Case Report
What Can We Learn About Deep Learning? An Orthopedic Systematic Review
Orthopedics and Traumatology, Spanish Hospital, Mexico.
*Corresponding Author: Ramón González Pola,Orthopedics and Traumatology, Spanish Hospital, Mexico.
Citation: Ramón G. Pola. (2024). What can we learn about Deep Learning? an orthopedic systematic review. Journal of Surgical Case Reports and Reviews. BioRes Scientia Publishers. 3(1):1-7. DOI: 10.59657/2993-1126.brs.24.020
Copyright: © 2024 Ramón González Pola, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 03, 2024 | Accepted: February 22, 2024 | Published: February 24, 2024
Abstract
Introduction: Artificial intelligence and machine learning in orthopedic surgery has gained mass interest over the last years. In prior studies, researchers have demonstrated that machine learning in orthopedics can be used for different applications, from radiographic assessment to bone tumor diagnosis. As time goes on, the utility of artificial intelligence and machine learning algorithms, such as deep learning, continues to grow and expand in orthopedic surgery. The purpose of this review is to provide an analysis of the current literature for AI and deep learning tools in order to identify the most commonly use application in risk assessment, outcomes assessment, imaging, and basic science fields.
Method: Searches were conducted in PubMed, EMBASE and Google scholar up to October 31st, 2023. We identified 717 studies, of which 595 were included in the systematic review. 282 studies about radiographic assessment, 97 about spine-oriented surgery, 102 about basic science application, 79 about outcomes assessment and 35 about basic AI orthopedic education were included for review. Peer-reviewed studies that reported on the accuracy of DL algorithms to identify pathology using medical imaging were included. Primary outcomes were measures of diagnostic accuracy, study design and reporting standards in the literature. Estimates were pooled using random-effects meta-analysis.
Results: 53 different imageology measures for radiographic aspects were identified. 185 different machine learning (ML) algorithms were used, being the convolutional neural network architecture the most common one (63%). To improve diagnostic accuracy and speed were the most commonly uses (72%).
Conclusion: Heterogeneity was high between studies and extensive variation in methodology, terminology and outcome measures was noted. This can lead to an overestimation of the diagnostic accuracy of DL algorithms on medical imaging. There is an immediate need for the development of artificial intelligence-specific guidelines, in order to provide guidance around key issues in this field.
Keywords: deep learning; artificial intelligence; orthopedics; convolutional network; imageology
Introduction
Deep learning (DL), a subset of machine learning and artificial intelligence (AI), has made significant advances in the field of healthcare, particularly orthopedic surgery. By comprehending complex algorithms, deep learning has the potential to revolutionize diagnoses, treatment plans, prediction of surgical outcomes, and even surgical procedures.
Advancements in Deep Learning in Orthopedics
Preoperative Planning
Deep learning algorithms have been successful in preoperative planning, particularly in predicting outcomes and complications. It also helps in selecting the most appropriate surgical approach [1].
Image Interpretation
Deep learning algorithms have been developed for image interpretation and have achieved expert-level accuracy in diagnosing different orthopedic conditions through MRI, X-ray, or CT scan readings [2].
Prosthesis Design
AI can aid in the customization of prosthetics, leading to a better fit and increased functionality for individual patients [3].
Robotic Orthopedic Surgery
Artificial intelligence-based robotics systems have gained ground in orthopedic surgery such as hip and knee replacements, leading to greater precision and potentially better patient outcomes [4].
Methods
This systematic review was carried out following the procedures highlighted in the 'PRISMA-DTA' extension for diagnostic validity studies, as well as a systematic review guideline [5].
Selection Criteria
We sought studies that reveal the diagnostic accuracy and uses of DL algorithms in identifying pathology or diseases of orthopaedic interest. The primary objective was identifying type of studies and range of diagnostic accuracy metrics. Secondary objectives encompassed study design and the quality of reporting.
