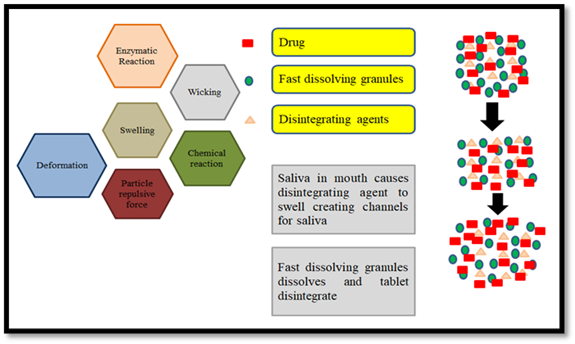
Tablets break up into little bits and dissolve in water or other liquids. The following phase is known as de-aggregation, which occurs when the granules break apart into their parent particles. Primary particles dissolve rapidly due to their huge surface area, whereas aggregates and whole tablets that form during tablet breakdown take longer to dissolve. The FDTs should dissolve or disperse in less than three minutes. Super disintegrates such sodium starch glycolate, cross povidone, and croscarmellose provide the basis of FDTs [7].
Figure 3: Mechanism of Fast Dissolving Tablets.
Important Characteristics of Fast-Dissolving Tablet
- Eases medication intake for individuals with mental impairments, disabilities, or reluctance to take pills.
- Solid doses can be ingested without the need for water.
- The dosage form disintegrates and dissolves quickly.
- Saliva in the stomach allows drugs to be absorbed through the mouth, throat, and esophagus, enhancing bioavailability.
- Effectively masks unpleasant drug tastes, improving the overall experience.
Advantages of Fast-Dissolving Tablets
- Unlike conventional dose forms, fast-dissolving tablets (FDTs) do not require liquid for ingestion.
- This feature makes them more convenient for patients who are traveling or lack access to water.
- The rapid disintegration and dissolution of FDTs enhance the bioavailability of poorly soluble medications.
- Manufacturing fast-dissolving tablets is cost-effective, as it utilizes standard processing and packaging equipment.
- FDTs dissolve and are absorbed swiftly in the mouth, providing quicker therapeutic effects [8].
Sustained-release (SR) Dosage
Sustained-release (SR) formulations are carefully designed to release active pharmaceutical ingredients over time, which helps maintain consistent therapeutic effects and minimizes the need for frequent dosing. By smoothing out variations in plasma levels, these formulations greatly improve patient compliance. Utilizing cutting-edge controlled-release technologies, including matrix systems, reservoir designs, and osmotic pumps, SR formulations take advantage of hydrophilic polymers and lipophilic waxes to control the rate of drug release. This approach optimizes pharmacokinetic profiles and ensures effective, long-lasting results [9,10].
Mechanism of Sustained Release Dosage Forms
Sustained-release (SR) formulations are carefully crafted to gradually release the active pharmaceutical ingredient over a longer period, ensuring that the therapeutic effects last. The release of the drug occurs through processes such as diffusion, dissolution, or erosion. Different systems like matrix-based, reservoir, and osmotic pumps play an essential role in controlling how the drug is released, which helps to minimize variations in plasma levels. Polymers like hydroxypropyl methylcellulose and ethylcellulose are intentionally used to manage the release rate, maintaining the drug's prolonged therapeutic effectiveness.
Important Characteristics of Sustained Release Dosage Forms
- Provides prolonged drug release, maintaining steady plasma concentration and reducing dosing frequency.
- Enhances patient compliance, especially for chronic conditions requiring long-term therapy.
- Reduces fluctuations in drug levels, minimizing peak-related side effects and improving therapeutic outcomes.
- Optimizes drug absorption by ensuring controlled and gradual release over time.
- Improves bioavailability by extending drug presence in the systemic circulation.
Advantages of Sustained Release Dosage Forms
- Unlike immediate-release formulations, sustained-release (SR) dosage forms provide a controlled and prolonged drug release, reducing the frequency of administration.
- This feature enhances patient convenience, particularly for individuals managing chronic conditions requiring long-term medication.
- The gradual release mechanism helps maintain steady plasma drug levels, minimizing fluctuations and reducing adverse effects.
- SR formulations improve bioavailability by ensuring consistent drug absorption over an extended period.
- Manufacturing sustained-release dosage forms is cost-effective, as it optimizes drug utilization and reduces the overall dosage requirement [11].
