Research Article
Adsorption of Copper from the Metal Industry Wastewaters using Fe2O3 Nanoparticles
- Delia Teresa Sponza *
- Rukiye Oztekin
Dokuz Eylül University, Engineering Faculty, Environmental Engineering Department Buca, İzmir- Turkey.
*Corresponding Author: Delia Teresa Sponza, Dokuz Eylül University, Engineering Faculty, Environmental Engineering Department Buca, İzmir, Turkey.
Citation: Delia T Sponza, R Oztekin. (2022). Adsorption of Copper from the Metal Industry Wastewaters using Fe2O3 Nanoparticles. Journal of BioMed Research and Reports, BRS Publishers. 1(1); DOI: 10.59657/2837-4681.brs.22.004
Copyright: © 2022 Delia Teresa Sponza, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 05, 2022 | Accepted: December 13, 2022 | Published: December 20, 2022
Abstract
Background:Ferric oxide (hematite, Fe2O3) nanoparticles were developed under laboratory conditions by co-precipitation method for the adsorption of copper from the metal industry wastewaters. The characteristics of the synthesized nanoparticles were assessed using fourier transform infrared spectroscopy (FTIR), X-ray difraction (XRD), field emission scanning electron microscope (FESEM) and energy-dispersive X-ray (EDX) analyses.
Results: Batch adsorption experiments were performed by adjusting the pH (3.0, 7.0 and 9.0), the contact time (10 min, 20 min and 30 min), stirring speed (5000 1/sn, 10000 1/sn and 20000 1/sn), and Fe2O3 concentration (2 mg/l, 5 mg/l and 10 mg/l). The magnetic nanoparticle showed an excellent performance in removing copper.
Conclusıon: For maximum copper removal (98%), 10 min of the optimum pH contact time and 5 mg/l Fe2O3 concentration are among increasing concentration of copper (5 mg/l, 25 mg/l and 30 mg/l), at a stirring speed of 150 rpm. The recovery yield of Fe2O3nanoparticle was 89% after 2 years.
Keywords: co-precipitation; energy-dispersive X-ray (EDX); field emission scanning electron microscope (FESEM); fourier transform infrared spectroscopy (FTIR); heavy metal pollution; magnetic nanoparticles.
Introduction
The wastewaters should be free of toxic heavy metals, because of their high toxicity and non-biodegradability. Ingestion of heavy metals can result in bioaccumulation, which not only causes health problems in animals and humans, but also contaminates the environment [1,2]. Heavy metals like arsenic, copper, major pollutants of fresh water reservoirs because of their toxic, non-biodegradable, and persistent nature. The industrial growth is the major source of heavy metals introducing such pollutants into different segments of the environment including air, water, soil, and biosphere. Heavy metals are easily absorbed by fishes and vegetables due to their high solubility in the aquatic environments. Hence, they may accumulate in the human body by means of the food chain. Various methods have been developed and used for water and wastewater treatment to decrease heavy metal concentrations. These technologies include membrane filtration, ion-exchange, adsorption, chemical precipitation, nanotechnology treatments, electrochemical and advanced oxidation processes. Heavy metal ions are elements from the fourth period of the periodic table, mostly chromium (Cr), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), and sometimes lead (Pb), mercury (Hg) [3,4]. Heavy metal ions can naturally be found in the environment, but these days their concentration is getting high because of increased industrial wastes [5]. The toxic ions enter the food chain and then the human body [6,7]. Once they accumulate in an organ to more than standard limits, they may cause serious health-related diseases [8]. For example, too much Zn may cause health problems like skin irritations, vomiting, and stomach cramps [9,10] high concentration of Ni bring cancer of lungs and kidney [9,11]. Since wastewater is the main source of diseases and hinders the sustainable growth for the human population, it needs to be treated to degrade the pollutants to less toxic forms [12,13,14]. Heavy metals remain in the human body persistently for a long time since they are not biodegradable. There are three kinds of nanoparticles: adsorptive, reactive, and hybrid magnetic. Nano magnetic oxides (NMOs) are adsorptive nanomaterials. They are widely used in wastewater treatment systems due to their high surface area, stability, and the mesoporous structure [15,16]. The adsorption mechanism is based on the sequestration of heavy metal ions which leads to deep removal of heavy metals to reach the maximum contaminant limits (MCL) [16]. Adsorption efficiency obviously relies on physicochemical conditions of the system and the chemical nature of the used absorbent [17].
Nano zero-valent iron (nZVI) is another noticeable nanoparticle used for wastewater treatment and heavy metal removal. It removes heavy metal ions by using reactive technologies, which make degradation and transformation of contaminants into harmless products possible [16,18]. In situ use of nZVI mostly starts by injecting it to the wastewater solution. It increases the ambient pH and decreases the redox potential of the solution. In addition to the changes applied to the wastewater after injecting the nZVI, the nZVI particles themselves undergo a chemical transformation by coating with natural organic matters in the waste solution [18].
The last kind of nanoparticles are hybrid magnetic nanoparticles. They are called hybrid because they contain two or more nanometer-scaled components and magnetic because at least one component is magnetic [12]. They are widely used in a variety of applications not only because of their convenient magnetic properties, low-toxicity, and low price, but also because they possess a high surface-to-volume ratio which leads to a high adsorption capacity and high removal rates of contaminants [17,19]. At diameters less than 20 nm, these particles have the maximum surface area and are in the super-magnetic state at room temperature [20]. Among the magnetic nano-sized materials, iron oxides like magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the main representatives of magnetic nanoparticles. [16,19,21]. Surface modification of magnetic nanoparticles to obtain more stabilized particles is essential [17,18]. There are various kinds of materials that can be used to coate magnetic nanoparticles, like polymer coating materials of lipids, poly(vinylpyrolidone) (PVP), poly(vinylalcohol) (PVA), etc [22]. The magnetic nanoparticles can be recovered and regenerated through a desorption process in a magnetic field.
