Research Article
Urate Crystal Induced Arthropathies-A Comparative Study Of 146 Patients with Gout
1Department of Pathology, Hospital of the Order of the brothers of St. John of God in Budapest, Hungary.
2Department of Rheumatology, St. Margaret Clinic, Budapest, Hungary.
*Corresponding Author: Miklos Bely, Department of Pathology, Hospital of the Order of the brothers of St. John of God in Budapest, Hungary.
Citation: Bely M, Apathy A. (2024). Urate Crystal Induced Arthropathies-A Comparative Study Of 146 Patients with Gout, International Journal of Clinical and Surgical Pathology, BioRes Scientia Publishers. 1(2):1-10. DOI: 10.59657/3067-0462.brs.24.009
Copyright: © 2024 Miklos Bely, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 05, 2024 | Accepted: August 26, 2024 | Published: September 02, 2024
Abstract
Gouty arthritis induced by MSU crystals (monosodium urate monohydrate [NaC5H3N4O3·H2O]) is one of the most important crystal induced arthritis. Identification of MSU crystals in tissue sections of gouty tophi or in synovial fluid is diagnostic. The diagnosis of gout may be difficult in case of atypical clinical symptoms and/or without demonstrable MSU crystals. The absence of MSU crystals does not rule out gout; the MSU crystals dissolve, and are not demonstrable in tissues fixed in aqueous formaldehyde solution and/or tissue sections stained with aqueous dyes. The non-staining technique of Bély and Apáthy’s is more effective, and the probability of identifying crystals is much higher in spite the aqueous formaldehyde fixation of tissue samples than with the classic stains and reactions.
Keywords: gout; monosodium urate crystals; arthritis
Introduction
Gout is characterized by crystal deposits of monomorphic monosodium urate monohydrate (MSU) [NaC5H3N4O3·H2O] and is generally considered as an independent metabolic entity of great clinical significance [1-3]. Surgical tissue specimens are traditionally fixed n 8% aqueous formaldehyde solution for microscopic examination. In most cases the MSU crystals dissolve in this aqueous fixative or in water containing dyes, and are not demonstrable in traditionally fixed and HE stained tissue sections. Occasionally, especially in deeper layers of tissue samples or in large amounts of MSU deposits, the MSU crystals can be preserved and remain demonstrable with HE or staining specific for MSU. McManus and Mowry (1960) concluded that in clinically known or suspected cases of gout-because of solubility of MSU in water-the surgical tissue specimens should be fixed in absolute ethyl alcohol [4]. According to our analytical studies, one of the main reasons of the loss of urate is the dehydration by acetone (before embedding in paraffin), but the most important is staining of nuclei with aqueous hematoxylin (Bély and Krutsay, 2013) [5]. HE stained tissue sections may appear negative for MSU crystals but these may be successfully demonstrated in unstained tissue sections by Bély and Apáthy’s non-staining technique (2013), especially in deeper layers of tissue samples [6-11]. The essence of this non-staining technique is avoidance of staining with water-based dyes, and viewing the unstained tissue sections with polarized light [6-11]. The clinical significance of this method is that not only more, but also other unknown crystals can be detected than with traditional staining procedures and histochemical reactions.
Objective
The authors demonstrate the histological characteristics of tophi (urate deposits) in conventionally processed tissue samples, furthermore illustrate the sensitivity of their non-staining technique.
Materials and Methods
Between 1985 and 2010 surgical specimens of 101855 patients were processed in the Department of Pathology of the National Institute of Rheumatology (ORFI) and of the Hospital of the Order of Brothers of Saint John of God (BIK). Among these, gout was diagnosed clinically in 146 (0.14 %) patients. Two hundred-eleven (211) paraffin embedded tissue blocks of 146 patients with gout, were available. The tissue samples were fixed in an 8% aqueous solution of formaldehyde at pH 7.6 for at least 24 hours at room temperature (20 Co) and embedded in paraffin. Serial tissue sections were examined without staining [6-11], with HE staining [12], as well as with special stains recommended in the literature. All slides were examined with the light microscope and under polarized light, respectively. For identification of MSU crystals Gömöri's methenamine-silver method [12,13] and the staining method of Schultz [14,15] were used. Possible deposits of amorphous calcium phosphate [Ca3(PO4)2] and/or calcium carbonate [CaCO3] were identified by Alizarin red S staining (specific for calcium) [4,16] and the von Kossa reaction (specific for phosphate and/or carbonate) [4,15]. For the description of non-staining” technique according to Bély and Apáthy (2013) for conventionally fixed tissue sections see the Appendix below.
