Review Article
The Role Of COX-2 In Knee Osteoarthritis: A Comprehensive Analysis of Cytokines, Inflammation and Signaling Pathways
- You Zhou *
- Chaoxin Liang
Guangxi Orthopedic Hospital, Nanning, China.
*Corresponding Author: You Zhou, Guangxi Orthopedic Hospital, Nanning, China.
Citation: Zhou Y., Liang C. (2024). The Role Of COX-2 In Knee Osteoarthritis: A Comprehensive Analysis of Cytokines, Inflammation, And Signaling Pathways. Clinical Case Reports and Studies, BioRes Scientia Publishers. 7(4):1-8. DOI: 10.59657/2837-2565.brs.24.194
Copyright: © 2024 You Zhou, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 22, 2024 | Accepted: November 05, 2024 | Published: November 09, 2024
Abstract
Knee osteoarthritis (KOA)of the knee is a prevalent joint disorder closely associated with multiple factors, among which cyclooxygenase-2 (COX-2) plays a pivotal role in inflammatory responses and cytokine release. This review aims to elucidate the role of COX-2 in the pathogenesis of knee osteoarthritis, analyze its interplay with key cytokines, and examine the signaling pathways involved in this process. By employing immunohistochemical techniques, we intend to gain a deeper understanding of the expression patterns of COX-2 and its functions within the inflammatory microenvironment, thereby providing new insights for the treatment of knee osteoarthritis.
Keywords: cox-2; knee osteoarthritis; cytokines; inflammation; signal transduction; immunohistochemistry
Introduction
Knee osteoarthritis (KOA) is a prevalent degenerative joint disease characterized by the progressive deterioration of articular cartilage, subchondral bone remodeling, and synovial inflammation. Epidemiological studies indicate that KOA affects approximately 10% of men and 18% of women over the age of 60 globally, with a significant burden observed in the Middle East and North Africa (MENA) region, where the prevalence has increased markedly from 1990 to 2019 [1] Clinically, KOA presents with symptoms such as joint pain, stiffness, swelling, and decreased range of motion, significantly impairing the quality of life and functional capacity of affected individuals [2]. The disease's multifactorial nature, influenced by age, obesity, joint injury, and genetic predisposition, necessitates comprehensive understanding and management approaches [3]. Cyclooxygenase-2 (COX-2) is an enzyme that plays a crucial role in the inflammatory response and pain pathways. It is induced during inflammatory processes and is responsible for the conversion of arachidonic acid into prostaglandins, which mediate inflammation and pain [4]. In the context of KOA, elevated COX-2 expression has been associated with increased levels of inflammatory cytokines, contributing to the synovial inflammation characteristic of the disease [5] This highlights the potential of COX-2 as a therapeutic target, as selective COX-2 inhibitors have been shown to alleviate pain and improve function in KOA patients [6].
Cytokines, particularly pro-inflammatory cytokines such as interleukins (IL-1β, IL-6) and tumor necrosis factor-alpha (TNF-α), play a pivotal role in the pathophysiology of KOA. These cytokines are involved in the inflammatory cascade that leads to cartilage degradation and synovial inflammation [7] The infrapatellar fat pad, a significant source of inflammatory cytokines, contributes to the local inflammatory environment in KOA, further exacerbating joint damage [8]. Understanding the intricate interplay between COX-2 and cytokines in KOA is essential for developing targeted therapies aimed at mitigating inflammation and preserving joint function. The objective of this review is to elucidate the epidemiology and clinical manifestations of KOA, examine the biological functions of COX-2 in inflammation, and explore the critical role of cytokines in the disease's progression. By synthesizing current literature, this review aims to highlight potential therapeutic avenues and inform clinical practice in managing KOA effectively.
The relationship between COX-2 and KOA
The expression of COX-2 and its regulatory mechanisms
COX-2 is a key enzyme involved in the inflammatory process and is significantly upregulated in osteoarthritis (OA). The expression of COX-2 is primarily regulated by various pro-inflammatory cytokines, such as interleukin-1 (IL-1) and TNF-α, which are often elevated in OA. Studies have shown that the activation of signaling pathways, including NF-κB and MAPK, plays a critical role in inducing COX-2 expression in chondrocytes and synovial cells under inflammatory conditions. For instance, the aberrant expression of COX-2 correlates with the downregulation of microRNA-758-3p in synovial tissues of OA patients, indicating a complex regulatory network involving both transcriptional and post-transcriptional mechanisms [9]. Additionally, the role of oxidative stress in modulating COX-2 expression has been highlighted, where reactive oxygen species (ROS) can activate transcription factors that enhance COX-2 gene expression [10]. Understanding these regulatory mechanisms is crucial for developing targeted therapies aimed at reducing COX-2 levels and mitigating inflammation in OA.