Copper heavy metals are the third most widely used industrial metal, and this heavy metal is considered an eco-toxicological hazard because of its increasing accumulation in organisms.23,24,25 A significant amount of copper metal ions is found in industrial wastewater [26,27] resulting from cardboard processing, metal cleaning smelting, paints, pigments, mining, fertilizers, electroplating baths, petroleum refining rinses for brass, wood pulp, printed circuit board production, and etching [28]. Generally, wastewater from these industries contains tolerable amounts of copper ions, which diffuse into habitats through soil and water flow, although these eventually accumulate in the food chain, posing a serious threat to human health and causing serious environmental problems [29,30,31]. Studies have shown that drinking water containing 30 g of copper ions is lethal, and the accumulation of 1.3 mg/l of copper ions can cause diarrhea, abdominal cramps, and nausea, especially in newborns [32,33]. Copper ions are extremely toxic even at low concentrations, and the maximum allowable discharge of polluted copper ions in surface and well water in the industry is 1 mg/l, while the maximum allowable copper ion concentration values for drinking water are 0.2 and 1.2 mg/l, according to the WHO and EPA standard reports, respectively. Therefore, copper wastewater must be treated before being discharged into the environment, to protect human health and the environment [34,35]. Several methods have been used to remove copper ions from the effluent of various industries, including ion exchange, reverse osmosis, precipitation, electrolysis, polymer flocculants, biochemical methods, macromolecule organic chelating agents, adsorption, and membrane filtration [36,37]. However, these heavy metal removal methods have some major shortcomings, such as high operating costs, insufficient removal efficiencies, and production of large amounts of toxic residue (sludge) [38,39]. In contrast, the adsorption method is more advantageous compared to other techniques, because of its simple design, zero sludge environment, low-cost, and high removal efficiency, even at low heavy metal concentrations [40]. Many adsorbents can be used to remove copper ions such as activated carbon, owing to their excellent adsorption properties, but they have relatively high operating costs [38,41]. As a result, there is an urgent demand for improved, low-cost, and high removal efficiency adsorbents for metal ion removal [42].
Many researchers have used nanoparticles to remove heavy metals from wastewater, especially iron oxide (hematite, Fe2O3) nanoparticles, which are commonly used in laboratories, and are the most stable natural compound in the Earth’s crust. Furthermore, iron oxides are promising, low-cost, and environmentally friendly materials, and can be used for mineralogy, biological proposals, materials engineering, wastewater treatment, catalysis, storage, and energy conservation [31,43]. Most iron oxides are only available in fine powder or ore form, because of their low hydraulic conductivity and increased project costs. Thus, it is difficult to use this material alone as an adsorbent filter. However one technique, which involves coating iron oxide on the surface of sand, has been developed to overcome the above-mentioned problems [44]. Research on the treatment of wastewater pollutants with iron oxide coated sand has been well documented.
In this study, Fe2O3 nanoparticles were developed under laboratory conditions by co-precipitation method for the adsorption of copper from the metal industry wastewaters. Batch adsorption experiments were performed by adjusting the pH (3.0, 7.0 and 9.0), the contact time (10 min, 20 min and 30 min), stirring speed (5000 1/sn, 10000 1/sn and 20000 1/sn), and Fe2O3 concentration (2 mg/l, 5 mg/l and 10 mg/l). The characteristics of the synthesized nanoparticles were assessed using Fourier transform infrared spectroscopy (FTIR), X-ray difraction (XRD), Field emission scanning electron microscope (FESEM) and Energy-dispersive X-ray (EDX) analyses.
Materials and Methods
Preparation of Fe2O3 nanoparticles
To prepare Fe2O3 nanoparticles, 0.1 M, 0.2 M and 0.3 M of FeCl3 solution was prepared in 200 ml of distilled water, separately and adjusted to three different pH levels, 2.00, 6.50, and 9.00, by adding 0.1 M of HCl or NaOH solution dropwise. These solutions were stirred for 30 min and rotated for 2 h in a rotary evaporator. The rotation speed was approximately 30 rpm and the temperature was approximately 80oC. After 10%–20% of the water remained.
Analytical methods
pH, COD, TSS, Oil and grease, Fe+3, NH4-N, NO2-N, Cl+2, Zn+2, S-2, Pb+2, Total-CN, Cr+5, Total Cr, Cd, Al+3, Ni+2, DOC, Cu+2, electrical conductivity were monitored following Standard Methods.45
Characterization of Fe2O3 nanoparticles
The mineral composition and surface morphology of the Fe2O3 nanoparticles were characterized by X-ray diffraction, SEM and EDX analysis. FTIR was also used to confirm the Fe2 deposition on the Fe2O3 nanoparticles and copper during adsorption. Table 1 showed that metal industry wastewater characterisation values.
| Parameters | Values |
| pH | 8.0 |
| COD | 200 mg/l |
| Total Suspended solids (TSS) | 110 mg/l |
| Oil and Grease | 25 mg/l |
| Fe+3 | 5 mg/l |
| NH4-N | 100 mg/l |
| NO2-N | 10 mg/l |
| Cl+2 | 0.5 mg/l |
| Zn+2 | 6 mg/l |
| S-2 | 2 mg/l |
| Pb+2 | 5 mg/l |
| Total CN | 2 mg/l |
| Cr+5 | 1.0 mg/l |
| Total Cr | 2 mg/l |
| Cd | 0.6 mg/l |
| Al+3 | 3 mg/l |
| Ni+2 | 5.8 mg/l |
| Dissolved organic carbon (DOC) | 775 mg/l |
| Cu+2 | 30 mg/l |
| Electrical conductivity | 8.5 ms/cm |
Table 1. Metal industry wastewater characterisation values
Batch studies for adsorption
Batch adsorption experiments were conducted for the adsorption of Cu+2 in a 250 ml conical flask, with an initial Cu+2 concentration of 30 mg/l, at an pH of 6, and at agitation speed of 200 rpm at 25oC.