Appendix - Bely and Apáthy’s “non-staining” technique [6-11].
- Tissue blocks of surgically removed specimens are fixed in 8% neutral buffered formalin (at pH 7.6 for >24 hours at 20 Co room temperature).
- Tissue blocks are dehydrated in ethyl alcohol, and are embedded in paraffin using acetone as well as xylene - 5 µm sections are cut.
- Prolonged deparaffinization (3-5 days) in a thermostat at 56°C (daily changing xylene).
- Chloroform – methanol I. (1:1) solution for 1 hour.
- Chloroform – methanol II. (1:1) solution for 1 hour or overnight.
- Dehydration in ethyl alcohol (two changes of 96% alcohol I-II. 30-30 min.), and using terpene xylene, as well as xylene, mounting in Canada balsam, cover slip.
Results
- In deparaffinized tissue sections of formaldehyde fixed and paraffin embedded surgical specimens-without staining with aqueous dyes-the MSU, CPPD and HA crystals furthermore the cholesterol crystals and crystalline lipids are preserved, and are well detectable with polarized light.
- In unstained sections MSU and CPPD crystals are more abundant than in sections stained with HE or with other staining.
- HA and cholesterol crystals or crystalline lipids cannot detect in conventional fixed tissue section stained with aqueous dyes.
The effectivity of the non-staining technique was characterized with Pearson's chi-squared (χ2) test comparing the prevalence of deposited crystals in unstained tissue sections, with HE or with other suited stains. The difference between two cohorts of samples was regarded “significant” at an alpha level of 0.05. Standard and unstained tissue sections were examined with a professional high-brightness (100-Watt) microscope (Olympus BX51); in selected cases, electron microscopy and electron diffraction were also performed (JEM 100CX).
Results
Demographics of patients with clinically diagnosed gout
Demographics of patients with clinically diagnosed gout are summarized in Table 1.
Table 1: Sex, mean age with SD and range (in years) of 146 patients with clinically diagnosed tophaceous gout.
| Clinical Diagnosis | Number of Patients (Tissue Samples) | Mean Age in Years at Surgery ± SD | Range (In Years) |
| Gout (MSU) | 146 (211) | 53.97±10.12 | 32-85 |
| Female | 14 (9) | 61.79±10.18 | 36-76 |
| Male | 132 (202) | 53.11±9.78 | 32-85 |
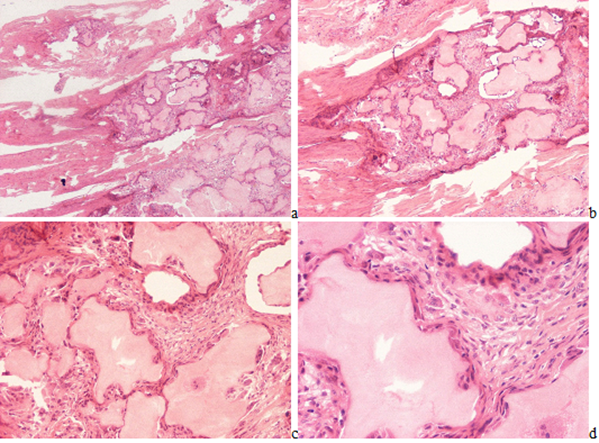
The ratio of males to females was 9.43 to 1. The mean age of females was higher (61.79 year) compared to the mean age of male (53.97 year); the difference was significant (p lessthan 0.0079). Microscopic characteristics of MSU (monosodium urate monohydrate [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3]) crystal deposits in patients with the clinical diagnosis of gout. Gouty tophi in HE stained tissue sections existed as irregular intra or extra articular eosinophilic formations surrounded by inflammatory cellular infiltrates and/or by a more or less organized cellular rim, later fibrotic zone, depending of the stage of the pathological process (Figure 1(a-d)).