Data Collection and Searches
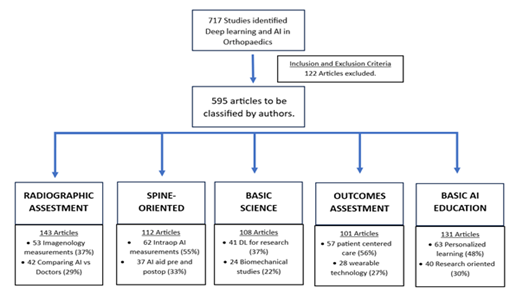
Electronic literature searches in Pubmed, EMBASE and Google scholar were conducted up to October 31st, 2023. Search terms consisted of MESH terms and all-field search expressions for “orthopaedic deep learning” and “neural networks” (examples are DL, convolutional networks, etc.) and specific termas as “imaging” (such as magnetic resonance, computed tomography, ultrasound, or X-ray) and final results as “diagnostic accuracy metrics” (sensitivity and specificity). We identified 717 studies, of which 595 were included in the systematic review. 282 studies about radiographic assessment, 97 about spine-oriented surgery, 102 about basic science application, 79 about outcomes assessment and 35 about basic AI orthopedic education were included for review. Peer-reviewed studies that reported on the accuracy of DL algorithms to identify pathology using medical imaging were included. Primary outcomes were measures of diagnostic accuracy, study design and reporting standards in the literature. Estimates were pooled using random-effects meta-analysis (Fig. 1)
Figure 1: Study explanation and key oriented classification by authors. DL, Deep Learning. AI, Artificial Intelligence
Inclusion Criteria
Eligible studies included those evaluating the diagnostic accuracy of a DL algorithm on orthopaedic field in general and in subspecialties. Only studies that reported either diagnostic accuracy raw data or sensitivity, specificity or accuracy data were incorporated in the systematic review. No restrictions were imposed on the date range, and the most recent search took place in November 2023.
Exclusion Criteria
Articles not written in English were excluded. Abstracts, conference articles, pre-prints, reviews, and meta-analyses were not considered, as the aim of this review was to assess the methodology, reporting standards, and quality of primary research studies published in peer-reviewed journals.
Study Selection
The study selection process entailed an initial review by two different reviewers of all titles and abstracts. This was followed by a second review, by the third and fourth author of the full text articles. This ensured that the remaining articles meet the inclusion criteria.53 different imagenology measures for radiographic aspects were identified. 185 different machine learning (ML) algorithms were used, being the convolutional neural network architecture the most common one (63%). To improve diagnostic accuracy and speed were the most commonly uses (72%).
Review
This review focuses on five principal applications of machine and deep learning methodologies in Orthopaedics. The key areas of focus include: (1) Evaluation through Radiography, (2) Spine-focused surgical interventions, (3) Fundamental orthopaedic science, (4) Evaluation of outcomes in general orthopaedic surgeries, and (5) Fundamental AI instruction for orthopaedic surgeons. Given the expansive use of AI in Orthopaedic surgery, it is impractical to cover every facet in this review. Instead, we have opted to concentrate on a select few applications that we believe encapsulate and effectively summarize the enhancements ushered in by AI and Deep Learning.
Application 1: Radiographic assessment
DL an AI technology possesses the ability to analyze and interpret complex medical imaging, a capability that paves the way for more accurate diagnoses and patient-specific treatments [6]. Studies show that DL can process X-rays, CT scans, and MRI images effectively, to identify specific pathologies, such as fractures, osteoarthritis, bone tumors, deformities, and degenerative diseases [7.8]. It proves instrumental in accurately determining the progression of diseases, planning surgeries, and predicting post-operative outcomes by assessing radiographic parameters [9,10]. Moreover, it provides the potential to analyze biomechanical data and perform automized measurements, which were traditionally time-consuming [11,12].
