Case Report
A Role for Isoniazid Prophylaxis Against Mycobacterium Tuberculosis During Treatment for Acute Myeloid Leukaemia in Highly Endemic Areas?
- Elton Roman *
Muhimbili University of Health and Allied Sciences, Tanzania.
*Corresponding Author: Elton Roman, Muhimbili University of Health and Allied Sciences, Tanzania.
Citation: Roman E., Leak S., Gasper L., Sadiq A., Mkwizu. (2023). A Role for Isoniazid Prophylaxis Against Mycobacterium Tuberculosis During Treatment for Acute Myeloid Leukaemia in Highly Endemic Areas? Clinical Case Reports and Studies, BRS Publishers. 2(5); DOI: 10.59657/2837-2565.brs.23.042
Copyright: © 2023 Elton Roman, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 30, 2023 | Accepted: June 13, 2023 | Published: June 21, 2023
Abstract
Hematological malignancy is an increasing burden on the Tanzanian healthcare system accounting for around 10% of all cancer diagnosis [1]. Acute myeloid leukemia (AML) is particularly challenging in the context of limited diagnostic services, restricted blood product availability, high cost of chemotherapy drugs and the complete absence of bone marrow transplantation. Despite this Kilimanjaro Christian Medical Centre (KCMC) has attempted to overcome these barriers and has commenced induction therapy with daunorubicin and cytarabine in a number of suitable candidates in an attempt to treat their disease. However, to date treatment during nadir poses an unresolved threat despite the use of prophylactic antiviral, anti-fungal and antibiotic therapies and broad-spectrum antibiotics in the case of infection.
Keywords: leukaemia; tuberculosis; prophylaxis
Introduction
Mycobacterium tuberculosis (TB) is also a significant health challenge in Tanzania having one of the highest TB burdens in the developing world with an incidence of 269 per 100000 population in 2017 [3]. Here we present a case of AML in a 33-year-old lady who died 23 days post 5+2 induction with a working diagnosis of invasive fungal infection versus pulmonary TB. We will describe the challenges in managing these cases in our setting and also review current literature on the use of isoniazid for TB prophylaxis as part of the neutropenic prophylactic measures in highly endemic regions.
Case Report
A 33-year-old lady was referred to the obstetrics and gynecology team at the regional referral center with a 7-day history of heavy menstrual bleeding with symptomatic anemia. She reported a 7-day history of easy fatigability, palpitations, dizziness and pre syncope on standing. Initial full blood picture revealed hemoglobin (Hb) 3.1g/dl, Platelets (Plts) 3 x 109/L and a total leucocyte count (WCC) of 25.06 x 109/L with a differential of neutrophils 18.17 x 109/L, lymphocytes 5.46 x 109/L and monocytes 1.08 x 109/L. Electrolytes, renal and liver function were all within normal range.
The patient was commenced on oral contraception and transfused 5 units of whole blood over a 1-week period at which point full blood picture was repeated revealing Hb 9.1g/dl, Plts 26 x 109 /L and a WCC of 1.98 x 109/L. Given the ongoing low platelets and development of leucopenia the patient was then referred to the medical team for review. Peripheral blood film was reviewed at this stage revealing a normochromic normocytic anemia, marked leucopenia, and a true marked thrombocytopenia and decision to proceed with bone marrow aspiration was made.
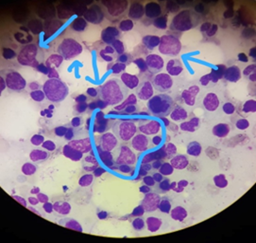
Bone marrow aspiration was performed on the posterior superior iliac spine (figure 1) and was cytomorphologic ally suggestive of acute myeloid leukemia. Unfortunately flow cytometry, immunohistochemistry and cytogenetic analysis were not available at the center to further delineate disease type. Long discussions were therefore held with the patient and family regarding disease prognosis, treatment, the need for platelet donors’ mobilization, and the financial implications which would come with a prolonged hospital admission and supportive medications. Chemotherapy was offered for free on a compassionate basis. The decision was made for induction with a 5+2 induction regimen with the hope of reducing time in nadir given the difficulties in managing patients during this window. Baseline chest X ray was performed prior to treatment and was normal. No other latent TB testing was available or appropriate. The patient was managed in a single room with gloves, masks and aprons used by all staff and visitors. The room had open windows to the outside due to a lack of air conditioning or other facilities.