Figure 1(a-d): Gouty arthritis (tophaceous gout), right hand, third finger, flexor tendon, MSU [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3] crystal deposits. Tissue samples were fixed in an 8% aqueous formaldehyde solution, stained with HE, and viewed with the light microscope. (a) x20, (b) same as (a) x40, (c) same as (a) x100, (d) same as (a) x200
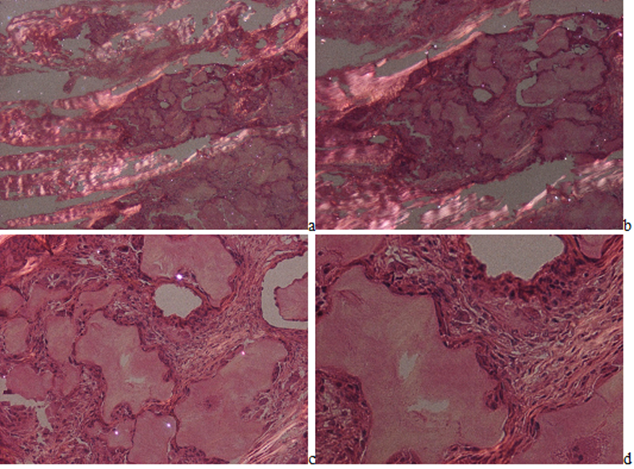
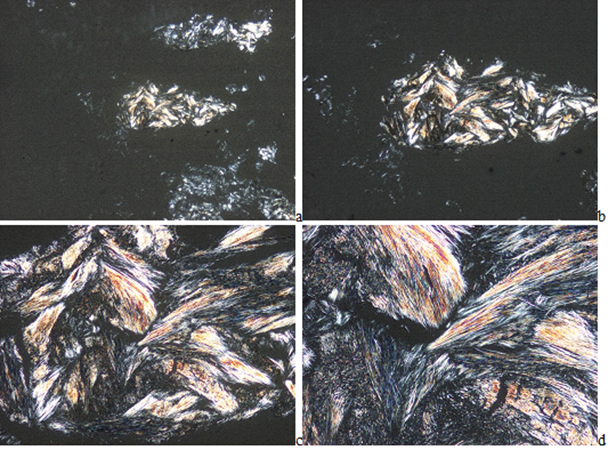
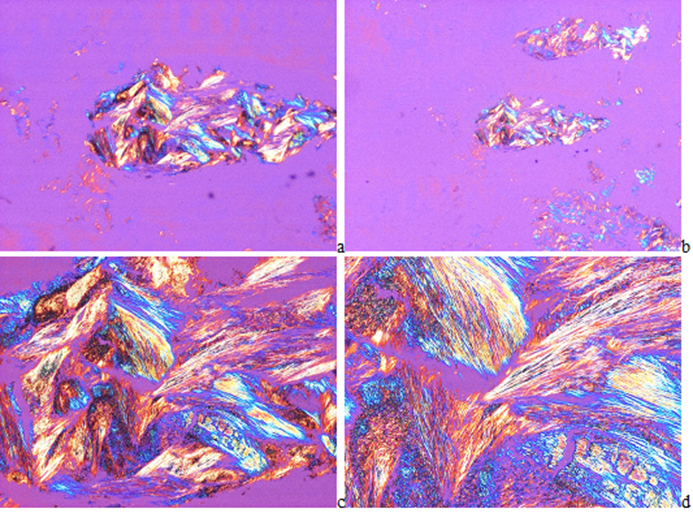
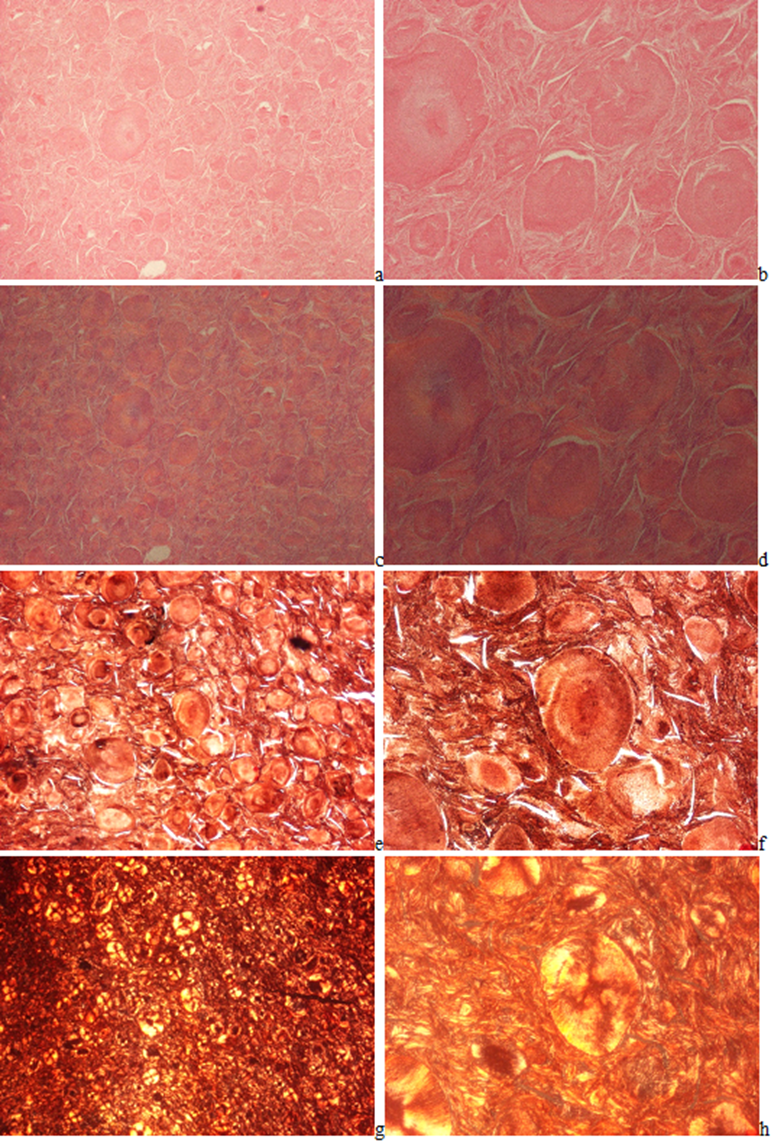
In HE stained tissue section of gouty tophi with a few crystals MSU cannot be detected under polarized light (Figure 2(a-d)), but in unstained sections they may remain and may be demonstrated (Figures 3-5(a-d)). In unstained sections viewed with the light microscope, great amounts of MSU crystals of swart natural color may retained, arranged in characteristic bundles, or sporadically as globules (Figure 3(a-d)). Viewed under polarized light the monomorphic needle-shaped crystals are arranged in characteristic bundles, and show intensive birefringence (Figure 4(a-d)). Using Red I compensator the birefringence is negative (Figure 5(a-d)).
Figure 2a-d: Gouty arthritis (tophaceous gout), right hand, third finger, flexor tendon, MSU [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3] crystal deposits. Tissue samples were fixed in an 8% aqueous formaldehyde solution, stained with HE, and viewed under polarized light. MSU crystals are not demonstrable.in characteristic tophi, they dissolved in the aqueous formaldehyde solution and/or during the water-based HE staining. The tophi are surrounded by birefringent dense collagen fibers or more cellular demarcating zone. (a) x20, (b) same as (a) x40, (c) same as (a) x100, (d) same as (a) x200