In addition to improving diagnostic accuracy, its application also brings forth the potential for expedited and optimized medical workflows. With its ability to swiftly analyze large volumes of radiographic data, clinicians can rapidly plan treatment trajectories and focus intensively on critical patient care [13,14]. Moreover, DL models can easily facilitate the detection of subtle or complex patterns in imaging that may be challenging even for experienced radiologists [15]. This could enhance early detection and intervention for debilitating conditions, eventually improving patient outcomes. Additionally, DL can serve as an educational tool to aid trainees and junior clinicians in enhancing their understanding and interpretation of orthopaedic imaging [16]. Certainly, there remain key challenges to be considered such as ensuring the ethical use of patient data, maintaining transparency in AI decision-making processes, and ironing out interoperability issues among varying healthcare systems. However, despite its promising advantages, DL implementation in orthopaedics must be approached with caution due to its dependency on the quality and size of the dataset used for training. Hence, more research is necessary to overcome the potential limitations and standardize the use of DL in orthopaedic radiographic assessment in pursuit of precision medicine. Overcoming these obstacles will be crucial to fully realize the potential benefits and advancements DL offers to the field of orthopaedic radiographic assessment.
Application 2: Spine-oriented surgery
DL has presented significant advancements particularly in spine-oriented surgical procedures. Researchers and practitioners are actively exploring and utilizing DL to assist in areas ranging from diagnosis to surgical planning and prognosis prediction [17]. Specific examples include the detection and classification of spinal disorders such as scoliosis, spondylolisthesis, and intervertebral disc degeneration from radiographic images [18-20]. DL models have also been designed to assist in surgical planning by predicting pedicle screw placements, reducing chances of iatrogenic injuries [21-23]. On top of diagnostics and treatment planning, DL's role in enhancing intraoperative guidance and postoperative rehabilitation in spinal surgeries also demonstrates significant promise. With its advanced pattern recognition capabilities, DL can potentially provide real-time feedback during spinal surgeries thereby increasing the precision and safety of procedures.(2) For instance, DL algorithms can aid in identifying anatomic landmarks, enhancing visualization, and assisting tool navigation, subsequently reducing the risk of medical errors [24-26]. As for postoperative care, DL algorithms may enable personalized rehab plans by predicting individual recovery trajectories, thereby expediting patient's return-to-function and improving overall quality of life [27-29].
Nevertheless, complete adoption of DL in spine-related orthopaedics practices remains a challenging task. Despite the considerable progress, issues like data privacy, algorithmic transparency, and the need for interdisciplinary collaboration between data scientists and clinicians need to be adequately addressed. As DL's potential continues to be explored, more comprehensive guidelines governing its application in spinal surgeries are yet to be established to leverage its benefits effectively.
Application 3: Basic science
In the realm of basic science in orthopaedics, the incorporation of DL has amplified the possibility of understanding the intricate details and processes at a molecular, cellular, and tissue level. Established DL algorithms can supplement traditional research methods by analyzing complex patterns and interrelationships among numerous biological variables. They can handle vast multi-dimensional datasets, aiding in biomarker discovery, modeling disease progression, and identifying potential therapeutic targets [25].
For instance, DL can aid genomic research in orthopaedics, where it could be used to understand patterns of gene expression pertaining to bone growth, learning the underlying genetic causes behind orthopaedic diseases, or predicting patient responses to treatments on a genetic level. (30,31) Similarly, at the tissue level, DL-powered image analysis can provide a more in-depth analysis of histopathological samples, potentially enabling early detection of degenerative diseases and comprehensive assessment of cellular responses to different interventions [32,33].
Moreover, DL can complement biomechanical studies by facilitating the analysis of complex motion patterns and forces, providing deeper insights into the effects of various physical activities on the musculoskeletal system [34,35].
Application 4: Outcomes assessment
DL is proving to be a critical tool in orthopaedics for assessing patient outcomes. By analyzing vast sets of patient data, DL models can predict patient-specific outcomes following various interventions, thereby enabling a more personalized approach to patient care. It can also help identify factors contributing to optimal and sub-optimal outcomes, thereby driving improvement in therapeutic strategies [36-38]. For instance, DL algorithms can be used to mine data to uncover complex, non-intuitive correlations between patient characteristics, intervention details, and postoperative outcomes. Such correlations could be used to predict future patient recovery patterns, incidence of complications, or even the likelihood of rehospitalization, allowing clinicians to effectively plan and adjust treatments and follow-up schedules [39,40].