Comparison of Formulation, Pharmacokinetics Parameter
Table 1: Formulation of fast dissolving and sustained release.
| Aspect | Fast Dissolving Tablets | Sustained Release Tablets |
| Purpose | To provide rapid dissolution and absorption for fast onset of action. | To release the drug over an extended period for prolonged therapeutic action. |
| Drug Release Mechanism | Drug dissolves quickly in the mouth or stomach. | Drug is gradually released over hours. |
| Excipients | Super-disintegrants- Sodium starch glycolate, Cross-povidone | Polymers- Hydroxypropyl methylcellulose, ethyl cellulose |
| Binders- Guar gum, PVPK-30 | Fillers- Microcrystalline cellulose | |
| Lubricants- Magnesium stearate | Binders- PVPK-30 | |
| Glidant- Talc | Lubricants- Magnesium stearate | |
| Coating agents- Ethyl cellulose coating | ||
| Tablet size | Smaller and thinner due to rapid dissolution mechanism | Larger size to accommodate controlled-release mechanisms and higher drug content |
| Tablet Compression | needs to be compressed under low force to ensure quick dissolution. | Requires higher compression forces to ensure integrity and sustained release. |
| Drug Release Profile | Rapid drug release within minutes, typically within 3-5 minutes of contact with water or saliva. | sustained drug release over several hours (typically 8-10 hours). |
| Preparation Methods | Direct compression or molding | Compression with controlled-release |
| Advantages | Fast onset of action for immediate relief. | Reduced frequency of dosing, often once daily. |
| Convenient for patients with swallowing difficulties. | Stable plasma drug concentration with minimal fluctuations. | |
| Enhanced patient compliance for medications that need to be taken urgently. | Improves patient adherence for long-term treatments [12-13]. |
Table 2: Pharmacokinetic parameter of fast dissolving and sustained release.
| Pharmacokinetic Parameter | Fast Dissolving Tablets (FDTs) | Sustained Release (SR) Tablets |
| Absorption | Rapid absorption due to immediate dissolution in the oral cavity and gastrointestinal tract. | Slow and controlled absorption over an extended period due to gradual drug release. |
| Time to Peak Concentration (Tmax) | Shorter (typically 0.5 - 2 hours) due to fast dissolution. | Longer (typically 3 - 12 hours) due to slow drug release. |
| Bioavailability | Generally higher, as some drugs are absorbed through the oral mucosa before reaching the stomach. | Moderate to high, depending on formulation and drug properties. |
| Onset of Action | Rapid onset | Delayed onset, typically 1-3 hours. |
| Metabolism | First-pass metabolism in the liver if absorbed via the gastrointestinal tract. | bypass first-pass metabolism depending on formulation |
| Excretion | Rapid elimination, requiring frequent dosing. | Slower elimination, leading to sustained drug action. [14-16] |
Materials and Methods
Glipizide was provided as a sample by USV Private Limited, located in the 2nd Stage of Peenya Industrial Estate, Bangalore, Karnataka. Microcrystalline cellulose was sourced from Maple Biotech Pvt. Ltd., Pune, Maharashtra, while saccharin was obtained from Ranbaxy, New Delhi. Talc and magnesium stearate were supplied by S.D. Fine Chem Ltd., Mumbai, Maharashtra. All other solvents and reagents for the formulation were procured from the market. These ingredients were carefully selected from reputable suppliers to ensure the final product's quality and effectiveness, maintaining high standards within the pharmaceutical industry. The attention to sourcing and quality control emphasizes the commitment to producing reliable and effective medications for end-users.
Table 3: Physical and chemical parameters of Glipizide.
| S. No. | Parameter | Predicted value |
| Molecular formula | C21H27N5O4S | |
| Molecular structure | ||
| IUPAC name | N-(4-[N-(cyclohexyl carbamoyl) sulfonyl] phenethyl)-5-methylpyrazine-2-carboxamide | |
| Molecular weight | 445.536 g/mol | |
| BCS class | Class II | |
| pH and pKa | 5.9 and 6.0 | |
| Log P | 1.91 or 1.83 | |
| Crystallinity | Crystalline solid | |
| Melting point | 208-209 0C (406 to 408 °F) | |
| Solubility | Soluble in organic solvents like methanol, acetone |
Pre-Compression Parameters
Angle of Repose: The maximum angle at which a build-up of loose powder remains stable without collapsing is known as the angle of repose. It acts as a gauge of the cohesiveness and flow properties of the powder. Better flow is indicated by lower angles, and worse flow is indicated by greater angles. A mound of powder is progressively built up on a level surface until it starts to slump in order to be measured.