All of the experiments were conducted in a 100 ml Cu+2 fixed volume, in a 250-ml conical flask, with different Fe2O3 nanoparticle concentrations (0.1, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 g), initial pH values (2.0, 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0), and initial Cu+2 concentrations (10, 30, 50, 100, 150, 200 and 250 mg/l) and were evaluated for up to 180 min.
The amount of Cu+2 adsorbed into Fe2O3 nanoparticles was calculated according to Eq. (1):
 (1)
(1)
Where, C0 (mg/l) is the initial concentration of Cu+2, Ce (mg/l) is the concentration of Cu+2 at equilibrium, m (g) is the amount of Fe2O3 coated sand, and V is the volume of the solution (100 ml). The removal efficiency, in percentage (%R) of Cu+2, was calculated by Eq. (2):
 100 (2)
100 (2)
Results and Discussions
Effect of pH and Fe+3 content on the removal of copper
The solubility of the Fe+3 compound regulated by the pH solution, which showed that Fe+3 readily precipitated at low pH values while at alkaline conditions high hydroxyl ion concentrations cause to precipitate of Fe+3 as Fe(OH)3. This precipitate settled down to the bottom and did not come into contact with the copper. The precipitation of iron oxide in a solution starts at pH=3.5 as reported by Morales [46]. Therefore, at this pH value, less iron was adsorbed to copper and decreased the removal efficiency of the copper. As a result, less surface area having Fe2O3 nanoparticle reduce the adsorption yield (Table 2). The Fe+3 content in the Fe2O3 nanoparticle was 80 mg/g with a copper removal efficiency of 92% at pH=3.00 and at a constant Fe2O3 nanoparticle of 2.0 mg/l. When the Fe+3 content in the Fe2O3 nanoparticle was 43 mg/g the copper yield was 85% at pH=6.7 while the copper yield was 78% at pH=8.9 at a Fe+3 content of 38 mg/g.
pH
| Fe+3 content in the Fe2O3 nanoparticle (mg/g) | Cu+2 removal efficiency (%) | Fe2O3 nanoparticle concentration |
| 3.00 | 80 | 92.00 | 2 mg/l |
| 6.70 | 43 | 85.00 | 2 mg/l |
| 8.90 | 38 | 78.00 | 2 mg/l |
Table 2: Effects of pH and Fe+3 content in the Fe2O3 nanoparticle on the copper removal yields
Characterization of Fe2O3 nanoparticle
X-ray Analysis
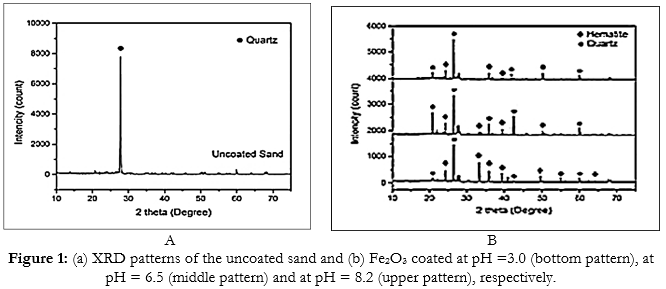
X-ray powder dispersion analysis of the Fe2O3 nanoparticle was performed to determine the crystalline textures. In Fig. (1a), the XRD analysis showed that the Fe2O3 nanoparticles exhibited very thin peaks indicating the fine nature and small crystallite size of the iron particles. The new intensity peaks appeared, increased, as the Fe+3 amount increased in the Fe2O3 nanoparticle deposited (Fig. (1b)). At pH=3.0 a number of high-intensity pure crystal peaks of Fe2O3 was detected while, SiO2, FeSiO2 and Fe(OH)3 were found with a large amount of NaOH. All detected peaks at hexagonal sructure of Fe2O3. Approximately seven peaks was detected at pH= 3.0, while five peaks at pH=6.5, and four peaks at pH=8.2 tabelled as at 24.21●, 33.1●, 35.7●, 39.52●, 49.57●, 54.3● and 64.4● were detected. These were also listed as (012), (104), (110), (024), (116), (214), and (118) deflections.
FTIR analysis
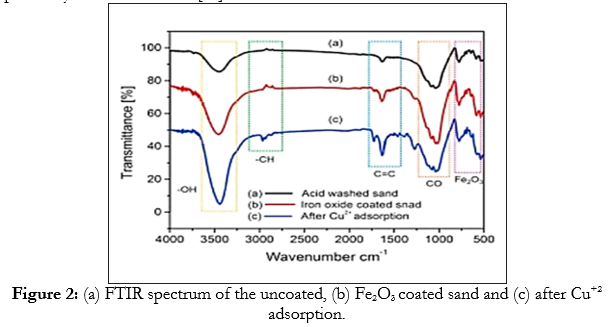
In Figure 2, the FTIR spectra of the synthesized of Fe2O3 nanoparticle. During the adsorption of copper in the range of 500–4000 1/cm were used to identify the chemical bonds and groups in the functional compounds. The bands at 3500–500 1/cm were assigned to the stretching vibrations of Si-Si-O or Al-Al-OH, since bending of the hydroxyl group (Fig. (2a)). During adsorption, all the Fe2O3 nanoparticle peak positions slightly shifted from 1775 to 540 1/cm. The preence of some new peaks at 3712, 1705, 1452, 1260, and 875 1/cm appeared can be explained by the stretching vibrations of the hydroxyl groups on the surface of Fe2O3 nanoparticle.
