Research Article
A Study on The Antibiotic Use Pattern Among Patients with Urinary Tract Infections at a County Referral Hospital in Kenya
1Department of Pharmacy, Chuka County Referral Hospital, Chuka, Kenya.
2Department of Pharmacology and Clinical Pharmacy, Mount Kenya University, Thika, Kenya.
*Corresponding Author: Opwoko D. J., Department of Pharmacology and Clinical Pharmacy, Mount Kenya University, Thika, Kenya.
Citation: Kithuka D.K, Wainaina S. M, Opwoko D. J. (2023). A Study on The Antibiotic Use Pattern Among Patients with Urinary Tract Infections at a County Referral Hospital in Kenya. Clinical Case Reports and Studies, BioRes Scientia Publishers. 3(5):1-8. DOI: 10.59657/2837-2565.brs.23.085
Copyright: © 2023 Opwoko D. J, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 14, 2023 | Accepted: November 02, 2023 | Published: November 10, 2023
Abstract
Urinary tract infections are a prevalent cause of hospital visits, particularly among females and the elderly. Timely diagnosis and adherence to clinical guidelines are crucial for effective management. This study aimed to evaluate antibiotic usage patterns in urinary tract infection patients at Thika Level 5 Hospital, providing insights into prescribing practices and guideline adherence. A cross-sectional study enrolled 308 participants using purposive sampling. Data were extracted from patient files and supplemented with information on clinical guideline availability and supplies for UTI diagnosis and treatment. Descriptive analysis was conducted using Microsoft Excel, with strict adherence to ethical data protection protocols. The study found that a significant proportion of participants (n=78, 25.4%) fell within the 39-49 years age group. Patients under 49 years old constituted the majority (n=200, 65.1%). Cystitis (41.4%) was the most common specific urinary tract infection diagnosis, followed by pyelonephritis (23.5%) and urethritis (18.9%). Common comorbidities included diabetes mellitus (n=31, 27.0%), benign prostatic hyperplasia (BPH) (n=16, 13.9%), and hydrocele (n=11, 9.6%). Ceftriaxone was the predominant antibiotic prescribed in both outpatient (single STAT doses) and inpatient settings, followed by cefixime (n=105, 34.2%) and azithromycin (n=70, 22.8%). Notably, most prescribed antibiotics belonged to the World Health Organization Watch list. Among the 307 prescriptions reviewed, only 112 (36.7%) adhered to Kenyan Clinical Management and Referral Guidelines Volume III. Non-compliant prescriptions involved incorrect antibiotic choice, inappropriate duration and lack of indication. However, prescription frequency, route of drug administration and total dose generally aligned with recommendations.
Keywords: adherence; comorbidities; cystitis; pyelonephritis; urethritis
Introduction
Urinary tract infections (UTIs) occur when microorganisms invade the urinary tract. UTIs are among the most common infections globally, affecting over 400 million people and causing approximately 236,000 deaths annually[1]. Additionally, UTIs contribute to approximately 5.2 million DALYs worldwide. Notably, Southern Sub-Saharan Africa recorded one of the lowest age-standardized death rates at 0.8 per 100,000 persons in 2019 [2]. The number of deaths, incidences, and DALYs has increased significantly by 60.4% from 1990 to 2019, from 252.3 million to 404.6 million [3]. Similarly, the mortality rate has risen by 1240.2%, from 98,590 in 1990 to 236,279 in 2019 [2]. UTIs are ranked as the second most common infections globally, causing a health burden of more than $6 billion annually [4].
Appropriately prescribing antibiotics for UTIs reduces the chances of resistance, recurrence, complications and cost [5]. However, incorrect selection, duration, dosing of antibiotics for treating UTIs, as well as misuse, could lead to the development of antimicrobial resistance and clinical complications. Currently, antimicrobial resistance is a major global concern driven by the inappropriate use of antibiotics by healthcare providers and patients [6]. Using evidence-based guidelines enables healthcare providers to optimize available antibiotics judiciously. International standards require clinicians to use urine culture and sensitivity testing as the gold standard for diagnosis [7]. Accurate diagnosis would support clinicians in using the most effective and narrow-spectrum antibiotics that target specific sensitive microorganisms [8]. However, many clinicians opt for empirical treatment with broad-spectrum coverage due to various limitations. Auditing antibiotic use is essential for improving therapeutic standards, with the goal of evaluating and suggesting improvements in prescribing habits among clinicians [5].