Figure 3(a-d): Gouty arthritis (tophaceous gout), right hand, third finger, flexor tendon, MSU [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3] crystal deposits. Tissue samples were fixed in an 8% aqueous formaldehyde solution, unstained section, viewed with the light microscope. MSU crystals of swart natural color arranged in characteristic bundles. (a) x20, (b) same as (a) x40, (c) same as (a) x100, (d) same as (a) x200
Figure 4(a-d): Gouty arthritis (tophaceous gout), right hand, third finger, flexor tendon, MSU [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3] crystal deposits. Tissue samples were fixed in an 8% aqueous formaldehyde solution, unstained section, viewed under polarized light. The needle-shaped crystals are arranged in characteristic bundles, and show a strong birefringence. (a) x20, (b) same as (a) x40, (c) same as (a) x100, (d) same as (a) x200
Figure 5(a-d): Gouty arthritis (tophaceous gout), right hand, third finger, flexor tendony, MSU [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3] crystal deposits. Tissue samples were fixed in an 8% aqueous formaldehyde solution, unstained section, viewed under polarized light using Red I compensator. The birefringence of the needle-shaped crystals is negative. (a) x20, (b) same as (a) x40, (c) same as (a) x100, (d) same as (a) x200
Typically, the MSU deposits in gouty tophi were not accompanied by amorphous calcium phosphate [Ca3(PO4)2] and/or calcium carbonate [CaCO3] deposits. In chronic tophi irritative MSU crystals were isolated by fibrotic, more or less cellular tissues.
