Research Article
A Correlation between Glymphatic System Weakening and Epileptic Seizure Duration
1Department of Physics, Ben-Gurion University, Beer-Sheva, Israel.
2Department of Physics, Sami Shamoon College of Engineering, Beer-Sheva, Israel.
3Makif YudAlef, Rishon Lezion, Israel.
*Corresponding Author: Avinoam Rabinovitch, Department of Physics, Ben-Gurion University, Beer-Sheva, Israel.
Citation: A Rabinovitch, Biton Y, Braunstein D, Rabinovitch R, Thieberger R. (2023). A Correlation between Glymphatic System Weakening and Epileptic Seizure Duration. Journal of Brain Research and Neurology, BRS Publishers. 1(1); DOI: 10.59657/2992-9768.brs.23.003
Copyright: © 2023 Avinoam Rabinovitch, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 03, 2022 | Accepted: January 31, 2023 | Published: February 10, 2023
Abstract
It has recently been assumed that a surge of Glymphatic system cleaning activity may be the direct cause of seizure termination. The aim of this work is, therefore, to determine whether an impaired system would slow the cleaning processes and thus increase seizure durations. Since no direct measurements of the relation between seizure durations and the speed of the Glymphatic system exists, we can only infer this result indirectly. Accordingly, we have analyzed published literature outcomes of seizure durations in cases of reduced Glymphatic-system function, caused by ageing, neurodegenerative diseases and traumas. These show that seizure durations are indeed positively correlated with markers of such reduction in Glymphatic conditions. Moreover, in the two cases showing a functional relation, this dependence is found to be above linear, i.e., the functional increase, y(x), of seizure duration, y, with the reduction, x, of the Glymphatic system, is faster than y = ax. Namely, there should be an additional term, y = ax + b with p > 1; here we assumed p = 2. Thus, under extreme circumstances, this dependence can lead to severe prolongation of seizures which may turn into perilous dysfunctions. The understanding of the crucial task of the Glymphatic conditions in controlling epileptic seizure lengths and the possible major failures which may be induced by the system's deterioration, can be of clinical importance. The positive correlation between a Glymphatic system deteriorations and epileptic seizure durations calls for new focused clinical treatments of the system in order to prevent these malfunctions.
Keywords: Glymphatic system; epileptic seizures; cerebrospinal fluid
Introduction
Epilepsy is a group of neurological disorders categorized by spontaneous recurrent seizures. As of 2020, about 50 million people suffer from epilepsy worldwide [1]. This malfunction causes morbidity and even mortality. 20-30% of epilepsy isrefractory to drugs [1]. Thus, understanding this malfunction and thereby finding ways of treating it, is crucial for life improvement of a large population.
One of the unsolved problems in epilepsy is how seizures terminate. None of the previous attempts to explain seizure termination have proved to be consistent with all of the experimental data. Recently, termination of epileptic seizure was assumed [2-4] to arise from the operation of the Glymphatic system in the brain by cleansing the glutamate or K+ debris (K+ concentration in the extracellular space is denoted by [K+]o) from the parenchyma. Thus, if the function of this system is slowed down, it would cause an increase of the time until seizure termination, i.e., an increase of seizure duration. It is well known that the Glymphatic system capability is weakened during ageing and also as a result of traumas and neurogenerative brain malfunctions such as Alzheimer's decease (AD). See e.g., [5]. It would therefore be interesting to compare these capability reductions with seizure duration.