Urinary tract infections (UTIs) rank among the most prevalent bacterial infections globally, impacting approximately 150 million individuals annually [9]. The incidence and mortality from UTIs have risen globally over the past three decades. The absolute number of cases of UTIs increased by 60.40% from 252.25 million in 1990 to 404.61million in 2019 [3]. UTIs are associated with a significant economic and clinical burden, as well as a reduced quality of life. The disability-adjusted life years and mortality rates associated with UTIs have been increasing worldwide [10]. Among them is the growing burden of chronic kidney disease (CKD), a serious public health concern in the twenty-first century. UTIs in patients with CKD are considered complicated. Urosepsis, a clinically manifested severe infection of the urinary tract, can lead to kidney damage[11]. The global prevalence of UTIs is about 25%[12] which closely resembles the estimated prevalence in Kenya [13].
In Kenya, most of the treatment approaches are empirical and vary across practitioners [14]. Although UTIs have been quite responsive to treatment for a long time, this has been quickly overturned by the emerging antibiotic resistance of uropathogens [15]. The indiscriminate exposure to antibiotics through misuse or inappropriate use has accelerated the development of resistance to the commonly used, available and affordable antibiotics. This has made UTIs less responsive to common treatments, costlier to treat and more severe [16]. Most clinicians lack access to up-to-date clinical guidelines based on antibiotic sensitivity data [17]. Therefore, UTIs are becoming a burden on the healthcare system, necessitating the need for a review of current treatment patterns.
Material & Methods
Study Design and Site
This was a cross-sectional study that was conducted at Thika Level 5 Hospital (TL5H), which is a regional referral hospital located at Thika town, in Kiambu County. The county covers an area of 2,538 km^2 with a population of approximately 2,417,735 individuals and 795,241 households [18]. About 80% of residents in this county have access to improved sanitation and more than three-quarters have access to improved water sources. This county referral hospital serves a population of 3-5 million people from Kiambu and neighboring counties and has a capacity of 265 beds, catering for approximately 20,000 inpatients annually. According to 2015 records, the hospital saw more than 217,000 patients, averaging 1,935 patients per month. The study population comprised adult inpatients and outpatients diagnosed with UTI at TL5H in the study period.
Sampling and Sample Size
Purposive sampling was employed to collect data from 307 individuals, comprising 30% of the participants from inpatient files and 70% from outpatient files. The sample size of 307 was determined using the Cochrane formula:
n = (Z^2 * p * (1 - p)) / E^2
Where: n (the desired sample size) was calculated with a confidence interval (Z) of 1.96 (for a 95% confidence level) and a prevalence rate (p) of 27.6%, as reported by Wanja et al. in 2021 [13].
Data collection
Data were collected from patient records using a standardized questionnaire. The variables under investigation included specific diagnoses, the sociodemographic profiles of patients, comorbidities and prescribed medications. Additionally, we gathered supplementary information from departmental heads in clinical, laboratory and pharmaceutical departments to assess the availability of clinical guidelines for UTI’s medications, diagnostic tests, and capacity-building activities.
Ethical Considerations
Authorization for the study was granted by the Mount Kenya University Institutional Ethics and Research Committee (Approval Number 1731). Additional permissions were obtained from NACOSTI (NACOSTI/P/23/25491) and the Thika Level 5 Ethics Committee. To protect participant information, data were anonymized, questionnaires were securely stored and digital copies were password-protected.
Data Analysis
Data were entered into Microsoft Excel and analyzed descriptively including percentages, means, graphs, and tables. Patient profiles, commonly prescribed antibiotics and prescribing patterns were summarized. Antibiotics were classified according to the WHO Aware classification[19], and cumulative results were presented in the form of percentages.
Results
Sociodemographic Profile of the Participants
From the sample size of 307 participants, there was an almost equal distribution of patients between the inpatient and outpatient settings. Most of these patients were female (n=175, 57.0%), among whom 15 (4.9%) were pregnant (Table 1). The basis of diagnosis was mainly through microscopy (n=199, 64.8%) followed by the dipstick method (n=65, 21.2%). None of the sampled patients benefited from culture and sensitivity testing.