Bulk Density: The mass of a powder divided by its whole volume, which includes the empty spaces between particles, is known as its bulk density. It is commonly given in quantities such as g/cm³ or kg/L and indicates how well the powder packs together. Using tools like a graduated cylinder, a known volume of the powder-including its vacant spaces-must be weighed in order to make a determination.

Tapped Density: The maximum bulk density attained when a powder is tapped or vibrated to remove interparticle gaps is known as "tapped density." The powder is tapped repeatedly in a container until no more volume loss takes place, indicating the powder's packing capabilities under compaction.

Hausner's Ratio: Hausner's ratio is the ratio of tapped density to bulk density, calculated using the formula:

It offers information about the packing efficiency and flow behavior of the powder. Good flowability is indicated by a ratio near 1, and poor flowability is indicated by values greater than 1.
Carr's Index: Carr's index, or compressibility index, quantifies the powder's compressibility and is calculated as follows:

It shows the extent of volume reduction brought about by compression or tapping. Superior flowability is indicated by lower Carr's index values, whereas reduced flow or enhanced cohesion are implied by higher values.
Partition Coefficient: Glipizide, a second-generation sulfonylurea used to treat type 2 diabetes, is a lipophilic compound with low solubility in water and a partition coefficient (Log P) between 2.4 and 2.6. This property aids its passage through lipid membranes, improving absorption; however, excessive lipophilicity may hinder its dissolution and bioavailability.

Glipizide identification studies: Glipizide 10mg was accurately weighed and dissolved in 10ml methanol and subsequent dilution was made to get required concentrations (1000µg/ml). The wave length of maximum absorbance (λmax) of this clear solution was determined from 200-400nm methanol used as blank.
Preparation of stock solution: Glipizide 10 mg was dissolved in methanol 10 ml (1000 µg/ml) Stock solution I. From this solution diluted with methanol up to 100ml (100 µg/ml) Stock solution II was prepared.
Preparation of sample solution: Aliquots of 0.5, 1, 1.5, 2, and 2.5 ml were taken from Stock Solution II and diluted to a total volume of 10 ml, leading to concentrations of 5, 10, 15, 20, and 25 µg/ml, respectively. The absorbance for each diluted solution was recorded at 268 nm using a UV-visible spectrophotometer (Model 1800), comparing each measurement to the corresponding blank solution. Standard curves were created by plotting the known concentrations along the X-axis against the associated absorbance values on the Y-axis. These curves served to define the relationship between concentration and absorbance, facilitating the precise determination of unknown concentrations in later analyses.
Melting point of Glipizide: The melting point of Glipizide was established using an electric melting point device. A capillary tube, sealed at one end, was filled with the drug sample and positioned in the appropriate slot of the apparatus. The device was turned on, and the tube was monitored closely through the built-in magnifying glass for any indication of melting. The temperature at which the sample-initiated melting was carefully recorded from the thermometer. This initial temperature was noted as the melting point of the sample. To guarantee accuracy and reliability, the procedure was repeated three times, and the average of the three measurements was taken as the definitive melting point value. This technique yielded dependable and precise results for characterizing Glipizide [17-21]. Formulation of Fast dissolving tablets and Sustained release tablets.
Table 4: List of formulation fast dissolving tablets.
| S. No. | Excipients | Role |
| 1. | Glipizide | Active pharmaceutical ingredients |
| 2. | Isabgol husk | Super-disintegrants |
| 3. | Cross-povidone | Super-disintegrants |
| 4. | Guar gum | Binder |
| 5. | Talc | Glidant |
| 6. | Magnesium Stearate | Lubricant |
| 7. | Sodium Saccharine | Sweating agent |
| 8. | Microcrystalline cellulose | Filler, Diluent |
Table 5: List of formulation Sustained release tablets.