The water management in the brain Glymphatic system is known to be modulated by Aquaporin-4 (AQP4), which is the principal expressed aquaporin in the cerebrospinal fluid (CSF) and has been shown to regulate the brain's water homeostasis.Thus, a damage in AQP4 can lead to accumulation of harmful materials in the extracellular space (ECS) and to exacerbating many neurodegenerative diseases such as Alzheimer’s disease (AD), Amyotrophic Lateral Sclerosis (ALS), Parkinson’s disease (PD), Multiple Sclerosis (MS), Neuromyelitis Optica (NMO), traumatic brain injury (TBI) and stroke. Reference [6] discusses the role of AQP4 in such malfunctions. It turns out that in patients with such health problems and additional epileptic seizures, a reduction of the Glymphatic system's functioning also correlates positively with an increase of seizure durations. However, there exist no direct measurements of the relation between seizure duration and the Glymphatic speed at the end of a seizure, so we infer such relation indirectly, reporting relations of seizure durations to aging and to neurodegenerative disfunctions when deterioration of the general conditions of the system under these malfunctions are known.
Materials and Methods
We have analyzed published literature outcomes of seizure durations in cases of reduced Glymphatic-system function, caused by ageing and neurodegenerative diseases. As databases we used both PubMed and the Web of Science during the last 15 years, since the Glymphatic-system's function was not really understood previously. The keywords used were an "and" combination of: 1. seizure-duration*, 2. (g-lymphatic or glymphatic), 3. (aging or ageing) or particular neurodegenerative ailments (s) such as AD, PD and MS, or traumatic brain injury (TBI) or knocked out animal models. However, "and" combination of all three items yielded zero results, and "and" combination of items 1 and 2 yielded only two printed papers, which we have incorporated in this study. Therefore, we looked for additional indirect inferences by carrying out separate combinations of items 1 and 3 and items 2 and 3, but only a few of those showed relative, even if no direct, connection to our hypothesis. For example, "and" combination of seizure-duration* and (aging or ageing) in the Web of Science yielded 157 published papers, of which we found only four to be of interest to us. Due to these difficulties, we do not claim to have carried out an exhaustive search according to the PRISMA directions, but hope to have made a real thorough try.
Results
A model of AQP4 knocked-out mice AQP4−/−mice showed memory impairments [7] simulating a neurodegenerative state, and, more relevant to the present work, a high increase of induced seizure durations [8, 9] compared to wild-type controls (33 ± 2 s vs. 13 ± 2 s). A possible explanation, at the time, of such an increase was "altered ECS volume", which could be one of the factors decreasing the speed of the Glymphatic cleaning. A different cause could be gleaned from their results that [8]: "the rise time to peak [K+]o (t1/2, 2.3 ± 0.5 s) as well as the recovery to baseline [K+]o (t1/2, 15.6 ± 1.5 s) were slowed in AQP4 -/- mice compared to WT mice (t1/2, 0.5 ± 0.1 and 6.6 ± 0.7 s, respectively)". These results evidently imply a decreased glymphatic system function, supporting our hypothesis.
New measurements [10] of the Glymphatic system's efficiency, show that after application of substances (lithium chloride and pilocarpine) in order to induce status epilepticus (SE) in mice, the system's function deteriorated for several days and could be renewed by another substance (glibenclamide). The authors concluded that SE caused the system's impairment, however, their results can be understood also in a reverse direction, namely, that the substances applied to the mice gradually caused edema, leading to the Glymphatic system decline, which in turn prolonged the epileptic seizure duration turning it into the SE state.
Following a traumatic brain injury (TBI) even a mild TBI (mTBI) the function of the Glymphatic system declines [11]. Simultaneously seizure duration was enlarged, e.g., see Ref. [12], in which seizures were induced by subconvulsive dose of pentylenetetrazole (PTZ) 15 days after focal mild TBI. Moreover, post-traumatic rats were shown [13] to incur seizures, whose durations "correlated with corticosterone (CS) levels elevation, levels of IL-1 in the hippocampus", i.e., with the decline of the Glymphatic system. The spread of the actual measurements of [13], however, makes it hard to assess this correlation more quantitatively.