Table 1: SociodemographicCharacteristics of the Patients Diagnosed with Urinary Tract Infections
| Variable | Frequency (%) |
| Setting Outpatient Inpatient | 158 (51.5%) |
| Gender | 149 (48.5%) |
| Male | 132 (43.0%) |
| Female | 175 (57.0%) |
| Pregnant | |
| No | 292 (95.1%) |
| Yes | 15 (4.9%) |
| Laboratory Tests | |
| Dipstick | 65 (21.2%) |
| Microscopy | 199 (64.8%) |
| Microscopy and Dipstick | 31 (10.1%) |
| Not done | 12 (3.9%) |
| Culture &Sensitivity | 0 |
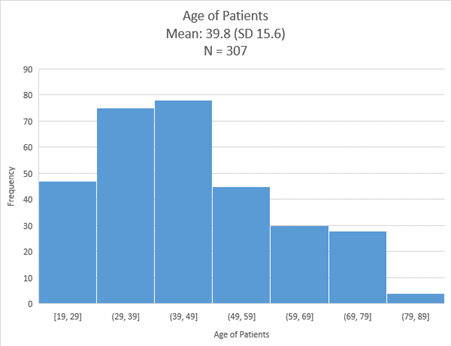
Most of the participants (n=78, 25.4%) were aged 39 to 49 years (Figure 1). Patients below 49 years old (n=200, 65.1%) constituted the majority in this study.
Figure 1: Age of the Patients Diagnosed with Urinary Tract Infections.
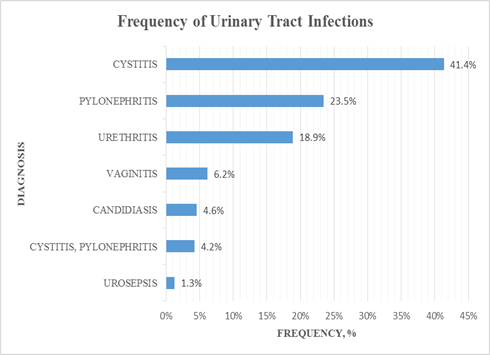
From the analysis of the specific UTI diagnosis, cystitis (41.4%) was the most common, followed by pyelonephritis (23.5%) and urethritis (18.9%) (Figure 2).
Figure 2: Frequency of the Urinary Tract Infections.
The most common comorbidities among the patients with UTIs were diabetes mellitus (n=31, 27.0%), benign prostatic hyperplasia (BPH) (n=16, 13.9%) and hydrocele (n=11, 9.6%) (Table 2).
Table 2: Most Common Comorbidities among Patients with Urinary TractInfections
| Comorbidities | Frequency (%) |
| Diabetes Mellitus | 31(27.0%) |
| Benign prostatic hyperplasia | 16(13.9%) |
| Hydrocele | 11 (9.6%) |
| Hypertension | 9 (7.8%) |
| Retroviral disease | 8 (7.0%) |
| Septicaemia | 7 (7.0%) |
| Incomplete abortion | 6 (5.2%) |
| Cervical cancer | 5 (4.3%) |
| Tuberculosis | 4 (3.5%) |
| Pelvic Inflammatory Disease | 3 (2.6%) |
| Chronic wound | 2 (1.7%) |
Commonly Prescribed Antimicrobial Agents
Ceftriaxone was the most frequently prescribed drug, both in the outpatient setting (single STAT doses) and for inpatients. Other antibiotics included cefixime (n=105, 34.2%) and azithromycin (n=70, 22.8%) (Table 3).
Table 3: List of the most Prescribed Antimicrobials
| Drug name | frequency (%) |
| Ceftriaxone | 123(40.1%) |
| Cefixime | 105(34.2%) |
| Azithromycin | 70(22.8%) |
| Metronidazole | 49(16.0%) |
| Ciprofloxacin | 46(15.0%) |
| Amoxiclav | 22(7.2%) |
| Clotrimazole | 20(6.5%) |
| Fluconazole | 17(5.5%) |
| Amoxicillin | 16(5.2%) |
| Levofloxacin | 16(5.2%) |
| Gentamicin | 15(4.9%) |
| Flucloxacillin | 14(4.6%) |
| Nitrofurantoin | 13(4.2%) |
| Cefuroxime | 10(3.3%) |
| Doxycycline | 7(2.3%) |
| Norfloxacin | 5(1.6%) |
| Amikacin | 1(0.3%) |
Aware Classification of Prescribed Antibiotics
Majority of the prescribed antibiotics were from the WHO Watch list of antibiotics (Table 4). None of the drugs was from the Reserve category of antibiotics.