| S. No. | Excipients | Role |
| 1. | Glipizide | Active pharmaceutical ingredients |
| 2. | Hydroxypropyl Methylcellulose (HPMC) | Polymer for sustained drug release |
| 3. | Ethyl cellulose | Hydrophobic polymer for controlling release |
| 4. | Polyvinylpyrrolidone (PVP K30) | Binder |
| 5. | Magnesium Stearate | Lubricant |
| 6. | Talc | Glidant |
| 7. | Microcrystalline Cellulose (MCC) | Filler, Diluent |
Direct Compression Method
Direct compression is a straightforward and effective method for producing tablets, in which the active pharmaceutical ingredient (API) and excipients are mixed and compressed directly into tablets, eliminating the need for granulation. This approach has numerous benefits, such as its simplicity, savings, and fewer processing steps. By not requiring either wet or dry granulation, the direct compression method cuts out extra phases, which greatly lowers both production time and expenses. It utilizes standard tablet compression machinery and commonly used excipients, making it an appealing choice for large-scale pharmaceutical manufacturing. The avoidance of intricate granulation processes enables a more efficient production workflow while ensuring the final product maintains high quality and uniformity. Furthermore, the shorter processing time can assist in fulfilling the increasing demand for expedited production. This technique is especially advantageous for APIs that exhibit good flow and compressibility, facilitating effective tablet formation without sacrificing the quality or effectiveness of the end product.
Direct compression can be done in several ways
- Direct compression technique using induced die feeders.
- Direct compression technique using dry binders.
- Direct compression technique using direct compression excipients.
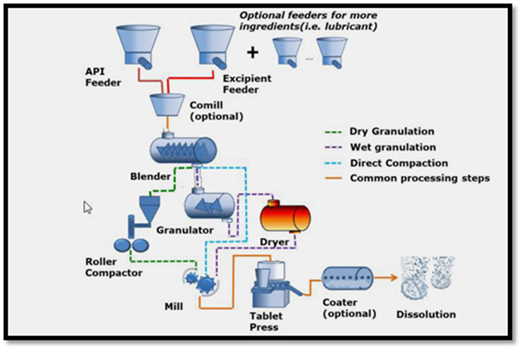
Figure 4: Steps involved in direct compression method of tablet manufacturing.
Direct compression method for fast-dissolving tablets
- Pre-milling of formulation ingredients (Active drug substance and excipients).
- Mixing of active drug substance with the powdered Excipients (including the lubricant). Compression of the mixed powders into Tablet [22].
Result and Discussion
Organoleptic properties: Organoleptic properties of the drug sample were found to be as given in table below.
Table 6: Organoleptic properties of Glipizide.
| S. No. | Organoleptic Characteristics | Result |
| 1. | Color | White to off-white crystalline compound |
| 2. | Nature | Crystalline powder |
| 3. | Odour | Odorless |
| 4. | Taste | Slightly bitter |
Melting point of Glipizide: The final reading was obtained by averaging the results of three independent experiments, resulting in a temperature of 209°C.
Partition Coefficient: The partition coefficient of Glipizide is found to be 1.91.
pH determination of Glipizide: The pH range glipizide is adjusted to 6.8 to 7.0 during preparation to ensure that it is stable and effective in treating diabetes.
Solubility study of Glipizide: A solubility analysis was undertaken to elucidate the dissolution characteristics of Glipizide in methanol. During the procedure, various aliquots of Glipizide were introduced into flasks containing a constant volume of methanol. The mixtures were subjected to continuous agitation for a duration of 24 hours to ensure complete solubilization of the compound. Upon completion of the stirring period, the solutions were meticulously filtered to eliminate any residual undissolved Glipizide particles, yielding a transparent solution. The concentration of the dissolved Glipizide was subsequently quantified using a UV-Vis spectrophotometer, with absorbance measurements compared to a pre-established calibration curve that correlated absorbance with known concentrations of Glipizide. By integrating the initial mass of Glipizide, the volume of methanol utilized, and the resulting absorbance data, the solubility of Glipizide in methanol was precisely calculated in milligrams per milliliter (mg/mL). This methodology affords an accurate assessment of Glipizide’s solubility in a designated solvent. Moreover, the approach is adaptable to examine the influence of additional variables, including temperature and solvent selection, on Glipizide’s solubility, thereby offering valuable insights for the refinement of pharmaceutical formulations. Such findings are critical for optimizing drug delivery systems, enhancing bioavailability, and ultimately improving the therapeutic efficacy of the drug.