Furthermore, DL can be used to analyze real-time patient data collected through wearable technology, to provide a comprehensive understanding of patient function and recovery in real-world settings. By mining this rich data source, DL can potentially uncover non-traditional metrics of orthopaedic outcomes, which may prove relevant in achieving patient-centered care [41].
For instance, DL can decipher patterns in movement data to accurately assess rehabilitation progress following joint replacement or reconstructive surgeries. Likewise, it can provide insights into the adherence of patients to prescribed rehabilitation protocols and enable tailored interventions for improving patient compliance [37,42].
While this fusion of DL and wearable technology opens a new dimension in outcomes research in orthopaedics, key issues including patient acceptance, data privacy, and data validity need careful deliberation. Regardless, with continuous refinement and stringent validation practices, the integration of DL can undoubtedly drive a paradigm shift in outcomes assessment, taking orthopaedic care a step closer to the ultimate aim of optimized, patient-centered care. As we move forward, the effective application of DL can significantly enhance the quality and efficacy of orthopaedic care by ensuring the interventions align with individual patient's expectations and desired outcomes.
Application 5: Basic AI education for orthopaedic surgeons
DL, being a subset of AI, has been progressively weaving its way into medical education, including orthopaedics. With its capacity to process vast and complex data sets, DL can contribute significantly to bolstering both theoretical knowledge and practical skills among medical students and professionals in the orthopaedic field. For example, DL algorithms can assist in creating immersive, personalized learning experiences by identifying individual learning patterns and offering tailored educational content [43,44].
Beyond direct educational functions, DL can serve as an integral tool in fostering research literacy among medical students and professionals in orthopaedics. With increasing focus on evidence-based practice in the current healthcare landscape, having skills to conceive, conduct, and interpret research is now considered equally important as clinical skills. DL can equip learners to handle big data analytic tasks, analyze complex research data, and more accurately interpret the findings of a study [44,45], For instance, DL can be used as a pedagogical tool to teach foundational concepts of bioinformatics relevant to orthopaedics, such as genomic studies in osteoarthritis or proteomic data analysis in bone healing.
The introduction of AI-driven research concepts in medical education faces several hurdles, including the need for curricular adaptations, lack of skilled educators, and the risk of over-reliance on algorithms at the expense of contextual decision-making. Despite these challenges, the incorporation of DL in medical education is a necessary advancement that can mold a generation of medical professionals competent in both clinical and research domains of orthopaedics.
Importantly, familiarizing future orthopaedic practitioners with AI concepts and its uses also becomes fundamental to nurture a workforce that is adept at leveraging DL in clinical problem-solving.
Limitations and Challenges
Certain challenges surfaced in the literature under review include the 'black box' nature of neural network models, which makes it difficult for users to comprehend and explain the basis of the outcomes produced. Moreover, there are hindrances to the applicability and implementation of these models across various healthcare institutions due to variations in standardization, procedures, and data set parameters availability. Even when data set parameters are available, their values may differ because of varying demographics and geographical locations. These limitations may influence the overall accuracy and predictive potency of the Artificial Intelligence model and could potentially pose a risk to patients if implemented indiscriminately. Thus, further investigations and trials are indispensable to confirm the efficiency of the developed model.
Despite its impressive potential, it's crucial to note that the success of DL application primarily relies on the quality, size, and diversity of data used for model training. The full integration of DL into surgery, therefore, requires more studies, robust data handling techniques, and stringent validation measures to ensure accurate and reliable utilization in clinical practice.
Future Considerations
Despite considerable progress, the use of deep learning in orthopedic surgery is still in its early stages with large untapped potential. There are several areas where deep learning still can add significant value:
Postoperative rehabilitation: Using deep learning algorithms to predict and plan the most effective rehabilitation regime tailored to individual patients.
IoT Integration: Combining deep learning algorithms with IoT devices such as wearables could provide real-time feedback, enable monitoring, and influence treatment and rehabilitation plans.
Virtual Assistants: Deep learning enables virtual assistants for physicians, doing mundane work (like paperwork), which could save time for patient-care.