The specific adsorption bands, from 826 to 553 1/cm, were also correlated to Fe-O stretching and vibration bending of the Fe2O3 nanoparticle (Fig. (2b)). After the copper adsorbed on the Fe2O3 the observation of some new peaks at 2945, 2904, 1492, 1438, and 1356 1/cm during slight band changes (Fig. (2c)). These bands corresponded to the aliphatic -CH and -OH, C=C, and CO stretching vibrations, and indicated that Cu+2 was present on the surface of the Fe2O3 nanoparticle as reported by Kuterasinski et al [47].
FESEM analysis
The surface morphology, surface texture and basic physical properties of the Fe2O3 nanoparticle and Cu+2 adsorbed were evaluated by FESEM analysis. The surface morphology of the Fe2O3 nanoparticle was smooth, and no other compounds were deposited on the surface. However, some irregular compounds did nor clearly showed the deposition of the iron oxide (Fig. 3) After the adsorption of copper, the surface contained newly formed cracks and cubes appeared. It is important to note that copper was successfully adsorbed to the Fe2O3 nanoparticle surface.
EDX analysis results
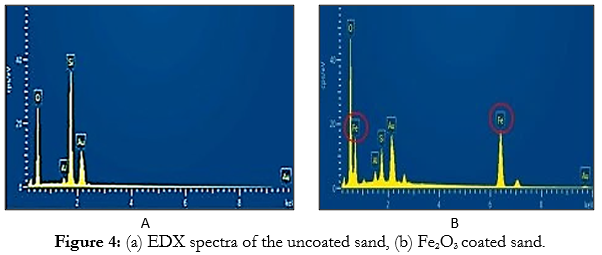
EDX analysis results showed the elemental distribution of the Fe2O3 nanoparticle coated with copper (Fig. (4a)) while indicates, the adsorption of copper by Fe2O3 nanoparticle Fig. (4a), a large amount Si-O2 was detected; while no individual Fe2O3 was found on the surface of the copper sample. The copper ion peak was observed in the enlarged area of the Fe2O3 nanoparticle surface. This result thus confirmed the adsorption of copper ions onto the Fe2O3 nanoparticle (Fig. (4b)).
Effect of adsorption time on the copper adsorption on the Fe2O3 nanoparticles
Time is an important factor for wastewater purification during adsorption. Table 3 showed that the concentration of copper ions decreased rapidly over time. The removal efficiency during the first 20 min was very low, but increased to 95 after 30 min, indicating that time had a significant impact on the removal efficiency of 18.0 mg/l copper at constant Fe2O3 nanoparticle of2.0 mg/l. The adsorption and removal rates of copper increased quickly. This indicates that the adsorption rate was carried out quickly. After 40 min, the curve became straight and the removal rate slowed. This can be explained as follows: At the beginning of the adsorption enough pores available on the surface of the Fe2O3 nanoparticle to adsorb copper ions, resulting in initial rapid adsorption. Over time, most of the pores on the surface became saturated with copper ions. Therefore, the adsorption of the adsorbent decreased over time, resulting in a lower adsorption rate. Consequently, the equilibrium adsorption contact time was choosen as 30 min.
| Adsorption time (min) | Cu+2 removal efficiency (%) | Removed Cu +2 adsorption concentration (mg/l) |
| 5 | 0 | 0 |
| 10 | 8 | 1.44 |
| 15 | 9 | 1.62 |
| 20 | 11 | 1.98 |
| 25 | 32 | 5.76 |
| 30 | 95 | 17.10 |
| 40 | 95 | 17.10 |
| 50 | 95 | 17.10 |
| 60 | 95 | 17.10 |
| 70 | 95 | 17.10 |
| 80 | 95 | 17.10 |
| 90 | 95 | 17.10 |
| 100 | 95 | 17.10 |
Table 3: Effect of adsorption time on the 18.0 mg/l initial copper adsorption concentration on the 2.0 mg/l Fe2O3 nanoparticle concentration
Effect of increasing Fe2O3 nanoparticles concentration on the adsorption of copper
As shown in Table 4 the effect Fe2O3 nanoparticle concentration in the metal industry wastewater on copper removal efficiency and Cu+2 adsorption percentage was found to be in the range of 0.1–7.0 mg/l at 30 min adsorption time. As the Fe2O3 nanoparticle concentration increased from 0.1 to 2.0 mg/l, the adsorption yield for Cu+2 increased from 56% to 98% This was attributed to the greater the mass of the adsorbent, as it provided a larger surface area and therefore provided more available binding sites to bind to Cu+2, which increased the removal percentage of Cu+2 through increased of Fe2O3nanoparticle. As reported by Pu et al [28], when the Fe2O3 nanoparticle concentration was very high, the adsorption percentage of Cu+2 was very fast, which reduced the Cu+2 concentration in the solution. However, the Cu+2 concentration dropped to a low value when the sorption sites on the Fe2O3 nanoparticle coated fully remained unsaturated. Therefore, the amount of Cu+2 per unit mass declined with increased Cu+2 concentration. For 30 mg/l of Cu+2, the maximum adsorption dose of the was 2.50 mg/l Fe2O3 nanoparticle and approximately 99.3% of Cu+2 was removed. However, owing to the low concentration of copper ions in the remaining solution, the removal efficiency of Cu+2 with more adsorbent addition was very low, and the removal efficiency of 4.0 mg /l Fe2O3 nanoparticle reached 94%.