Table 7: Solubility of glipizide in solvents.
| S. No. | Name of Solvent | Solubility |
| 1. | Methanol | Freely soluble |
| 2. | Ethanol | Practically soluble |
| 3. | Acetone | Slightly soluble |
| 4. | Methylene chloride | Slightly soluble |
| 5. | Water | Practically insoluble |
Table 8: Flow properties of powder blend.
| S. No | Angle of Repose (θ) | Bulk Density (g/cm2) | Tapped Density (g/cm2) | Hausner’s Ratio | Carr’s Ratio (%) |
| 1. | 33.26 | 0.52 | 0.64 | 1.23 | 18.40 |
| 2. | 32.55 | 0.47 | 0.53 | 1.12 | 11.35 |
| 3. | 31.74 | 0.52 | 0.60 | 1.15 | 13.38 |
| 4. | 34.45 | 0.48 | 0.57 | 1.16 | 14.03 |
| 5. | 33.23 | 0.55 | 0.64 | 1.12 | 10.93 |
| 6. | 32.66 | 0.52 | 0.62 | 1.16 | 14.51 |
| 7. | 30.36 | 0.48 | 0.55 | 1.14 | 12.77 |
| 8. | 32.54 | 0.51 | 0.59 | 1.15 | 14.85 |
| 9. | 33.18 | 0.49 | 0.62 | 1.15 | 16.92 |
| 10. | 34.47 | 0.48 | 0.57 | 1.15 | 14.02 |
| 11. | 33.23 | 0.53 | 0.65 | 1.23 | 18.40 |
| 12. | 31.74 | 0.52 | 0.59 | 1.16 | 13.32 |
| 13. | 33.22 | 0.56 | 0.64 | 1.11 | 10.92 |
| 14. | 33.25 | 0.52 | 0.65 | 1.22 | 18.48 |
| 15. | 32.52 | 0.51 | 0.58 | 1.19 | 14.84 |
Post-compression Studies
Organoleptic Characters
Table 9: Organoleptic properties of Glipizide Tablets.
| S. No. | Organoleptic Characteristics | Result |
| 1. | Color | White to off-white |
| 2. | Shape | Circular |
| 3. | Odour | Odorless |
| 4. | Taste | Palatably sweet |
Identification of Glipizide by UV spectrophotometry
Derivation of drug spectrum: The methanol stock solution of Glipizide, with a concentration of 10µg/ml, exhibited a peak absorption wavelength (λmax) at 268 nm. The absorbance observed at this wavelength was measured to be 0.1404.
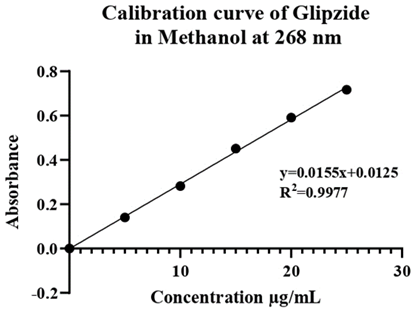
Preparation of calibration curve of Glipizide in water: The calibration curve of glipizide dissolved in methanol was plotted using a UV spectrophotometer and measured at a wavelength of 268 nm. This curve was created from 5 dilutions of stock solution, each at 5 µg/ml. The resulting curve is shown in Table 13.
Table 10: Calibration curve data of Glipizide in methanol.
| S. No. | Concentration (μg/ml) | Absorbance at 268 |
| 1. | 5 | 0.1404 |
| 2. | 10 | 0.2824 |
| 3. | 15 | 0.4513 |
| 4. | 20 | 0.5914 |
| 5. | 25 | 0.7170 |
Figure 5: Calibration curve of Glipizide.
Table 11: Post compressional data of optimized batch (FDT 1).
| S. No. | Post-compression evaluation parameter | Results |
| 1. | Thickness | 2.98±0.2mm |
| 2. | Hardness | 2.357 kg/cm2 |
| 3. | Disintegration time | 13.8 seconds |
| 4. | Friability | 0.49 % |
| 5. | % Drug release | 97.981 % |
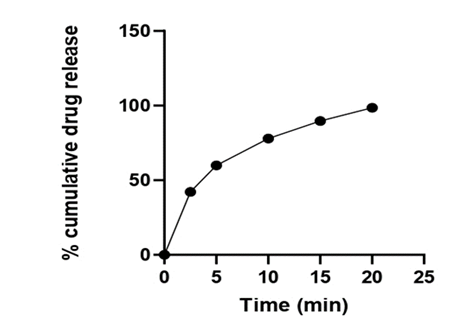
Table 12: In vitro drug release for optimized batch (FDT 1).
| Formulation batch | 2.5 min | 5 min | 10 min | 15 min | 20 min |
| FDT 1 | 42.17 | 59.89 | 77.89 | 89.69 | 97.98 |
Figure 6: Percent drug release of batch FDT 1 (Optimized Batch).