In a recent paper [14] the cerebral vascular compliances (VCs) of young and older people were measured at different brain arteries. The average ratio between the VC of older people to that of the younger ones (see table 1 of Ref. 10) was 68%. Assuming that VC is a measure of the "driving force of the recently discovered glymphatic system" [14], the 68% ratio should provide an estimate of the ratio of seizure durations for these age groups. According to [15], the Glymphatic system: "relative to the young, clearance of intraparechamally injected amyloid β was impaired by 40% in the old mice" and "a 27% reduction in the vessel wall pulsatility…" which is of the same order as the ~32% reduction of [14].
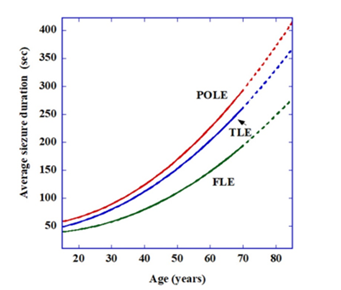
On the other hand, changes of seizure durations as a function of age for human patients with temporal lobe epilepsy (TLE), frontal lobe epilepsy (FLE) and parieto-occipital lobe epilepsy (POLE) were measured in [16]. Average results (though with extensive standard deviations) are given in Fig. 4 of [16] and show that the increase in seizure duration with increase in age is faster than linear. We therefore approximated them here to a polynomial of the second degree: SD=a+bA+cA2
Where SD is the seizure duration in seconds, and A is the age in years (no such mathematical relations appear in [16]). The results for the specific cases yield the following numerical equations:
FLE: SD=40-0.6A+0.04A2
TLE: SD=38+0.05A+0.045A2
POLE: SD=52-0.4A+0.058A2
Figure 1 is a representation of these relations. A similar graph (only up to age ~ 70) appears in [16], therefore the >70 part of the figures here appears dashed.
Figure 1: Seizure duration (in seconds) as a function of age for the three epilepsy types: temporal lobe epilepsy (TLE), frontal lobe epilepsy (FLE) and parieto-occipital lobe epilepsy (POLE). Note the faster than linear dependence.
Seizure termination in people with status epilepticus is impaired, prolonging seizure duration. Measuring the Glymphatic system characteristics in these patients indicates that it has been compromised as well. For example, [17] states that at least 60% of patients had CSF inflammatory signs. This could mean that weakened systems may be the cause of such extension in the temporal seizure durations.
In [18], the impairment of the brain's cleaning mechanism was assessed by measuring the presence and levels of substances such as Albumin, Lactate, Tau etc. in the cerebrospinal fluid (CSF) . Seizure durations of people with epilepsy were correlated with these levels. It turned out that there was a valid positive correlation between seizure durations and Albumin quotients and Tau concentrations. We assume that these durations are the result of the deterioration of the Glymphatic cleansing system and that this deterioration is demonstrated by the levels of the existing materials in the CSF. Evidently, a prolonged period of epileptic seizures can also have a reciprocal effect in causing damage to the cleaning system. Figure 3 of [18] shows that the increase in seizure duration with increase in Albumin quotient is again faster than linear. We therefore assume that the average seizure duration (SD, in minutes) depends on the average Albumin quotient (AQ, in units) as: SD=a+b(AQ)+c(AQ)2
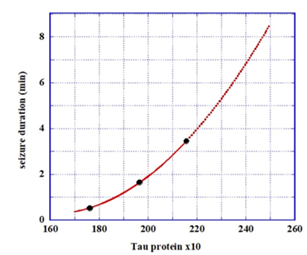
Similarly, according to the measurements of Fig. 3 of [18], the seizure duration, SD, dependence on Tau concentration (TC, in pg/ml) leads to: SD=25.9-0.323(TC)+0.00103(TC)2
Figure 2 depicts the SD dependence on Tau concentration. E.g., for Tau concentration of 230 pg/ml, the duration would be ~ 5 min.
Figure 2: Seizure duration (in minutes) as a function of Tau concentration (TC, in pg/ml). Note the faster than linear dependence.