| Fe2O3 nanoparticles concentration (mg/l) | Cu+2 adsorption percentage (%) | Cu+2 adsorption concentration (mg/l) | Remaining Cu+2 adsorption concentration (mg/l) | Cu+2 Adsorption yield (mg/g) |
| 0.10 | 56.00 | 16.80 | 13.20 | 0.40 |
| 0.50 | 78.00 | 23.40 | 6.60 | 0.50 |
| 1.00 | 82.60 | 24.60 | 5.40 | 0.60 |
| 1.50 | 90.00 | 27.00 | 3.00 | 0.95 |
| 2.00 | 98.00 | 29.40 | 0.60 | 1.05 |
| 2.50 | 99.30 | 29.79 | 0.21 | 1.30 |
| 3.00 | 99.00 | 29.70 | 0.30 | 1.28 |
| 3.50 | 99.00 | 29.70 | 0.30 | 1.28 |
| 4.00 | 94.00 | 28.20 | 1.80 | 1.08 |
| 4.50 | 94.00 | 28.20 | 1.80 | 1.08 |
| 5.00 | 94.00 | 28.20 | 1.80 | 1.08 |
| 7.00 | 94.00 | 28.20 | 1.80 | 1.08 |
Table 4: Effect of Fe2O3 nanoparticles concentration on the adsorption of copper
Effect of pH on the adsorption of copper on Fe2O3 nanoparticles
The effect of pH was a main variable addecting the metal surface of nanocomposite. Typically, metal adsorption can be increased by increasing the pH of the metal contamination solution. Thus, the pH effect will also vary according to the type of adsorption material, the behavior of the solution, and the type of ion adsorption [48]. In this study, (Table 5) it was shown that variations in pH significantly affect the adsorption efficiency of copper. However, only a small amount of metal ions were removed under alkaline pH=9.0. As the pH value increased from 4.0 to 9.0, the removal percentage rapidly decreased from 99.80% to 37%. As the pH value of the solution was high, the surface hydroxyl groups will compete with the protons and metal ions during adsorption, resulting in a low metal adsorption rate, at 30 min adsorption time, at 18 mg/l Cu+2 concentration and at 2.5 mg/l Fe2O3 nanoparticle concentration, respectively. However, by increasing the pH value from 7.0 to 9.0, no significant changes occurred, which indicated that Cu+2 was free at pH=9.0. At very high pH values (6.0–8.0), the removal percentage was very low, possibly because binding site energy could not be achieved in a highly alkaline medium. In addition, copper precipitation may also occur when the pH is higher than 6.0; thus, all adsorption experiments were performed between pH 4.0–5.0 [49,50].
| pH | Copper Adsorption yield (%) |
| 4.00 | 99.80 |
| 7.00 | 76.00 |
| 9.00 | 37.00 |
Table 5: Effect of pH on the adsorption of copper on Fe2O3 nanoparticle (30 min, 18 mg/l copper concentration, 2.5 mg/l Fe2O3 nanoparticle concentration)
Effect of copper concentrations in metal ındustry wastwaeter on the adsorption efficiency
To evaluate the optimum concentration of Cu+2, the sampless taken from the metal industry at different days were optimized to have copper concentrations varying between 0.1 and 8.0 mg/l, depending on the metal industry wastewater to check the maximum removal efficiency of the 2.3 g of Fe2O3nanoparticle. In this study, as the Cu+2 concentration was increased from 0.1 mg/l up tp 0.5 mg/l and the removal percentage and Cu+2 and adsorption rates increased, as shown in Table 6. This phenomenon was explained by the large amount of Cu+2, which had limited active adsorption sites, causing an increase in Cu+2 concentration in the bulk solution. With more Cu+2, it was disadvantageous to have sites involved in adsorption and thus reduce the removal capacity of Cu+2. In addition, at a higher Cu+2 concentration, the ratio of the initial number of moles of metal ions (Cu+2) to the available adsorption sites was very high, resulting in a low Cu+2 adsorption rate [51]. 4 mg/l Cu+2 can be adsorbed with a maximum yied of 99.90%. The maximum Cu+2 adsorption yield were 99.99% for Cu+2 concentrations vaying between 0.10 and 3.0 mg/l.
| Copper concentration in metal industry wastewater (mg/l) | Cu+2 adsorption yield (%) | Maximum adsorption (mg/g) |
| 0.1 | 99.99 | 2.00 |
| 0.2 | 99.99 | 2.00 |
| 0.3 | 99.99 | 2.00 |
| 0.5 | 99.99 | 2.00 |
| 1.0 | 99.99 | 2.00 |
| 2.0 | 99.99 | 2.00 |
| 3.0 | 99.99 | 2.00 |
| 4.0 | 99.90 | 1.99 |
| 5.0 | 99.00 | 1.90 |
| 6.0 | 98.00 | 1.80 |
| 7.0 | 97.00 | 1.70 |
| 8.0 | 97.00 | 1.70 |
Table 6: Effect of copper concentrations in metal industry wastwaeter on the adsorption efficiency in the presence of 2.30 mg/l Fe2O3 nanoparticle
Adsorption isotherms
Adsorption isotherm studies were carried out to determine an equation which can be accurately present the maximum adsorption capacities of the Fe2O3 nanoparticle. The data obtained from the Langmuir and Freundlich kinetics were plotted to determine the relavant constants:
Langmuir isotherm results
The Langmuir model is based on the presumption that the surface monolayer will adsorb homogeneous substances with a limited number of adsorption sites, and there is no relationship between the adsorbed substances, according to the linearized equation of the Langmuir isotherm model, according to Eq. (3): [52].
 (3)
(3)
Where, qe is the maximum equilibrium adsorption amount per unit mass of Fe2O3 nanoparticle coated copper (mg/g), Ce is the equilibrium concentration of copper ions (mg/l), qm (mg/g) is the amount of copper ions adsorbed, and KL (l/mg) is a constant in the Langmuir model related to the energy of adsorption.