Table 13: Post compressional data of optimized batch (SDT 1).
| S. No. | Post-compression evaluation parameter | Results |
| 1. | Thickness | 2.99±0.2mm |
| 2. | Hardness | 2.357 kg/cm2 |
| 3. | Disintegration time | 45.0 seconds |
| 4. | Friability | 0.55 % |
| 5. | % Drug release | 98.37 % |
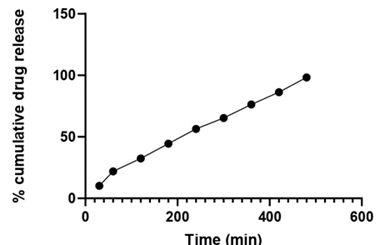
Table 14: In vitro drug release for optimized batch (SDT 1).
| Formulation batch | 30 min | 60 min | 120 min | 180 min | 240 min | 300 min | 360 min | 420 min | 480 min |
| SDT 1 | 10.23 | 21.93 | 32.39 | 44.39 | 56.46 | 65.32 | 76.36 | 86.37 | 98.37 |
Figure 7: Percent drug release of batch SDT 1 (Optimized Batch).
Conclusion
This study highlights the essential importance of advanced drug delivery systems in effectively managing Type 2 diabetes mellitus, specifically regarding glipizide. By comparing fast-dissolving tablets (FDTs) and sustained-release (SR) formulations, the research reveals how aspects like excipient composition, dissolution kinetics, and bioavailability significantly impact the results. FDTs allow for quick absorption, leading to an immediate therapeutic effect, while SR tablets provide a controlled and extended release, promoting better glycemic stability and enhancing patient adherence. A thorough understanding of these different release mechanisms, along with the optimization of pharmaceutical approaches, is crucial for improving treatment effectiveness, reducing the risk of complications, and advancing Type 2 diabetes management. Addressing this widespread global health issue requires ongoing improvements in drug delivery systems to foster better patient outcomes and promote long-term health advantages.
Declarations
Acknowledgement
I want to genuinely thank IPS Academy College of Pharmacy for offering the crucial resources, facilities, and assistance necessary for executing this study successfully. I am truly grateful for the continuous encouragement and guidance received during this journey. Furthermore, I extend my heartfelt appreciation to all my colleagues and peers for their meaningful discussions, helpful suggestions, and steadfast moral support, which were vital in bringing this work to fruition. Their collaboration and encouragement were key in navigating challenges and achieving the success of this project.
Competing Interest
The authors have declared that no competing interest exists.
References
- Husain A, Joshi S, Choudhary AN, Ajmal M, Khan M, et al. (2024). Computational Design and Toxicity Prediction of Oxazole Derivatives Targeting Pparγ as Potential Therapeutics for Diabetes Mellitus in Compare to Rosiglitazone and Pioglitazone. Journal of the Chilean Chemical Society. 69(1):6056-6064.
Publisher | Google Scholor - Parasuraman S, Thinagaran JN. (2023). Evaluation of the Antidiabetic Activity of Hesperidin on Streptozotocin-Induced Diabetes Mellitus in Swiss Albino Mice. Free Radicals and Antioxidants. 13(1):46-49.
Publisher | Google Scholor - Tiwary N, Sharma N, Singh S, Behl T, Zahoor I. (2024). Understanding The Pharmacological and Nanotechnological Facets of Dipeptidyl Peptidase-4 Inhibitors in Type II Diabetes Mellitus: A Paradigm in Therapeutics. Bionanoscience. 14(1):211-229.
Publisher | Google Scholor - Ozkan CK, Esim O, Savaser A, Ozkan Y. (2021). An Overview of Excipients Classification and Their Use in Pharmaceuticals. Current Pharmaceutical Analysis. 17(3):360-374.
Publisher | Google Scholor - El-Dakroury WA, Zewail MB, Amin MM. (2023). Design, Optimization, and In-Vivo Performance of Glipizide-Loaded O-Carboxymethyl Chitosan Nanoparticles in Insulin Resistant/Type 2 Diabetic Rat Model. Journal of Drug Delivery Science and Technology. 79:104040.