Note that these results (by [18]) are based only on three average measurements, and therefore should be used only as a crude estimate, if at all. However, the mere positive correlation between the SD and these levels, indicates that the Glymphatic cleaning speed can be the important factor in seizure termination, and that the SD can be looked upon as an indication of the damage.
Discussion
Epileptic seizure duration is looked at from a new perspective, relating it to the performance of the Glymphatic system in the brain. This approach can have implications both for understanding the phenomena involved and for possible clinical treatments.
To our knowledge, there are no direct measurements of the dependence of seizure duration as a function of the speed of the Glymphatic system. Recently an associated measurement has appeared [19] in which seizure durations were correlated with the observed capacities (numbers and volumes) of young epilepsy patients' perivascular spaces (so called Virchow-Robin spaces VRS). The VRS are a major part of the Glymphatic system. They appear as tubular spaces around blood vessels in the brain which are filled with liquid (cerebrospinal fluid, CSF), and it is through these VRS that the fluid flows when the Glymphatic system is operating. The authors of [19] found positive relations between seizure durations and both the number of and the volume of the VRS (see Fig. 6 of [19]). There is no published data on connections between these VRS features and either the quality of the Glymphatic system's performance or the CSF velocities during seizures which are studied here. Nevertheless, the authors also measured a decrease of ~50% of the VRS capacity both in volume and in numbers during 7 days following a seizure. This reduction could mean that the augmented capacity is really needed only in the time of the seizure, during which, according to our hypothesis [2-4], the VRS is dedicated to seizure termination. The positive correlation between VRS capacity and seizure duration can imply that longer seizures are harder to reduce and require higher volumes of CSF for the task, hence higher VRS capacities.
These VRS results can be related to the following argumentation: A vast speeding up of the Glymphatic system was measured after an occurrence of stroke [20]. According to [20], the mechanism of this rate is based on vasoconstriction, obtained in the case of stroke by a spreading depolarization or by a spreading ischemia. This vasoconstriction causes the VRS to upsurge, thereby forcing a CSF "rush" into them, thus speeding up the Glymphatic cleansing process. We want to argue that if such a vasoconstriction occurred during seizure, it could trigger a similar rapid Glymphatic system brain cleansing, leading to seizure termination, as presumed in [2-4]. Indeed, in epileptic rats, such fast vasoconstriction/hypoperfusion/hypoxic is observed (see Fig. 1 in [21]) during the first minutes of an ictal, with a much slower return to normal oxygen performance. Such a slow return to normalcy is observed in recovery from "A prolonged vasoconstriction/hypoperfusion/hypoxic event" which "follows self-terminating focal seizures" [22]. Unfortunately for us, experiments ([23-26]) measuring seizure vasoconstriction/ hypoperfusion, mostly postictal, do not provide the exact time, during the ictal, of the process inception, so that, at the moment, we are not able to further analyze this assumption.
The possible higher than linear dependence (quadratic, as assumed here, or other) of the seizure duration on both age and CSF substance levels (Figs. 1,2) indicates that, beyond a certain age (or a certain Glymphatic system deterioration), a rather small additional decline of the system can lead to larger and larger increases in seizure durations. Such untreated failures can possibly lead to a state of Status Epilepticus (seizure duration > 5mi) or even to epilepsia-partialis-continua (seizure duration ~years). And since the life expectency of people in the world increases, this can become an acute problem worldwide.