Conclusion
The advancements in deep learning offer immeasurable opportunities in orthopedic surgery, from aiding in diagnosing complex conditions to performing surgical procedures with greater precision than ever before. However, integrating deep learning algorithms into orthopedics is met with numerous challenges including ethical concerns around data privacy, as well as regulating the use of AI in clinical environments. More research is needed to validate and refine these tools and strategies so they can be safely and effectively incorporated into practice. Heterogeneity was high between studies and extensive variation in methodology, terminology and outcome measures was noted. This can lead to an overestimation of the diagnostic accuracy of DL algorithms on medical imaging. There is an immediate need for the development of artificial intelligence-specific guidelines, in order to provide guidance around key issues in this field. DL is a burgeoning field with an immense potential across all areas of healthcare, particularly radiology. The present systematic review and meta-analysis scrutinized the quality of existing literature and provided summarized diagnostic accuracy for DL techniques. Despite the high diagnostic accuracy intimating the efficacy of DL at the moment, it is vital to interpret these findings considering the poor design, conduct, and reporting of studies, which could result in bias and overestimation of the power of these algorithms. Improving DL application necessitates standardized instructions related to study design and reporting, which can further clarify its clinical utility down the line.
References
- S taartjes VE, De Wispelaere MP, Vandertop WP, Schröder ML. (2019). Deep learning-based preoperative predictive analytics for patient-reported outcomes following lumbar discectomy: feasibility of center-specific modeling. The Spine Journal. 19(5):853-861.
Publisher | Google Scholor - Lalehzarian SP, Gowd AK, Liu JN. (2021). Machine learning in orthopaedic surgery, WJO. 12(9):685-699.
Publisher | Google Scholor - Kang YJ, Yoo JI, Cha YH, Park CH, Kim JT. (2020). Machine learning–based identification of hip arthroplasty designs. Journal of Orthopaedic Translation, 21:13-17.
Publisher | Google Scholor - Denecke K, Baudoin CR. (2022). A Review of Artificial Intelligence and Robotics in Transformed Health Ecosystems. Front Med, 9:795957.
Publisher | Google Scholor - McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, and the PRISMA-DTA Group, et al. (2018). Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 319(4):388.
Publisher | Google Scholor - Pinto-Coelho L. (2023). How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering, 10(12):1435.
Publisher | Google Scholor - Berg HE. (2017). Will intelligent machine learning revolutionize orthopedic imaging? Acta Orthopaedica, 88(6):577-577.
Publisher | Google Scholor - Pankhania M. (2020). Artificial Intelligence in Musculoskeletal Radiology. Past, Present, and Future. IJMSR. 2:89-96.
Publisher | Google Scholor - Meena T, Roy S. (2022). Bone Fracture Detection Using Deep Supervised Learning from Radiological Images: A Paradigm Shift. Diagnostics, 12(10):2420.
Publisher | Google Scholor - Zhang X, Yang Y, Shen YW, Zhang KR, Jiang Z kun, Ma LT, et al. (2022). Diagnostic accuracy and potential covariates of artificial intelligence for diagnosing orthopedic fractures: a systematic literature review and meta-analysis. Eur Radiol, 32(10):7196–216.
Publisher | Google Scholor - Bousson V, Benoist N, Guetat P, Attané G, Salvat C, Perronne L. (2023). Application of artificial intelligence to imaging interpretations in the musculoskeletal area: Where are we? Where are we going? Joint Bone Spine, 90(1):105493.
Publisher | Google Scholor - Moon KR, Lee BD, Lee MS. (2023). A deep learning approach for fully automated measurements of lower extremity alignment in radiographic images. Sci Rep, 13(1):14692.
Publisher | Google Scholor - Yang S, Yin B, Cao W, Feng C, Fan G, He S. (2020). Diagnostic accuracy of deep learning in orthopaedic fractures: a systematic review and meta-analysis. Clinical Radiology. 75(9): 713.e17-713.e28.
Publisher | Google Scholor - Lee J, Chung SW. (2022). Deep Learning for Orthopedic Disease Based on Medical Image Analysis: Present and Future. Applied Sciences, 12(2):681.