Table 4: WHO AWaRe Classification for the Prescribed Antibiotics
| Who classification | Drugs | Frequency |
| ACCESS | Amoxicillin | 137 (44.6%) |
| Amoxicillin/Clavulanic acid | ||
| Doxycycline | ||
| Flucloxacillin | ||
| Metronidazole | ||
| Nitrofurantoin | ||
| Gentamicin | ||
| Amikacin | ||
| WATCH | Azithromycin | 200 (64.9%) |
| Cefixime | ||
| Ceftriaxone | ||
| Cefuroxime | ||
| Levofloxacin | ||
| Norfloxacin | ||
| Ciprofloxacin | ||
| RESERVE | - | 0 |
Characteristics of Prescriptions
Out of the 307 prescriptions reviewed 112 (36.7%) adhered to the Kenyan Clinical Management and Referral Guidelines Volume III. The majority of non-compliant prescriptions included one or more of the following issues: the use of an antibiotic not recommended, incorrect antibiotic duration, inappropriate choice of antibiotic for a specific patient and the absence of an indication for the prescribed drug. Nevertheless, all the prescriptions followed the recommended guidelines for frequency, route of drug administration, and total dose (Table 5).
Table 5: Prescribing Pattern of Drugs for Urinary Tract Infections
| Drug | Total Daily Dose (mg) | Frequency | Route of administration | Duration of therapy |
| Ceftriaxone | 2000 | STAT/OD/BD | IV | STAT-5 DAYS |
| Cefixime | 400 | OD | PER ORAL | 5 DAYS |
| Azithromycin | 500 | OD | PER ORAL | 3 DAYS |
| Metronidazole | 1200 | TDS | IV/PER ORAL | 5 DAYS |
| Ciprofloxacin | 800-1000 | BD | PER ORAL/IV | 5-14 DAYS |
| Amoxicillin/Clavulanic acid | 1250 | BD | PER ORAL | 5-7 DAYS |
| Clotrimazole | 200-500 | OD | PER VAGINAL | 1-6 DAYS |
| Fluconazole | 200 | STAT | PER ORAL | STAT |
| Amoxicillin | 1500-2000 | TDS/BD | PER ORAL | 5-7 DAYS |
| Levofloxacin | 750-1000 | OD/BD | PER ORAL | 5-7 DAYS |
| Gentamicin | 3MG/KG | OD | IV | 5 -7 DAYS |
| Flucloxacillin | 2000 | QID | PER ORAL | 5 DAYS |
| Nitrofurantoin | 200 | BD/TDS | PER ORAL | 5 - 7 DAYS |
| Cefuroxime | 1000 | BD | PER ORAL/IV | 5 DAYS |
| Doxycycline | 200 | BD | PER ORAL | 5 DAYS |
| Norfloxacin | 800MG | BD | PER ORAL | STAT |
| Amikacin | 15MG/KG | OD | PER ORAL/IV | 5-7DAYS |
Discussion
From the sample size of 307 participants, the majority of the patients were female, accounting for 57.0%. The higher prevalence of UTIs among females correlates with a study conducted in India, where the prevalence of UTIs among females was 73.57% compared to 35.14% in males [20]. These findings are also supported by a study carried out in a hospital in Peru from 2017 to 2019, which reported a UTI prevalence in women of 79.82% [21] . This observation can be explained by female anatomy, where the urethra is close to the anus, allowing easier entry of bacteria from the anus into the urethra and bladder. Additionally, females have a shorter urethra compared to males, making it easier for bacteria to reach the bladder[8]. In our study, it was found that the most common comorbidity among the patients with UTIs was diabetes mellitus, affecting 27.0% of the participants. This finding is consistent with a study from 2017-2018, where 46.81% of patients had diabetes [21]. Diabetic patients often have poor metabolic control, compromised immune systems and incomplete bladder emptying due to autonomic neuropathy, all contributing to an increased risk of urinary tract infections. Other factors include weakened white blood cells, poor blood supply, and glucosuria [21].