There is, therefore, an urgent clinical need to find a treatment for such Glymphatic system malfunctions. However, while reducing the system's operation is accessible (for example, in the case of edema, see e.g., [27]), increasing it is less available. Such an increase can possibly improve the condition of people with refractory epilepsy by reducing their seizure durations and could perhaps advance also treatments of neurodegenerative deceases. Since this issue is quite new, efforts to address it have only recently been attempted. We mention here three recent attempts. Thus, the use of L-3-n-butylphthalide (NBP) in mice models, "In the wild-type mice, both CSF tracer influx and perivascular drainage increased after NBP treatment compared with vehicle treatment. Moreover, NBP promoted the perivascular drainage of Aβ via increased cerebral pulsation" [28]. A new work [28] discusses treatments to improve sleep quality by increasing the Glymphatic flow. Such a treatment can possibly help also in reducing seizure durations. Ref. [29] mentions that " In rodents, dexmedetomidine and xylazine (a2-noradrenergic autoreceptor agonists) cause a reduction in central noradrenergic output and increase glymphatic flow" [30, 31]. Other "non-pharmacological treatments have been proposed, including adjusting body posture and modulation of respiration and other integrative health approaches, although these approaches are still in the early validation stages" (see Refs. in [29]). And "exploring new non-pharmacological strategies, including the neuroprotective mechanisms of aerobic exercise, to enhance therapeutic treatment of AD (Alzheimer's decease, AR et al) are essential" [32].
Conclusion
In order to check the assumption that seizure durations increase when the Glymphatic system is impaired by ageing or during neurodegenerative malfunctions, published measured correlations between system-function's reductions and seizure-durations were analyzed and were indeed shown to increase under these conditions. Interestingly, this increase is possibly faster than linear with age and with CSF debris-contents. Such a state of affairs can be risky and shows a need for novel Glymphatic system repair mechanisms.
Declarations
Disclosure statement: Neither of the authors has any conflict of interest to disclose.
Acknowledgement: The authors would like to thank Dr. C. Howard for helpful discussions.
References
- Ghosh S et al. (2021). Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy, Biomedicines, 9:470.
Publisher | Google Scholor - Rabinovitch A, et al. (2019). A combined astrocyte G-lymphatic model of epilepsy initiation, maintenance and termination. Med Hypotheses, 133:109384.
Publisher | Google Scholor - Rabinovitch A, et al. (2020). Explaining recent postictal epilepsy EEG results by the G-lymphatic clearance hypothesis. Med Hypotheses, 137:109600.
Publisher | Google Scholor - Rabinovitch A, et al. (2021). Origin of post-ictal and post-anesthesia adverse effects and possibly of SUDEP. Med Hypotheses, 151:110591.
Publisher | Google Scholor - Tarasoff-Conway JM, Carare RO, Osorio RS et al. (2015). Clearance systems in the brain—implications for Alzheimer disease, Nat Rev Neurol. 11:457-470.
Publisher | Google Scholor - Hubbard J.A, J.I. Szu and D.K. Binder. (2018). The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull, 136:118-129.
Publisher | Google Scholor - Scharfman H.E and D.K. Binder. (2013). Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochemistry international, 63(7):702-711.
Publisher | Google Scholor - Binder D.K, et al. (2006). Increased seizure duration in mice lacking aquaporin-4 water channels. Acta Neurochir Suppl, 96:389-392.
Publisher | Google Scholor - Binder D.K, et al. (2006). Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia, 53(6):631-636.
Publisher | Google Scholor - Liu K, et al. (2021). Attenuation of cerebral edema facilitates recovery of glymphatic system function after status epilepticus. JCI Insight, 6(17).
Publisher | Google Scholor - Christensen J, Wright DK, Yamakawa GR, et al. (2020). Repetitive Mild Traumatic Brain Injury Alters Glymphatic Clearance Rates in Limbic Structures of Adolescent Female Rats, Scientific Reports, 10:6254.
Publisher | Google Scholor - Ghadiri T, Gorji A, Vakilzadeh G, et al. (2020). Neuronal injury and death following focal mild brain injury: The role of network excitability and seizure, Iran J Basic Med Sci, 23:63-70.
Publisher | Google Scholor - Komoltsev IG, Frankevich SO, Shirobokova NI, et al. (2021). Neuroinflammation and Neuronal Loss in the Hippocampus Are Associated with Immediate Posttraumatic Seizures and Corticosterone Elevation in Rats, Int. J. Mol. Sci, 22:5883.