Publisher | Google Scholor - Ubhe TS, Gedam P. (2020). A Brief Overview on Tablets and It’s Types. Journal of Advancement in Pharmacology. 1(1):21-31.
Publisher | Google Scholor - Ade N, Koirala Y, Mannan MS. (2019). Towards an Inherently Safer Bioprocessing Industry: A Review. Journal of Loss Prevention in The Process Industries. 60:125-132.
Publisher | Google Scholor - Nandhini J, Rajalakshmi AN. (2018). Dispersible Tablets: A Review. Journal of Pharmaceutical Advanced Research. 1(3):148-155.
Publisher | Google Scholor - Islam N, Irfan M, Zahoor AF, Syed HK, Khan IU, et al. (2020). Evaluation of Solubility and Dissolution of Lamotrigine Using Lipid Based Microparticulate Carriers: An In-Vitro Analysis. Pakistan Journal of Pharmaceutical Sciences. 3:33.
Publisher | Google Scholor - Bermejo M, Sanchez-Dengra B, Gonzalez-Alvarez M, Gonzalez-Alvarez I. (2020). Oral Controlled Release Dosage Forms: Dissolution Versus Diffusion. Expert Opinion on Drug Delivery. 17(6):791-803.
Publisher | Google Scholor - Jan N, Madni A, Rahim MA, Khan NU, Jamshaid T, et al. (2021). In Vitro Anti-Leukemic Assessment and Sustained Release Behaviour of Cytarabine Loaded Biodegradable Polymer Based Nanoparticles. Life Sciences. 267:118971.
Publisher | Google Scholor - Allaboun H, Alkhamis KA, Al-Nimry SS. (2023). Preparation of Sustained Release Formulation of Verapamil Hydrochloride Using Ion Exchange Resins. AAPS Pharmscitech. 24(5):114.
Publisher | Google Scholor - Balusamy B, Celebioglu A, Senthamizhan A, Uyar T. (2020). Progress in the Design and Development of “Fast-Dissolving” Electrospun Nanofibers Based Drug Delivery Systems-A Systematic Review. Journal of Controlled Release. 326:482-509.
Publisher | Google Scholor - Al-Kubati SS, Ahmed MA, Emad NA. (2022). Palatable Levocetirizine Dihydrochloride Solid Dispersed Fast-Dissolving Films: Formulation and In Vitro and In Vivo Characterization. Scientific World Journal. 1552602.
Publisher | Google Scholor - Giusti R, Bosco M, Lucchesi M, Ripamonti CI. (2021). Alternative Routes for Systemic Opioid Delivery. In textbook Of Palliative Medicine and Supportive Care, CRC Press. 255-270.
Publisher | Google Scholor - El-Deen EE, Yassin HA. (2019). In-Vitro and In-Vivo Relationship of Gabapentin from Floating and Immediate Release Tablets. Journal of Advances in Medicine and Medical Research. 30(6):1-2.
Publisher | Google Scholor - Butola M, Bhatt V, Nainwal N, Jakhmola V, Dobhal K, et al. Preparation and Evaluation of Polymer Fused Metformin Hydrochloride Sustained Release Tablet. Indian Journal of Pharmaceutical Education and Research. 57(3):711-717.
Publisher | Google Scholor - Kakad SB, Rachh PR. (2022). Effect of Hydrophilic Polymer and Binder on Drug Release of Metformin Hcl Sustained Release Tablet. International Journal of Health Sciences. 3343-6635.
Publisher | Google Scholor - Tomar A, Wadhwani S, Bhadauria RS. (2019). A Preformulation Study of Pure Metformin Hcl. International Journal of Pharmaceutical Erudition. 8(4):1-9.
Publisher | Google Scholor - Tyagia L. (2024). A Comprehensive Review on Superdisintegrants: Accelerating Dissolution for Enhanced Drug Performance: International Journal of Medical Science. 34-49.
Publisher | Google Scholor - Othman AM, Alburyhi MM, Al-Hadad GH. (2024). Formulation And Evaluation of Captopril Mouth Dissolving Tablets. European Journal of Pharmaceutical and Medical Research. 11(1):18-28.
Publisher | Google Scholor - Stavras FM, Partheniadis I, Nikolakakis I. (2023). Formulation of Taste-Masked Orodispersible Famotidine Tablets by Sequential Spray Drying and Direct Compression-Bitterness Evaluation. Journal of Drug Delivery Science and Technology. 81:56-67.
Publisher | Google Scholor