Ceftriaxone was the most prescribed antibiotic, accounting for 40% of prescriptions, both in the outpatient and inpatient settings. These results are similar to a study conducted in Singapore, where ceftriaxone was prescribed as empirical treatment for UTIs in 36.6% of cases. In contrast to our study, cefixime was the second most prescribed antibiotic at 34.2%, with azithromycin ranking third at 22.8%. The Singapore study reported that co-amoxiclav was the second most prescribed antibiotic for UTIs, followed by piperacillin tazobactam and ciprofloxacin [22]. These differences may be attributed to variations in antimicrobial resistance patterns between the two countries and differences in clinical practice guidelines. Most prescribed antibiotics belonged to the WHO Watch list of antibiotics. Access antibiotics accounted for 44.6% of prescriptions, while watch antibiotics were prescribed in 64.9% of cases. None of the drugs fell under the Reserve category of antibiotics. These results align with a study conducted in India, where the percentage of patients on access antibiotics (35%) was lower than those on watch antibiotics (78.7%), with reserve antibiotics being prescribed to the fewest patients (0.1%) [23]. The reverse usage of watch antibiotics compared to the WHO's target for using access antibiotics for 60% of patients raises concerns. Notably, third generation cephalosporins, particularly ceftriaxone, were excessively used among the antibiotics for treating UTIs. The overuse of third generation cephalosporins (Watch) is alarming, as it is considered a contributing factor to the emergence of extended-spectrum beta-lactamase (ESBL) producing microorganisms.
Among the 307 prescriptions reviewed, 112 (36.7%) adhered to the Kenyan Clinical Management and Referral Guidelines. This indicates that most prescribers did not follow the Kenyan guidelines for UTI treatment [16]. Non-compliant prescriptions often included the use of non-recommended antibiotics, incorrect antibiotic durations, inappropriate antibiotic choices for specific patients and the absence of an indication for the prescribed drug. These findings highlight irrational prescribing practices and a lack of standardization in UTI treatment, which could lead to drug misuse, adverse reactions and drug shortages at health facilities [24]. However, all prescriptions adhered to the recommendations regarding frequency, route of administration and total dosage.
Conclusion
This study revealed inadequate adherence to WHO recommendations for the treatment of urinary tract infections [25]. Most patients (78.7%) were prescribed WATCH antibiotics, particularly third generation cephalosporins, which raises concerns about the emergence of drug-resistant microorganisms, treatment failures, and increased therapy costs. Additionally, irrational prescribing practices were observed, with many patients receiving inappropriate antibiotics and incorrect treatment durations.
Declarations
Acknowledgement
The authors greatly appreciated the role of the healthcare workers and the Thika Level 5 Hospital who willingly participated in this study.
Author’s contribution
Doris Kithuka designed the study and collected data. The manuscript was written by Wainaina Samuel, and corrections and proofreading were carried out by Dennis Opwoko.
Conflict of interests
The authors declare that there is no conflict of interest in this published work.
References
- Z. Zeng, J. Zhan, K. Zhang, H. Chen, and S. Cheng. (2022). Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J Urol, 40(3):755-763.
Publisher | Google Scholor - D. Kasew et al., (2022). Antimicrobial resistance trend of bacterial uropathogens at the university of Gondar comprehensive specialized hospital, northwest Ethiopia: A 10 years retrospective study. PLoS One, 17(4):e0266878.
Publisher | Google Scholor - X. Yang, H. Chen, Y. Zheng, S. Qu, H. Wang, and F. Yi. (2022). Disease burden and long-term trends of urinary tract infections: A worldwide report. Front Public Health, 10.
Publisher | Google Scholor - M. S. Alam et al. (2020). Antibiotic Use Evaluation in Genitourinary Tract Infections in Female Patients at a Tertiary Care Hospital. J Evol Med Dent Sci, 9(8):532-538.
Publisher | Google Scholor - S. Govindarajan and P. Shenoy. (2020). Drug prescribing patterns in pediatric urinary tract infections: A retrospective drug utilization analysis in an urban tertiary care hospital. J Pharm Bioallied Sci, 12(4):423.
Publisher | Google Scholor - N. A. Okinda and G. Revathi. (2012). Urinary tract infections at aga khan university hospital nairobi - a one year experience. East Afr Med J, 89(5):147-153.