Publisher | Google Scholor - Li Y, et al. (2021). Three-dimensional assessment of brain arterial compliance: Technical development, comparison with aortic pulse wave velocity, and age effect. Magn Reson Med, 86(4):1917-1928.
Publisher | Google Scholor - Kress B.T, et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann Neurol, 76(6):845-861.
Publisher | Google Scholor - Kaufmann E, et al. (2020). Who seizes longest? Impact of clinical and demographic factors. Epilepsia, 61(7):1376-1385.
Publisher | Google Scholor - Gugger J.J, et al. (2020). New-onset refractory status epilepticus: A retrospective cohort study. Seizure, 74:41-48.
Publisher | Google Scholor - Tumani H, et al. (2015). Effect of epileptic seizures on the cerebrospinal fluid--A systematic retrospective analysis. Epilepsy Res, 114:23-31.
Publisher | Google Scholor - Salimeen M.S.A, et al. (2021). Exploring Variances of White Matter Integrity and the Glymphatic System in Simple Febrile Seizures and Epilepsy. Front Neurol, 12:595647.
Publisher | Google Scholor - Mestre H, Ting Du T, Sweeney AM, et al. (2020). Cerebrospinal fluid influx drives acute tissue swelling, Science, 367:6483.
Publisher | Google Scholor - Tran CHT, George AG, Teskey GC, et al. (2020). Seizures elevate gliovascular unit Ca2+ and cause sustained vasoconstriction, JCI Insight, 5(19):136469.
Publisher | Google Scholor - Gom RC, Bhatt D, Villa BR, et al. (2021). The ketogenic diet raises brain oxygen levels, attenuates postictal hypoxia, and protects against learning impairments, Neurobiology of Disease 154:105335,
Publisher | Google Scholor - Farrell JS, Gaxiola-Valdez I, Wolff MD, et al. (2016). Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. eLife 5.
Publisher | Google Scholor - Gaxiola-Valdez I, Singh S, Perera T, et al. (2017). Seizure onset zone localization using postictal hypoperfusion detected by arterial spin labelling MRI. Brain 140:2895-2911.
Publisher | Google Scholor - Li E, d’Esterre CD. (2020). Gaxiola tissue swelling, Science. 367:6483.
Publisher | Google Scholor - Valdez I, et al. (2019). CT perfusion measurement of postictal hypoperfusion: localization of the seizure onset zone and patterns of spread. Neuroradiology, 61:991-1010.
Publisher | Google Scholor - Ji C, et al. (2021). The role of glymphatic system in the cerebral edema formation after ischemic stroke. Exp Neurol, 340:113685.
Publisher | Google Scholor - Zhang B, et al. (2021). L-3-n-Butylphthalide Effectively Improves the Glymphatic Clearance and Reduce Amyloid-β Deposition in Alzheimer's Transgenic Mice. J Mol Neurosci, 71(6):1266-1274.
Publisher | Google Scholor - Piantino JA, Iliff JJ, and Lim MM. (2022). The Bidirectional Link Between Sleep Disturbances and Traumatic Brain Injury Symptoms: A Role for Glymphatic Dysfunction? Biological Psychiatry, 91:478-487.
Publisher | Google Scholor - Benveniste H, Lee H, Ding F, et al. (2017). Anesthesia with dexmedetomidine and low dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared to high dose isoflurane, Anesthesiology, 127(6):976-988.
Publisher | Google Scholor - Hablitz LM, Vinitsky HS, Sun Q, et al. (2019). Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia Sci.
Publisher | Google Scholor - Yin M, et al. (2018). Astroglial water channel aquaporin 4-mediated glymphatic clearance function: A determined factor for time-sensitive treatment of aerobic exercise in patients with Alzheimer's disease. Med Hypotheses, 119:18-21.
Publisher | Google Scholor