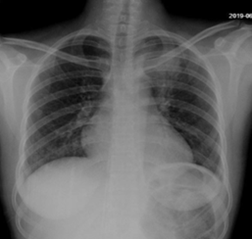
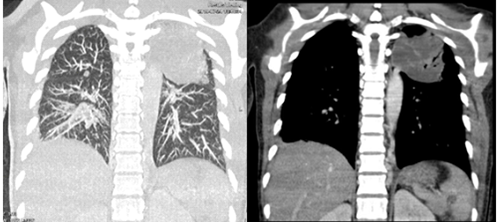
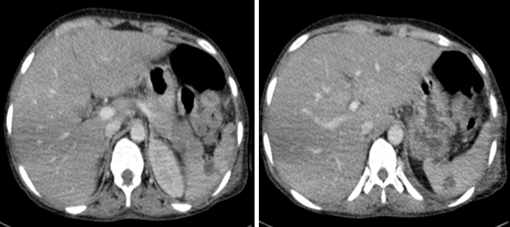
The patient was commenced on fluconazole prophylaxis 200mg once daily; of note funds could not cover itraconazole or Posaconazole. In addition, cotrimoxazole 960mg once daily, ciprofloxacin 500mg twice daily and chlorhexidine mouthwash QID from day 1 of treatment were started. Unfortunately, antiviral prophylaxis was not available. On day 8 of induction the patient spiked a fever, blood cultures and chest x-ray were performed and the patient was commenced on piperacillin-tazobactam 4.5g IV q8h and gentamicin 80mg IV q12h with no clear source, cultures showed no growth. Fevers continued and the patient was then switched to meropenem 1g IV q8h at day 14. Fevers settled and counts began to improve with day 20 bloods revealing Hb 12.2g/dl, Plts 36 x 109 /L and a WCC of 4.05x 109 /L with neutrophils 2.76 x109 /L. At this stage however the patient developed a productive cough with shortness of breath and chest tightness. Chest X-ray was performed (figure 2). Azithromycin was added to cover for probable atypical pneumonia while continuing with meropenem. Amphotericin B 70mg IV OD was also initiated for the possibility of pulmonary fungal infection. It was given for only four days due to availability issues. Despite this oxygen requirement continued to increase and we therefore proceeded to do a high-resolution CT scan of the chest on day 22 which can be seen in figure 3 and 4.
Figure 1: Bone marrow aspiration at diagnosis - prominent blasts indicated with nucleoli present.
Figure 2: Chest X-ray - Consolidation in the left upper lobe with patchy alveolar infiltrates in the right lower lobe.
Figure 3: Axial, sagittal and coronoal CT images shows consolidation in the apico-posterior segment of the left upper lobe with air bronchograms. Consolidation in the posterior basal segment of the right lower lobe. Scattered micronodules seen in the right upper and middle lobe. Features are suggestive of multi-lobe pneumonia (bacterial pneumonia). Tuberculosis and fungal infection cannot be ruled out.
Figure 4: CT images of the upper abdomen shows multiple hypodense cystic lesions in the splenic parenchyma suggestive of fungal abscesses. DDX: Granulomatous infection / lymphoma
Unfortunately, the patients passed away despite these interventions and we were unable to make a documented microbiological or mycotic diagnosis. Disseminated fungal infection was felt as the most likely in this setting despite the lack of improvement with amphotericin B. Immune reconstitution syndrome was also considered however the second most likely differential was felt to be mycobacterium tuberculosis infection. Given the above case we have conducted a literature review of the incidence of concurrent TB infection in hematological malignancy patients as well as reviewing the evidence for and against the use of isoniazid prophylaxis in these patients.
Literature Review
Mycobacterium is well recognized as an important opportunistic infection in immunosuppressed patients in highly endemic areas. Numerous cases of TB during treatment for hematological malignancy have been reported in the literature and a number of case reports document TB mimicking disseminated fungal infection in acute leukemia [10-11] [12]. It is estimated that the relative risk of TB infection in hematological malignancy patients is 2-40 times that of the general population [8]. AML patients are particularly at risk and have been shown to be at greater risk than those with acute lymphoblastic leukemia [10]. Current UK guidelines recommend the use of 6 months isoniazid prophylaxis (IP) in cancer patients who have a prior history of TB infection or evidence of TB scars on CXR [7] and would recommend latent TB testing with interferon gamma release assay and subsequent treatment in those with severe immunocompromise. This includes patients undergoing allogeneic bone marrow transplant as well as other hematological malignancies [2].
Accessing latent TB testing for hematological malignancy patients in highly endemic areas, which are typically low income, is challenging or often impossible. Even in instances where tuberculin skin testing is available there is a lack of sensitivity given concurrent immunodeficiency and it is further complicated by the high prevalence of BCG vaccination making result interpretation challenging [8]. Use of IP as part of AML treatment in this setting would require blind use as is employed in HIV guidelines. In the case of HIV treatment effectiveness in reducing mortality has been strongly demonstrated, [6] however data supporting blind IP in the setting of hematological malignancy is lacking. A study performed in Pakistan looking at the use of IP in allogeneic stem cell transplant patients required for the treatment of various hematological malignancies found that its use led to a statistically significant reduction in cases of active TB infection [4].