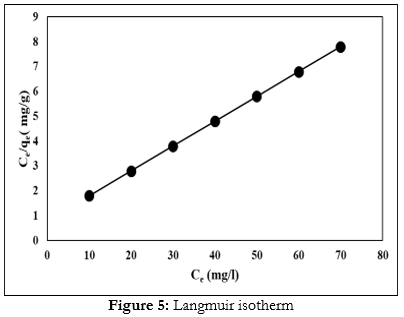
The values were determined from plotting (Ce/qe) versus (Ce) (Fig. 5) and the linear equation, respectively. In addition, we used the basic characteristics of the Langmuir isotherm model to test the suitability of the Langmuir equation, for copper ion adsorption on the surface of Fe2O3 nanoparticle. Langmuir isotherm results were determined at Table 7.
| Parameters | Values |
| Temperature | 21oC (ambient conditions) |
| qe | 2.89 mg/g |
| KL | 1.78 × 10−2 1/mg |
| R2 | 0.99 |
The Langmuir isotherm model was represented by a non-dimensional constant called the separation factor RL, and this relationship was expressed by Eq. (4):
 (4)
(4)
Where, C0 (mg/l) is the initial metal ion concentration and KL is the Langmuir constant. In addition, the RL separation factor indicates whether the adsorption is unfavorable or not, depending on RL > 1, or is linear if 0 < RL>
Freundlich isotherm results
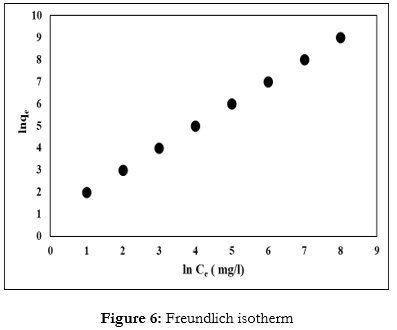
The Freundlich isotherm is an empirical equation that elucidates the adsorption process, where adsorbent surface changes are involved in the multi-layer diffusion of the adsorbate, and associations between the particles. This isotherm equation is commonly used to explain adsorption equilibrium, and the Freundlich isotherm equation model can according to Eq. (5): [53].
 (5)
(5)
Where, KF (mg/g) and 1/n are the Freundlich constants calculated from the slope and intercept of the plot of log qe vs log Ce (Fig. (6)).
As shown in Table 8, if the value n was n > 1, then it indicated favorable adsorption, but if the n value was n < 1>
The comparison of Table 7 and Table 8; and also (Fig. 5) and (Fig. 6) showed that the Langmuir model fit well and was more suitable than the Freundlich model. In addition, the R2 values in the Langmuir model were higher than in the Freundlich model for all temperatures, indicating that adsorption was related to monolayer adsorption.
The RL values v/s initial copper ions concentration at different temperatures in the Langmuir model were found to be greater than zero. This indicated that the adsorption of copper ions to Fe2O3 nanoparticle was more favorable. Boujelben et al [49]. and McKay et al [55] suggested that if the value of n is in the range of 1–10, then the adsorption is favorable, the results in this study clearly verified this.
| Parameters | Values |
| Temperature | 21oC (ambient conditions) |
| Kf | 0.197 |
| n | 1.98 |
| R2 | 0.976 |
Table 8: Freundlish isotherm results
Recovery of Fe2O3 nanoparticles
High-quality and highly efficient adsorbents require basic characteristics such as reusability and sufficient response to the desorption process, which are necessary for adsorbent regeneration. However, the regeneration and desorption of raw materials not only reduces the cost of the purification process for sewage treatment industries but also protects the environment, as it limits water pollution, decreases landfill need, is energy-saving, and protects natural habitats. Therefore, desorbing metals in the adsorbent from the used adsorbent are critical for the continued use of the adsorbent, recovering precious (valuable) compounds, and reducing operating wastewater treatment costs. Some studies have shown that metal ions adsorption on adsorbents cannot be completely reversed [56].
In this study, six different solutions (HCl, H2SO4, CH3OOH, NaOH, distilled water and tap water) were used for desorption, in an attempt to regenerate Cu+2 from the hydrophobic ionizable organic compounds (HIOCs) surface. The desorption results of the tap water, distilled water, acetic acid, and NaOH media were all weak. So, HCl and H2SO4 were more effective in recovering Cu+2 (60%–70%) from the surface of the hematite iron oxide coated sand. Thus, after desorption, the material could be reused five times. The removal efficiency decreased after each cycle, although this may be because some copper ions remained in the pores of HIOCs, or the tiny iron particles were repeatedly digested due to acid desorption. Thus, the results indicated that our prepared adsorbent served as a good recyclable adsorbent product for copper ion removal from wastewater, without any critical losses.
Conclusion
When 80 mg/g Fe+3 content in the Fe2O3 nanoparticles was with 92% Cu+2 yield, at pH=3.0 and at a constant 2.0 mg/l Fe2O3 nanoparticles. X-ray analysis; All detected peaks at hexagonal sructure of Fe2O3. Nearly seven peaks was detected at pH=3.0, while five peaks at pH=6.5, and four peaks at pH=8.2 tabelled as at 24.21●, 33.1●, 35.7●, 39.52●, 49.57●, 54.3● and 64.4● were detected These were also listed as (012), (104), (110), (024), (116), (214), and (118) deflections.
The removal efficiency of 18 mg/l Cu+2 was measured to 95
Acknowledgement
This research study was undertaken in the Environmental Microbiology Laboratury at Dokuz Eylül University Engineering Faculty Environmental Engineering Department, Izmir, Turkey. The authors would like to thank this body for providing financial support.
References
- Ahmad M, Wang J, Xu J, Zhang Q and Zhang B. (2020) Magnetic tubular carbon nanofibers as efficient Cu (II) ion adsorbent from wastewater. J Clean Prod 252:119825.
Publisher | Google Scholor - Demiral H and Güngor C. (2016) Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 124:103-113.
Publisher | Google Scholor - Kadirvelu K, Thamaraiselvi K and Namasivayam C. (2001) Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresource Technology 76(1):63-65.