Publisher | Google Scholor - R. Xu, N. Deebel, R. Casals, R. Dutta, and M. Mirzazadeh. (2021). A New Gold Rush: A Review of Current and Developing Diagnostic Tools for Urinary Tract Infections. Diagnostics, 11(3):479.
Publisher | Google Scholor - S. Reza Mortazavi-Tabatabaei, J. Ghaderkhani, A. Nazari, K. Sayehmiri, F. Sayehmiri, and I. Pakzad. (2019). Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis, Int J Prev Med, 10(1):169.
Publisher | Google Scholor - W. E. Stamm and S. R. Norrby. (2001). Urinary Tract Infections: Disease Panorama and Challenges. J Infect Dis, 183:S1–S4.
Publisher | Google Scholor - M. Medina and E. Castillo-Pino, An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol, 11:175628721983217.
Publisher | Google Scholor - Z. Dimitrijevic, G. Paunovic, D. Tasic, B. Mitic, and D. (2021). Basic, Risk factors for urosepsis in chronic kidney disease patients with urinary tract infections. Sci Rep, 11(1):14414.
Publisher | Google Scholor - W. G. Masika, W. P. O’Meara, T. L. Holland, and J. (2017). Armstrong, Contribution of urinary tract infection to the burden of febrile illnesses in young children in rural Kenya, PLoS One, 12(3):e0174199.
Publisher | Google Scholor - F. Wanja, C. Ngugi, E. Omwenga, J. Maina, and J. Kiiru. (2021). Urinary Tract Infection among Adults Seeking Medicare at Kiambu Level 5 Hospital, Kenya: Prevalence, Diversity, Antimicrobial Susceptibility Profiles and Possible Risk Factors, Adv Microbiol., 11(8):360-383.
Publisher | Google Scholor - L. Momanyi, S. Opanga, D. Nyamu, M. Oluka, A. Kurdi, and B. (2019). Godman, Antibiotic prescribing patterns at a leading referral hospital in Kenya: A point prevalence survey, J Res Pharm Pract, 8(3):149.
Publisher | Google Scholor - B. KOT. (2019). Antibiotic Resistance Among Uropathogenic Escherichia coli, Pol J Microbiol, 68(4):403-415.
Publisher | Google Scholor - F. Wanja, C. Ngugi, E. Omwenga, J. Maina, and J. Kiiru. (2021). Urinary Tract Infection among Adults Seeking Medicare at Kiambu Level 5 Hospital, Kenya: Prevalence, Diversity, Antimicrobial Susceptibility Profiles and Possible Risk Factors. Adv Microbiol, vol. 11(8):360-383.
Publisher | Google Scholor - M. Maina et al. (2020). Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. International Journal of Infectious Diseases, 99:10-18.
Publisher | Google Scholor - Kenya National Bureau of Statistics, Kenya population and housing census volume 1: Population by County and sub-County. In Kenya National Bureau of Statistic. 1.
Publisher | Google Scholor - V. Zanichelli et al. (2023). The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Organ, 101:4.
Publisher | Google Scholor - D. Prakash and R. S. Saxena. (2013). Distribution and Antimicrobial Susceptibility Pattern of Bacterial Pathogens Causing Urinary Tract Infection in Urban Community of Meerut City, India. ISRN Microbiol, 2013:1-13.
Publisher | Google Scholor - M. J. Bono, S. W. Leslie, and W. C. Reygaert. (2023). Urinary Tract Infection.
Publisher | Google Scholor - N. Y. Li et al. (2020). A Prediction Tool for the Presence of Ceftriaxone-Resistant Uropathogens upon Hospital Admission. Antibiotics, 9(6):316.
Publisher | Google Scholor - C. J. L. Murray et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet.
Publisher | Google Scholor - L. Melku, M. Wubetu, and B. (2021). Dessie, Irrational drug use and its associated factors at Debre Markos Referral Hospital’s outpatient pharmacy in East Gojjam, Northwest Ethiopia. SAGE Open Med, 9:205031212110251.
Publisher | Google Scholor - F. Ramírez et al. (2022). Clinical practice guideline for the diagnosis and management of urinary tract infections: 2022 update. Arch Argent Pediatr, 120(5).
Publisher | Google Scholor