Comparative Analysis of Conventional Methods and Non-Staining Technique
The comparative analysis of conventional methods and non-staining technique was performed on 105 of 211 serially sectioned tissue samples. In HE stained tissue sections MSU crystals were detected in 24 (22.86% of 105), and were not absent in 81 (77.14% of 105). In these 81 “urate negative” cases stained with HE, MSU crystals were demonstrable in 59 unstained tissue sections (72.84% of 81). With Gömöri’s methenamine silver method MSU crystals were found in 59 (56.19% of 105), and were not seen in 46 (43.81% of 105) tissue sections. In these 46 Gömöri negative tissue sections MSU crystals were seen in 24 with the non-staining technique (52.17% of 46). With the Schultz staining, MSU crystals were identified in 66 (62.86% of 105), and were not in 39 (37.14% of 105) tissue sections. In the 39 negative tissue section stained according to Schultz, MSU crystals were present in 17 unstained sections (43.59% of 39).
According to Schultz’s staining the MSU crystals and/or the not crystalline uric acid together were present n 81 tissue sections (77.14% of 105), and were not in 24 (22.86% of 105). According to Schultz’s staining the 24 negative MSU and/or not crystalline uric acid tissue sections (n=24) showed MSU positivity in 12 with non-staining technique (50.0% of 24) (the non-crystalline uric acid without birefringence is not visible in unstained sections under polarized light). In contrast with the classic stains and reactions the non-staining technic of Bély and Apáthy’s was more effective, MSU crystals were demonstrated in 83 (79.05% of 105) tissue sections, and were absent only in 22 (20.95% of 105). In the negative unstained sections MSU crystals were not found by HE, according to Schultz staining or by Gömöri methenamine-silver method.
Table 2 summarizes the prevalence of MSU crystals in tissue sections of patients with gout, stained by conventional staining’s and reaction in comparison with Bély and Apáthy’s “non-staining” technique, furthermore the level of significance (“p” values) between different staining’s and techniques.
Table 2: The prevalence of MSU crystals and uric acid in tissue sections of patients with gout and the statistical difference (“p” values of significance) between different stains and techniques.
| Presence of Crystals in Tissue Sections with Different Stains | MSU/Uric Acid N (% Of 105) | Gömöri MSU Versus | Schultz MSU Versus | Schultz MSU + Uric Acid Versus | Bély And Apáthy MSU Versus |
| HE MSU [12] | 24 (22.86 Of 105) | C=1.0, Χ2=22.004, P Lessthan 0.0000 | C=1.0, Χ2=16.379, P Lessthan 0.0000 | C=1.0, Χ2=7.614, P Lessthan 0.0058 | C=1.0, Χ2=6.688, P Lessthan 0.0097 |
| Gömöri MSU [12,13] | 59 (56.19 Of 105) | C=1.0, Χ2=75.999, P Lessthan 0.0000 | C=1.0, Χ2=36.999, P Lessthan 0.0000 | C=1.0, Χ2=32.867, P Lessthan 0.0000 | |
| Schultz MSU [14,15] | 66 (62.86 Of 105) | C=1.0, Χ2=43.7548, P Lessthan 0.0000 | |||
| Schultz [14,15] MSU + Uric Acid | 81 (77.14 Of 105) | C=0.7531, Χ2=15.849, P Lessthan 0.0000 | |||
| Bély And Apáthy MSU [7-11] | 83 (79.05 Of 105) |
Remarks to Table 2
- In the comparative study of patients with gout only 105 of 211 tissue samples was examined, and only the presence of crystals was registered; the amount of crystal deposits was not estimated, and was not compared.
- Schultz’s staining is specific for MSU crystals [NaC5H3N4O3·H2O], non-crystalline uric acid [C5H4N4O3], and cholesterol crystals [C27H46O].
- The Gömöri or Schultz stains were more effective in detection of MSU than the HE, and the non-staining technique of Bély and Apáthy’s was much more sensitive than all of these.
Figure 1-7 demonstrates a characteristic gouty tophus with MSU crystal deposits comparing the conventionally in formaldehyde solution fixed HE stained tissue sections with unstained ones according to Bély and Apáthy (2013).