The pathological role of COX-2 in osteoarthritis.
The pathological role of COX-2 in KOA is multifaceted, primarily contributing to inflammation, cartilage degradation, and pain. Elevated COX-2 levels are associated with increased production of prostaglandins, which are mediators of pain and inflammation in OA [11]. The persistent expression of COX-2 in the joint leads to a vicious cycle of inflammation and cartilage destruction, exacerbating the disease progression. Moreover, selective COX-2 inhibitors have been shown to possess chondroprotective effects, suggesting that managing COX-2 activity could be a viable therapeutic strategy [12]. Research indicates that COX-2 not only influences inflammatory pathways but also interacts with other signaling molecules that contribute to chondrocyte apoptosis and extracellular matrix degradation. Therefore, targeting COX-2 could potentially halt or reverse the degenerative processes associated with KOA.
The role of cytokines in KOA
Classification and function of major cytokines
Cytokines are pivotal in the pathophysiology of knee osteoarthritis, with both pro-inflammatory and anti-inflammatory cytokines playing significant roles. Major pro-inflammatory cytokines involved in OA include IL-1, TNF-α, and IL-6, which are known to promote inflammation, cartilage degradation, and pain [5]. These cytokines act by stimulating the production of matrix metalloproteinases (MMPs) and inhibiting the synthesis of cartilage matrix components, leading to the destruction of articular cartilage. Conversely, anti-inflammatory cytokines, such as IL-10 and transforming growth factor-beta (TGF-β), are crucial for maintaining cartilage homeostasis and promoting repair mechanisms [8]. The balance between these cytokines is essential for joint health, and dysregulation can lead to the progression of OA.
The interaction between cytokines and COX-2
The interaction between cytokines and COX-2 is a critical aspect of the inflammatory response in knee osteoarthritis. Pro-inflammatory cytokines, particularly IL-1 and TNF-α, have been shown to upregulate COX-2 expression in chondrocytes, thereby enhancing the production of inflammatory mediators such as prostaglandins [13]. This positive feedback loop amplifies the inflammatory response and contributes to the symptoms of OA. Furthermore, studies suggest that the interplay between cytokines and COX-2 can influence chondrocyte survival and apoptosis, affecting cartilage integrity [14]. Understanding these interactions provides insights into potential therapeutic targets, as modulating the effects of specific cytokines or inhibiting COX-2 could help alleviate inflammation and slow the progression of osteoarthritis.
Mechanisms of Inflammatory Response in Knee Osteoarthritis
Initiation and Maintenance of Inflammation
The inflammatory response in KOA is a complex process that involves the activation of various immune cells and the release of pro-inflammatory cytokines. This process is initiated by mechanical stress and damage to the cartilage, which leads to the release of damage-associated molecular patterns (DAMPs) that activate pattern recognition receptors (PRRs) on synovial cells and immune cells within the joint. The activation of these receptors triggers a cascade of inflammatory signaling pathways, including the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, which plays a crucial role in the transcription of pro-inflammatory cytokines such as IL-1 and TNF-α [15].
Moreover, the maintenance of inflammation in knee OA is sustained by the continuous presence of inflammatory mediators and the recruitment of additional immune cells, such as macrophages and T cells, to the joint. These cells not only produce inflammatory cytokines but also secrete MMPs that contribute to cartilage degradation [16]. Recent studies have highlighted the role of galectin-3 in promoting synovial inflammation through the activation of the phosphatidylinositol-3-kinase/Akt pathway, indicating a potential therapeutic target for managing inflammation in OA [17]. Additionally, the presence of chronic inflammation in the synovial fluid of OA patients has been associated with the severity of the disease, further emphasizing the need for effective anti-inflammatory strategies [18].
Role of COX-2 in the Inflammatory Response
COX-2 is a key enzyme involved in the inflammatory response in knee osteoarthritis, primarily responsible for the conversion of arachidonic acid into prostaglandins, which are potent mediators of inflammation. Elevated levels of COX-2 have been observed in the synovial tissue and fluid of OA patients, correlating with increased levels of prostaglandin E2 (PGE2), a pro-inflammatory mediator that exacerbates pain and inflammation in the joint [4]. The inhibition of COX-2 has been shown to reduce the levels of inflammatory cytokines and alleviate symptoms in OA patients, making it a target for therapeutic intervention [19].