Publisher | Google Scholor - Liu Y, Liu W, Chen H, Xie S, Wang C, Liang T, et al. (2023). Artificial intelligence versus radiologist in the accuracy of fracture detection based on computed tomography images: a multi-dimensional, multi-region analysis. Quant Imaging Med Surg, 13(10):6424-6433.
Publisher | Google Scholor - Adams SJ, Henderson RDE, Yi X, Babyn P. (2021). Artificial Intelligence Solutions for Analysis of X-ray Images. Can Assoc Radiol J, 72(1):60-72.
Publisher | Google Scholor - Malik AT, Khan SN. (2019). Predictive modeling in spine surgery. Ann Transl Med, 7(S5):S173–S173.
Publisher | Google Scholor - Fraiwan M, Audat Z, Fraiwan L, Manasreh T. (2022). Using deep transfer learning to detect scoliosis and spondylolisthesis from x-ray images. Gadekallu TR, editor. PLoS ONE, 17(5):e0267851.
Publisher | Google Scholor - Trinh GM, Shao HC, Hsieh KLC, Lee CY, Liu HW, Lai CW, et al. (2022). Detection of Lumbar Spondylolisthesis from X-ray Images Using Deep Learning Network. JCM, 11(18):5450.
Publisher | Google Scholor - Masood RF, Taj IA, Khan MB, Qureshi MA, Hassan T. (2022). Deep Learning based Vertebral Body Segmentation with Extraction of Spinal Measurements and Disorder Disease Classification. Biomedical Signal Processing and Control, 71:103230.
Publisher | Google Scholor - Fan X, Zhu Q, Tu P, Joskowicz L, Chen X. (2023). A review of advances in image-guided orthopedic surgery. Phys Med Biol, 68(2):02TR01.
Publisher | Google Scholor - Charles YP, Lamas V, Ntilikina Y. (2023). Artificial intelligence and treatment algorithms in spine surgery. Orthopaedics & Traumatology: Surgery & Research, 109(1):103456.
Publisher | Google Scholor - Rasouli JJ, Shao J, Neifert S, Gibbs WN, Habboub G, Steinmetz MP, et al. (2021). Artificial Intelligence and Robotics in Spine Surgery. Global Spine Journal, 11(4):556-64.
Publisher | Google Scholor - Ma L, Fan Z, Ning G, Zhang X, Liao H. (2018). 3D Visualization and Augmented Reality for Orthopedics. In: Zheng G, Tian W, Zhuang X, editors. Intelligent Orthopaedics [Internet]. Singapore: Springer Singapore, 193-205.
Publisher | Google Scholor - Zheng G, Nolte LP. (2018). Computer-Aided Orthopaedic Surgery: State-of-the-Art and Future Perspectives. In: Zheng G, Tian W, Zhuangm X, editors. Intelligent Orthopaedics [Internet]. Singapore: Springer Singapore, 1-20.
Publisher | Google Scholor - Chen H. (2023). Application progress of artificial intelligence and augmented reality in orthopaedic arthroscopy surgery. J Orthop Surg Res, 18(1):775.
Publisher | Google Scholor - Ogink PT, Groot OQ, Karhade AV, Bongers MER, Oner FC, Verlaan JJ, et al. (2021). Wide range of applications for machine-learning prediction models in orthopedic surgical outcome: a systematic review. Acta Orthopaedica, 92(5):526-531.
Publisher | Google Scholor - McDonnell JM, Evans SR, McCarthy L, Temperley H, Waters C, Ahern D, et al. (2021). The diagnostic and prognostic value of artificial intelligence and artificial neural networks in spinal surgery: a narrative review. The Bone & Joint Journal, 103-B(9):1442-1448.
Publisher | Google Scholor - Hornung AL, Hornung CM, Mallow GM, Barajas JN, Rush A, Sayari AJ, et al. (2022). Artificial intelligence in spine care: current applications and future utility. Eur Spine J. (8):2057-2081.
Publisher | Google Scholor - Tan J, Shi M, Li B, Liu Y, Luo S, Cheng X. (2023). Role of arachidonic acid metabolism in intervertebral disc degeneration: identification of potential biomarkers and therapeutic targets via multi-omics analysis and artificial intelligence strategies. Lipids Health Dis, 22(1):204.