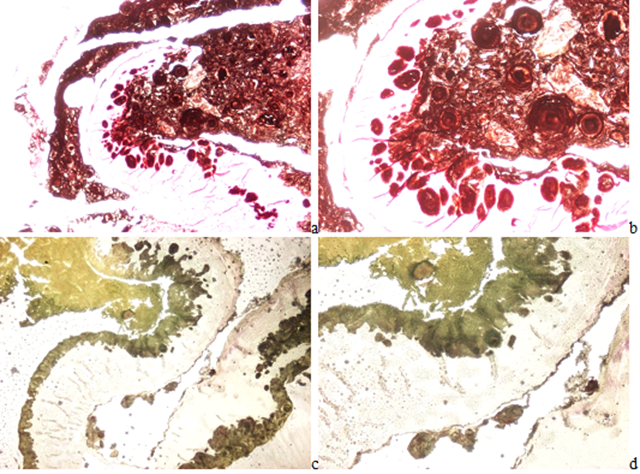
Original magnifications of all Figures correspond to the 24x36 mm transparency slide; the correct height: width ratio is 2:3. The printed size may be different; therefore, the original magnifications are indicated. Figures 6(a-h) demonstrates the globular arrangement of MSU crystals. Figures 7(a-b) illustrates the Gömöri's methenamine-silver method, and Figures 7(c-d) the staining method of Schultz.
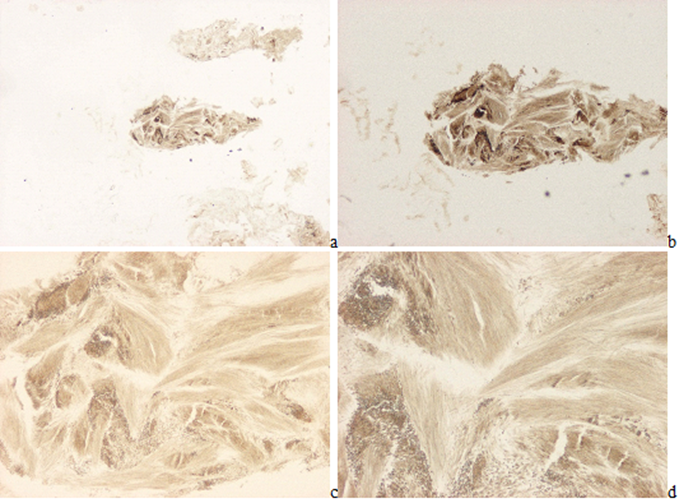
Figure 6(a-h): Gouty arthritis (tophaceous gout), left elbow, synovial membrane, MSU [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3] crystal deposits. Tissue samples were fixed in an 8% aqueous formaldehyde solution. In HE stained section, the MSU crystals are not detectable (a-d). (a) MSU crystals are arranged in characteristic bundles and sporadically as globules, HE, viewed with the light microscope, x40, (b) same as (a) x100, (c) same as (a) viewed under polarized light, x40, (d) same as (b) x100. (e) Gömöri’s methenamine silver method, the MSU crystals are arranged in characteristic bundles and sporadically as globules, x40, (f) same as (e) x100, (g) same as (e) viewed under polarized light, x40, (h) same as (f) x100
Figure 7(a-b):Gouty arthritis, left elbow, synovial membrane. Tissue samples were fixed in aqueous formaldehyde solution, Gömöri’s methenamine silver method [12,13]. (a) MSU[NaC5H3N4O3·H2O] crystal deposits, viewed with the light microscope, x40, (b) same as (a) x100. Figure 7(c-d): Gouty arthritis, left elbow, synovial membrane. Tissue samples were fixed in aqueous formaldehyde solution, Schultz’s staining [14,15]. (c) MSU [NaC5H3N4O3·H2O] crystal deposits, viewed with the light microscope, x40, (d) same as (c) x100
Discussion
Gouty arthritis induced by MSU crystals (monosodium urate monohydrate [NaC5H3N4O3·H2O] - monosodium salt of uric acid [C5H4N4O3]) is one of the most important crystal induced arthritis [1-3]. From a clinical and pathological point of view gout is a well-defined disease with a well-known metabolic background, and can be clearly distinguished from chondrocalcinosis (pseudogout), and apatite rheumatism (Milwaukee syndrome) [18,19].
‘The prevalence of gout ranges 1-4% worldwide and the incidence ranges 0.1-0.3%. Gout is more common in men vs. women and ranges 3:1 to 10:1. The incidence and prevalence of gout increases with each decade of life, with prevalence increasing to 11-13% and the incidence increasing to 0.4% in people older than 80 years’ [20]. Gout occurs more often in men than in women. The signs and symptoms start earlier in men usually between the ages of 30 and 50 - whereas in women they develop later, generally after menopause [21].