Also, Moriuchi et al. conducted a retrospective study in India on the use of prophylaxis in hematological malignancy patients and also found a significant reduction in the incidence of active TB when used although it is important to note that the number of cases in both groups was small [5]. A potential concern regarding the use of IP in this setting is the risk of hepatotoxicity when used in combination with chemotherapeutic agents. A small study conducted by Sanchez-Garcia E et al. reviewed 105 patients treated with latent TB treatment in the context of haematological malignancy 21 of whom at concurrent chemotherapy. No safety risk was noted in these patients when isoniazid was used concurrently with chemotherapeutic agents [9]. Data on this however is clearly limited. Also there have been concerns about the risk of increasing TB resistance to first line therapies with widespread use of IP in the context of HIV, however this is still an area of current debate with some studies suggesting that in this context the benefits on incidence and mortality are likely to outweigh the risk of drug resistance [13].
Conclusion
Clearly reactivation of TB in AML patients is an area requiring focus, however there is a paucity of literature evaluating potential interventions which could be employed to prevent its occurrence. Given the widespread use and effectiveness of isoniazid prophylaxis in patients with HIV, it seems reasonable to also trial its use in these high infection risk patients as a prophylactic measure during nadir, however a full trial is required to assess its potential benefit and risk. With regards our case it is essential in a high TB endemic setting to have a low threshold for considering TB in these patients and commence treatment early if there is clinical suspicion, and the patient is failing to improve with other treatments.
References
- Gopal S et al. (2012). Meeting the challenge of hematological malignancies in sub-Saharan Africa, Blood, 119:5078-5087
Publisher | Google Scholor - Mishra P, Kumar R, Mahapatra M, et al. (2006). Tuberculosis in acute leukemia: a clinico-hematological profile. Hematology, 11:335-340
Publisher | Google Scholor - Wu CY, Hu HY, Pu CY, et al. (2011). Aerodigestive tract, lung and hematological cancers are risk factors for tuberculosis: an 8-year population-based study. Int J Tuberc Lung Dis. 15:125-130
Publisher | Google Scholor - Lee D et al. (2001). Hepatosplenic tuberculosis mimicking disseminated candidiasis in patients with acute leukemia. International Journal of Hematology, 73(1):119-121.
Publisher | Google Scholor - Anibarro L et al, (2014). Tuberculosis in Patients with Haematological Malignancies. Mediterr J Hematol Infect Dis, 6(1).
Publisher | Google Scholor - (2019). London cancer North and East, Latent Tuberculosis Guidelines for Cancer Patients.
Publisher | Google Scholor - National Institute for Health and Care Excellence (2016). Tuberculosis (NICE Guideline 33).
Publisher | Google Scholor - Van Halsema et al. (2010). Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS, 24(7):1051-1055
Publisher | Google Scholor - Ahmed P et al. (2005). Role of isoniazid prophylaxis for prevention of tuberculosis in haemopoietic stem cell transplant recipients. J Pak Med Assoc., 55(9):378-381.
Publisher | Google Scholor - Moriuchi Y, Kamihira S, Satoh T, Yanagisako T, Miyazaki Y. (1991). Infection prophylaxis in patients with hematological malignancies (II) – Successful prophylaxis of tuberculosis with isoniazid. Rinsho Ketsueki, 32:199-204.
Publisher | Google Scholor - Sanchez-Garcia E et al, (2013). Toxicity and adherence to treatment for latent tuberculosis infection in patients with hematologic malignancies. Infection, 41(5):903-907
Publisher | Google Scholor - Kunkel A et al. (2016). Benefits of continuous isoniazid preventive therapy may outweigh resistance risks in a declining TB/HIV co-epidemic. AIDS, 30(17):2715-2723.
Publisher | Google Scholor - Moriuchi Y, Kamihira S, Satoh T, Yanagisako T, Miyazaki Y. et al. (1991). Infection prophylaxis in patients with hematological malignancies (II) – Successful prophylaxis of tuberculosis with isoniazid. Rinsho Ketsueki., 32:199-204.
Publisher | Google Scholor - Khalid Ahmed Al-Anazi, Asma Marzouq Al-Jasser, David Alan Price Evans. Infections caused by mycobacterium tuberculosis in patients with hematological disorders and in recipients of hematopoietic stem cell transplant, a twelve-year retrospective study.
Publisher | Google Scholor