Publisher | Google Scholor - Renu MA, Singh K, Upadhyaya S and Dohare R. (2017) Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater Today Proc 4(10):534-538.
Publisher | Google Scholor - Lasheen MR, El-Sherif IY, El-Wakee ST, Sabry DY and El-Shahat MF. (2017)Heavy metals removal from aqueous solution using magnetite Dowex 50WX4 resin nanocomposite. JMES 8(2):503-511.
Publisher | Google Scholor - Huang J, Cao Y, Liu Z, Deng Z and Tang F. (2012) Efficient removal of heavy metal ions from water system by titanate nanoflowers. Chem Eng J 180:75-80 (2012).
Publisher | Google Scholor - Adelere IA and Lateef A. (2016)A novel approach to the green synthesis of metallic nanoparticles: the use of agrowastes, enzymes and pigments. Nanotechnol Rev 5(6):567-587.
Publisher | Google Scholor - Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L and Muller RN. (2008)Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064-2110.
Publisher | Google Scholor - Tiwari JN, Tiwari RN and Kim KS. (2012) Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog Mater Sci 57:724-803.
Publisher | Google Scholor - Shin W-K, Cho J, Kannan AG, Lee Y-S and Kim D-W. (2016) Cross-linked composite gel polymer electrolyte using mesoporous methacrylatefunctionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci Rep 6:26332, 1-10.
Publisher | Google Scholor - Bhatnagar A and Sillanpaa M (2010) Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment: a review. Chem Eng J 157:277–296.
Publisher | Google Scholor - Chandrasekhar S, Pramada PN and Majeed J. (2006) Efect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J Mater Sci 41(23):7926-7933.
Publisher | Google Scholor - Dash S, Kamruddin M, Ajikumar P, Tyagi A and Raj B. (2000) Nanocrystalline and metastable phase formation in vacuum thermal decomposition of calcium carbonate. Thermochimica Acta 363(1):129-135.
Publisher | Google Scholor - Pokhrel M, Wahid K and Mao Y. (2016) Systematic Studies on RE2Hf2O7:5%Eu3+ (RE = Y, La, Pr, Gd, Er, and Lu) Nanoparticles: Effects of the A-Site RE3+ Cation and Calcination on Structure and Photoluminescence. J Phys Chem C 120:14828-14839.
Publisher | Google Scholor - Lykhach Y, Kozlov SM, Ska´ la T, Tovt A, Stetsovych V, Tsud N, Dvorˇa´ k F, Johanek V, Neitzel A, Myslivecek J, Fabris S, Matolın V, Neyman KM, Libuda J. (2016) Counting electrons on supported nanoparticles. Nat Mater 15(3):284-288.
Publisher | Google Scholor - Popoola LT, Aderibigbe TA. (2018) Yusuff AS. Synthesis of composite snail shell-rice husk adsorbent for brilliant green dye uptake from aqueous solution: Optimization and Characterization. Environmental Quality Management 28(2):79-88.
Publisher | Google Scholor - Kestens V, Roebben G, Herrmann J, Ja¨ mting A, Coleman V, Minelli C, Clifford C, De Temmerman P-J, Mast J, Junjie L, Babick F, Colfen H and Emons H. (2016) Challenges in the size analysis of a silica nanoparticle mixture as candidate certified reference material. J Nanopart Res 18:171.
Publisher | Google Scholor - Zainol MM, Asmadi M and Amin NAS. (2017) Preparation and characterization of impregnated magnetic particles on oil palm frond activated carbon for metal ions removal. Sains Malaysiana 46(5):773-782.
Publisher | Google Scholor - Ullah H, Khan I, Yamani ZH and Qurashi A. (2017) Sonochemicaldriven ultrafast facile synthesis of SnO2 nanoparticles: growth mechanism structural electrical and hydrogen gas sensing properties. Ultrason Sonochem 34:484-490.
Publisher | Google Scholor - Hammond C and Hammond C. (2009) The Basics of Crystallography and Diffraction, 3rd edition, Oxford, United States, Oxford University Press Inc., New York.
Publisher | Google Scholor - Iqbal N, Khan I, Yamani ZH and Qurashi A. (2016) Sonochemical assisted solvothermal synthesis of gallium oxynitride nanosheets and their solardriven photoelectrochemical watersplitting applications. Sci Rep 6:32319.
Publisher | Google Scholor - Sahu JN, Acharya J and Meikap BC. (2009) Response surface modeling and optimization of chromium(VI) removal from aqueous solution using tamarind wood activated carbon in batch process. J Hazard Mater 172(2-3):818-825.
Publisher | Google Scholor - Imamoglu M and Tekir O. (2008) Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 228(1-3):108-113.
Publisher | Google Scholor - Wuana RA and Okieimen FE. (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Notices 2011:402647, 1-20.
Publisher | Google Scholor - Yargiç AS, Yarbay Şahin RZ, Özbay N and Önal E. (2015) Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J Clean Prod 88:152-159.
Publisher | Google Scholor - Al-Saydeh SA, El-Naas MH and Zaidi SJ. (2017) Copper removal from industrial wastewater: a comprehensive review. J Ind Eng Chem 56:35-44.
Publisher | Google Scholor - Trakal L, Sigut R, Sillerova H, Faturíkova D and Komarek M. (2014) Copper removal from aqueous solution using biochar: effect of chemical activation. Arab J Chem 7(1):43-52.
Publisher | Google Scholor - Pu X, Yao L, Yang L, Jiang W and Jiang X. (2020) Utilization of industrial waste lithium-silicon-powder for the fabrication of novel nap zeolite for aqueous Cu (II) removal. J Clean Prod 265:121822.