In our population the male: female ratio was 9.43:1, and the mean age of males was 53.97 year versus females 61.79 year; the difference was significant (p< 0>
MSU and CPPD crystals may rarely coexist in synovial fluid [26,27] or in mixed tophi of the synovial membranes [2]. In our patient cohorts MSU and calcium pyrophosphate dihydrate (CPPD-[Ca2P2O7.2H2O]) or calcium hydroxyapatite (HA - [Ca5(PO4)3(OH)]) crystal combination was not detected; co-existent metabolic diseases (chondrocalcinosis, apatite rheumatism or primary synovial chondromatosis) did not occur with tophaceous gout.
Conclusions
Gout is an independent metabolic disease of great clinical significance. The histology of gouty tophi is characteristic and stage dependent; the irregular eosinophilic deposits (with or without demonstrable MSU crystals) are surrounded by inflammatory cellular infiltrates and/or by fibrotic demarcating dense connective tissue. Identification of MSU crystals in tissue sections of gouty tophi or in synovial fluid is diagnostic of gout, and it means a definitive diagnosis. The probability of identifying crystals is much higher in unstained histologic sections viewed under polarized light than in hematoxylin-eosin (HE) or other conventionally stained ones, in spite the aqueous formaldehyde fixation of tissue samples.
Abbreviations
MSU- Monosodium Urate Monohydrate [NaC5H3N4O3·H2O]
HE- Hematoxylin Eosin
Declarations
Conflict of Interests
No financial or non-financial competing interests
Funding
There is no financial support.
References
- Gardner DL, McClure J. (1992). Metabolic, Nutritional and Endocrine Diseases of Connective Tissue in Pathological Basis of The Connective Tissue Diseases, 1st edition. (Editor: Gardner DL), Edward Arnold: London, Melbourne, Auckland, Great Britain. 10:380-393.
Publisher | Google Scholor - Mohr W. (2013). Gicht In: Mohr W, editor. Gelenkpathologie, historische Grundlagen, Ursachen und Entwicklungen von Gelenkleiden und ihre Pathomorphologie. Springer-Verlag, Berlin, Heidelberg. 181-193.
Publisher | Google Scholor - Fassbender HG. (2002). Crystal-Associated Arthropathies. In: Pathology and Pathobiology of Rheumatic Diseases. Springer, Berlin, Heidelberg., 17(17.1): 353-369.
Publisher | Google Scholor - McManus JFA, Mowry RW. (1960). Methods of General Utility for The Routine Study of Tissues, Sodium Alizarin Sulfonate Stain for Calcium and Von Kossa’s Method for Phosphates and Carbonates In: Mcmanus JFA, Mowry RW, Editors. Staining Methods, Histologic and Histochemical. Hoeber PB Inc, New York. 55-72,193-194&201.
Publisher | Google Scholor - Bély M, Krutsay M. (2013). A Simple Method to Detect Urate Crystals in Formalin Fixed Tissue. LAM. 23(5-6):271-275.
Publisher | Google Scholor - Bély M, Apáthy Á. (2013). Mönckeberg‟S Sclerosis: Crystal-Induced Angiopathy. Orvosi Hetilap, 154(23):908-913.
Publisher | Google Scholor - Bély M, Apáthy Á. (2016). A Simple Method of Diagnostic Pathology for Identification of Crystal Deposits in Metabolic and Crystal Induced Diseases. Structural Chemistry & Crystallography Communication, 2:1-15.
Publisher | Google Scholor - Bély M, Apáthy A. (2018). Metabolic Diseases and Crystal Induced Arthropathies Technic of Non-Staining Histologic Sections - A Comparative Study of Standard Stains and Histochemical Reactions. Clinical Archives of Bone and Joint Diseases, 1(2).
Publisher | Google Scholor - Bély M, Apáthy A. (2018). Crystal Deposits in Tissue of Patients with Chondrocalcinosis and Apatite Rheumatism - Microscopic Identification of CPPD and HA With the Non-Staining Technique of Bely and Apáthy. BAOJ Clinical Trials, 4(1):18.