Publisher | Google Scholor - Anastasio AT, Zinger BS, Anastasio TJ. (2022). A novel application of neural networks to identify potentially effective combinations of biologic factors for enhancement of bone fusion/repair. Srinivasan K, editor. PLoS ONE, 17(11):e0276562.
Publisher | Google Scholor - Yang L, Coleman MC, Hines MR, Kluz PN, Brouillette MJ, Goetz JE. (2019). Deep Learning for Chondrocyte Identification in Automated Histological Analysis of Articular Cartilage. Iowa Orthop J, 39(2):1-8.
Publisher | Google Scholor - Melgoza IP, Chenna SS, Tessier S, Zhang Y, Tang SY, Ohnishi T, et al. (2021). Development of a standardized histopathology scoring system using machine learning algorithms for intervertebral disc degeneration in the mouse model—An ORS spine section initiative. JOR Spine, 4(2):e1164.
Publisher | Google Scholor - Konnaris MA, Brendel M, Fontana MA, Otero M, Ivashkiv LB, Wang F, et al. (2022). Computational pathology for musculoskeletal conditions using machine learning: advances, trends, and challenges. Arthritis Res Ther, 24(1):68.
Publisher | Google Scholor - Zhu W, Zhang X, Fang S, Wang B, Zhu C. Deep Learning Improves Osteonecrosis Prediction of Femoral Head After Internal Fixation Using Hybrid Patient and Radiograph Variables. Front Med, 7:573522.
Publisher | Google Scholor - Kunze KN, Krivicich LM, Clapp IM, Bodendorfer BM, Nwachukwu BU, Chahla J, et al. (2022). Machine Learning Algorithms Predict Achievement of Clinically Significant Outcomes After Orthopaedic Surgery: A Systematic Review. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 38(6):2090-105.
Publisher | Google Scholor - Jeyaraman M, Ratna HVK, Jeyaraman N, Venkatesan A, Ramasubramanian S, Yadav S. (2023). Leveraging Artificial Intelligence and Machine Learning in Regenerative Orthopedics: A Paradigm Shift in Patient Care.
Publisher | Google Scholor - Clement ND, Clement R, Clement A. (2024). Predicting Functional Outcomes of Total Hip Arthroplasty Using Machine Learning: A Systematic Review. JCM, 13(2):603.
Publisher | Google Scholor - Poduval M, Ghose A, Manchanda S, Bagaria V, Sinha A. (2020). Artificial Intelligence and Machine Learning: A New Disruptive Force in Orthopaedics., IJOO. 54(2):109-22.
Publisher | Google Scholor - Ahmed Z, Mohamed K, Zeeshan S, Dong X. (2020). Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database, baaa010.
Publisher | Google Scholor - Misic D, Zdravkovic M. (2022). Overview of AI-Based Approaches to Remote Monitoring and Assistance in Orthopedic Rehabilitation. In: Canciglieri Junior O, Trajanovic MD, editors. Personalized Orthopedics [Internet]. Cham: Springer International Publishing, 535-553.
Publisher | Google Scholor - Sumner J, Lim HW, Chong LS, Bundele A, Mukhopadhyay A, Kayambu G. (2023). Artificial intelligence in physical rehabilitation: A systematic review. Artificial Intelligence in Medicine, 146:102693.
Publisher | Google Scholor - Pears M, Konstantinidis S. The Future of Immersive Technology in Global Surgery Education. Indian J Surg, 84(S1):281-285.
Publisher | Google Scholor - Han ER, Yeo S, Kim MJ, Lee YH, Park KH, Roh H. (2019). Medical education trends for future physicians in the era of advanced technology and artificial intelligence: an integrative review. BMC Med Educ, 19(1):460.
Publisher | Google Scholor - Cheng CT, Chen CC, Fu CY, Chaou CH, Wu YT, Hsu CP, et al. (2020). Artificial intelligence-based education assists medical students’ interpretation of hip fracture. Insights Imaging, 11(1):119.
Publisher | Google Scholor