Publisher | Google Scholor - El-Massaoudi M, Radi S, Lamsayah M, Tighadouini S, Seraphin KK, Kouassi LK and Garcia Y. (2021) Ultra-fast and highly efficient hybrid material removes Cu (II) from wastewater: kinetic study and mechanism. J Clean Prod 284:124757.
Publisher | Google Scholor - Eren E. (2008) Removal of copper ions by modified Unye clay, Turkey. J Hazard Mater 159:235-244.
Publisher | Google Scholor - Yang Z, Chai Y, Zeng L, Gao Z, Zhang J and Ji H. (2019) Efficient removal of copper ion from waste water using a stable chitosan gel material. Molecules 24:1-14.
Publisher | Google Scholor - Pehlivan E, Altun T and Parlayici S. (2009) Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J Hazard Mater 164:982-986.
Publisher | Google Scholor - Salmani MH, Ehrampoush MH, Sheikhalishahi S and Dehvari M. (2012) Removing copper from contaminated water using activated carbon sorbent by continuous flow. J Heal Res 1:11-18.
Publisher | Google Scholor - Abdul MA, Shittu SO, Randawa JA and Shehu MS. (2009) The cervical smear pattern in patients with chronic pelvic inflammatory disease. Niger. J Clin Pract 12:289-293.
Publisher | Google Scholor - Florio M. (1997) The economic rate of return of infrastructures and regional policy in the European Union. Ann Public Coop Econ 68:39–64.
Publisher | Google Scholor - Bilal M, Shah JA, Ashfaq T, Gardazi SMH, Tahir AA, Pervez A, Haroon H and Mahmood Q. (2013) Waste biomass adsorbents for copper removal from industrial wastewater-A review. J Hazard Mater 263(2):322–333.
Publisher | Google Scholor - Hemavathy RV, Kumar PS, Kanmani K and Jahnavi N. (2020) Adsorptive separation of Cu (II) ions from aqueous medium using thermally/chemically treated Cassia fistula based biochar. J Clean Prod 249:119390.
Publisher | Google Scholor - Miretzky P, Saralegui A and Cirelli AF. (2006) Simultaneous heavy metal removal mechanism by dead macrophytes. Chemosphere 62:247-254.
Publisher | Google Scholor - Shahin SA, Mossad M and Fouad, M. (2019) Evaluation of copper removal efficiency using water treatment sludge. Water Sci Eng 12:37-44.
Publisher | Google Scholor - Pan J, Gao Y, Gao B, Guo K, Xu X and Yue Q. (2019) One-step synthesis of easilyrecoverable carboxylated biogas residues for efficient removal of heavy metal ions from synthetic wastewater. J Clean Prod 240:118264.
Publisher | Google Scholor - Nguyen HD, Tran HN, Chao H-P and Lin C-C. (2019) Activated carbons derived from teak sawdust-hydrochars for efficient removal of methylene blue, copper and cadmium from aqueous solution. Water 11(12):2581-2595.
Publisher | Google Scholor - Dinu MV and Dragan ES. (2010) Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: kinetics and isotherms. Chem Eng J 160:157-163.
Publisher | Google Scholor - Khalid, S, Shahid, M, Natasha, Bibi, I, Sarwar, T, Shah, AH and Niazi, NK. (2018) A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int J Environ Res Publ Health 15(5):895-930.
Publisher | Google Scholor - Lai CH and Chen CY. (2001) Removal of metal ions and humic acid from water by ironcoated filter media. Chemosphere 44:1177-1184.
Publisher | Google Scholor - Eaton AD, Clesceri LS and Rice EW. (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn), ed by Greenberg AE and Franson MAH. American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF). American Public Health Association 800 I Street, NW Washington, DC, 20001-3770, USA.
Publisher | Google Scholor - Morales Morales JA. (2017) Synthesis of hematite α-Fe2O3 nano powders by the controlled precipitation method. Cienc En Desarro 8:99-107.
Publisher | Google Scholor - Kuterasinski Ł, Podobinski J, Rutkowska-Zbik D and Datka J. (2019) IR studies of the Cu ions in Cu-faujasites. Molecules 24(23):4250-4263.
Publisher | Google Scholor - Bekenyiova A, Styriakova I and Dankova Z. (2015) Sorption of copper and zinc by goethite and hematite. Arch Tech Sci 1:58-66.
Publisher | Google Scholor - Boujelben N, Bouzid J and Elouear Z. (2009) Adsorption of nickel and copper onto natural iron oxide-coated sand from aqueous solutions: study in single and binary systems. J Hazard Mater 163:376-382.
Publisher | Google Scholor - Phuengprasop T, Sittiwong J and Unob F. (2011) Removal of heavy metal ions by iron oxide coated sewage sludge. J Hazard Mater 186:502-507.
Publisher | Google Scholor - Ben-Ali S, Jaouali I, Souissi-Najar S and Ouederni A. (2017) Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J Clean Prod 142:3809–3821.
Publisher | Google Scholor - Ayawei N, Ebelegi AN and Wankasi D. (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017:3039817, 1-11.
Publisher | Google Scholor - Freundlich HM. (1906) Over the adsorption in solution. J Phys Chem 57:385-470.
Publisher | Google Scholor - Jiang Y, Pang H and Liao B. (2009) Removal of copper (II) ions from aqueous solution by modified bagasse. J Hazard Mater 164:1-9.
Publisher | Google Scholor - McKay G, Otterburn MS and Sweeney AG. (1980) The removal of colour from effluent using various adsorbents-IV. Silica: equilibria and column studies. Water Res 14:21-27.
Publisher | Google Scholor - Igberase E, Osifo P and Ofomaja A. (2017) The adsorption of Pb, Zn, Cu, Ni, and Cd by modified ligand in a single component aqueous solution: equilibrium, kinetic, thermodynamic, and desorption studies. Int J Anal Chem 2017:6150209, 1-15.
Publisher | Google Scholor