Publisher | Google Scholor - Apáthy Á, Bély M. (2023). Amorphous Mineralization, Chondroid And/or Osteoid Formation in Crystal Induced Metabolic Disorders - A Comparative Study of Apatite Rheumatism, Chondrocalcinosis and Primary Synovial Chondromatosis. Annals of the Rheumatic Diseases, 82(Supplement 1):1857-1858.
Publisher | Google Scholor - Bély M, Apáthy Ά. (2023). A Comparative Microscopic Study of Apatite Rheumatism, Chondrocalcinosis and Synovial Chondromatosis - HA And CPPD Induced Metabolic Disorders. EC Pulmonology and Respiratory Medicine, 12(6):1-17.
Publisher | Google Scholor - Carson FL. (1990). Mayer’s Hematoxylin and Gömöri’s Methenamine Silver Method for Urates In: Carson FL, Editor. Histotechnology, ASCP Press, Chicago. 100-105,222-223.
Publisher | Google Scholor - Pearse AGE. (1985). Hexamine Silver Method for Uric Acid In: Pearse AGE, Editor. Histochemistry Theoretical and Applied, Churchill Livingstone, Edinburgh, London, Melbourne And New York, Editor: Pearse AGE. 1012-1026.
Publisher | Google Scholor - Schultz A. (1931). Zur Frage der Beziehungen zwischen Leukämie und Gicht. Virchows Arch. path Anat. 280:519-529.
Publisher | Google Scholor - Lillie RD. (1954). Von Kóssa’s Method and Uric Acid and Urates; Schultz Staining In: Lillie RD, Editor. Histopathologic Technic and Practical Histochemistry, The Blakiston Division McGraw-Hill Book Company, New York, Toronto, London, 264-265,153.
Publisher | Google Scholor - Vacca LL. (1985). Alizarin Red S In: Vacca LL, Editor. Laboratory Manual of Histochemistry, Raven Press, New York. 333-334,409.
Publisher | Google Scholor - Lentner C. (1982). Statistical Methods, Volume 2 In: Lentner C, Editor. Compiled By: Diem K, Seldrup J. Geigy Scientific Tables. Ciba-Geigy Limited, Basle, Switzerland. 227.
Publisher | Google Scholor - Schumacher HR. (1996). Crystal-Induced Arthritis: An Overview. American Journal of Medicine, 100(2A):46S-52S.
Publisher | Google Scholor - Gupta SJ. (2020). Crystal Induced Arthritis: An Overview. Journal of Indian Rheumatology Association, 5-13.
Publisher | Google Scholor - Singh JA, Gaffo A. (2020). Gout Epidemiology and Comorbidities. Seminars in Arthritis and Rheumatism, 50:S11-S16.
Publisher | Google Scholor - Gout: Diseases Conditions, Symptoms, Causes. Mayo Clinic.
Publisher | Google Scholor - Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, et al. (2015). 2015 Gout Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis and Rheumatology, 67(10):2557-2568.
Publisher | Google Scholor - Richette P, Doherty M, Pascual E, Barskova V, Becce F, et al. (2020). 2018 Updated European League Against Rheumatism Evidence-Based Recommendations for The Diagnosis of Gout. Ann Rheum Dis. 79(1):31-38.
Publisher | Google Scholor - Reginato AJ, Reginato AM. (2001). Diseases associated with deposition of calcium pyrophosphate or hydroxyapatite in: Ruddy Sh, Harris ED Jr, Sledge CB, editors. Crystal-associated synovitis, Section XV, Kelly’s Textbook of Rheumatology, 6th ed. WB Saunders Company: A division of Harcourt Brace and Company, Philadelphia, London, New York, St. Louis, Sydney, Toronto. 90:1377-1390.
Publisher | Google Scholor - Poh YJ, Dalbeth N, Doyle A, McQueen FM. (2011). Magnetic Resonance Imaging Bone Edema is Not a Major Feature of Gout Unless There is Concomitant Osteomyelitis: 10-Year Findings from A High Prevalence Population. Journal of Rheumatology, 38:2475-2481.
Publisher | Google Scholor - Mituszova M, Gáspárdy G, Tanka D, Neumark T, Bély M. (1981). Köszvény és Rheumatoid Arthritis Együttes Elôfordulása. Orvosi Hetilap, 122:339-342.
Publisher | Google Scholor - Apáthy Á, Bély M. (2022). Crstal Induced Arthropathies - Retrospective Analysis of Synovial Fluids Aspirated Between 2005 and 2017. EC Cardiology, 9(8):15-31.
Publisher | Google Scholor