Moreover, recent research has indicated that COX-2 not only contributes to the inflammatory process but also plays a role in the resolution of inflammation. Specialized pro-resolving mediators derived from omega-3 fatty acids can modulate COX-2 activity, promoting the resolution of inflammation and restoring tissue homeostasis [20]. The dual role of COX-2 in both promoting and resolving inflammation highlights the complexity of targeting this enzyme in therapeutic strategies. Inhibition of COX-2 has been associated with adverse effects, including gastrointestinal complications, necessitating a careful consideration of the therapeutic approaches used in managing KOA [21]. Understanding the precise mechanisms by which COX-2 influences inflammation in KOA will be critical for developing effective treatments that balance pain relief and inflammation resolution.
The Role of Signal Transduction Pathways in COX-2 Regulation
Overview of Major Signal Transduction Pathways
Signal transduction pathways are crucial for cellular communication and play a significant role in regulating various biological processes, including inflammation and cancer progression. The primary pathways involved in the regulation of COX-2 include the mitogen-activated protein kinase (MAPK) pathway, phosphoinositide 3-kinase (PI3K)/Akt pathway, and NF-κB signaling. The MAPK pathway, which consists of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, is activated by various extracellular stimuli and is involved in regulating gene expression, cell proliferation, and apoptosis. The PI3K/Akt pathway is essential for cell survival and growth, and its activation leads to the upregulation of COX-2 expression in response to inflammatory signals. NF-κB is a transcription factor that, when activated, translocates to the nucleus to promote the transcription of pro-inflammatory genes, including COX-2. The interplay among these pathways is complex, and their dysregulation can lead to pathological conditions such as cancer and chronic inflammation [22-24].
Interaction Between Signal Transduction Pathways and COX-2
The interaction between signal transduction pathways and COX-2 is pivotal in mediating inflammatory responses and tumorigenesis. For instance, TNF-α, a pro-inflammatory cytokine, activates the NF-κB pathway, leading to increased COX-2 expression in various cell types, including macrophages and epithelial cells [25,26]. Additionally, studies have shown that the PI3K/Akt pathway can enhance COX-2 expression by promoting the stability of COX-2 mRNA and increasing its translation [27]. Furthermore, the activation of the MAPK pathway, particularly ERK, has been implicated in the upregulation of COX-2 during inflammatory responses, indicating a multifaceted regulatory mechanism [28,29]. In cancer, COX-2 is often overexpressed, and its regulation by these signaling pathways contributes to tumor progression and resistance to apoptosis. Targeting these pathways may provide therapeutic strategies for conditions characterized by elevated COX-2 levels, such as colorectal cancer and other malignancies [30,31]. Understanding the intricate relationship between COX-2 and signal transduction pathways is essential for developing novel anti-inflammatory and anticancer therapies.
Immunohistochemistry in Research Applications
Basic Principles of Immunohistochemistry
Immunohistochemistry (IHC) is a critical technique in biomedical research and clinical diagnostics, leveraging the specificity of antibodies to detect particular antigens in tissue sections. The fundamental principle of IHC involves the binding of an antibody to its target antigen, followed by visualization through various detection systems, which can be chromogenic or fluorescent. This process typically begins with the fixation of tissue samples to preserve cellular structures, followed by embedding in paraffin or freezing for cryosectioning. The antibodies used in IHC can be monoclonal or polyclonal, each with unique advantages depending on the specificity and sensitivity required for the study. The use of secondary antibodies conjugated to enzymes or fluorophores enhances the detection signal, allowing for the visualization of antigen distribution and expression levels within the tissue microenvironment. Recent advancements have introduced multiplexed IHC techniques, enabling the simultaneous detection of multiple antigens in a single tissue section, which is particularly beneficial for understanding complex biological processes and cellular interactions in situ [32,33]. Furthermore, the integration of imaging technologies with IHC has opened new avenues for tissue analysis, providing high-dimensional data that can elucidate the spatial organization and heterogeneity of cellular populations within tissues [34,35].
Applications of Immunohistochemistry in KOA Research
In the context of KOA, immunohistochemistry has emerged as a powerful tool for elucidating the pathophysiological mechanisms underlying this degenerative joint disease. IHC allows researchers to visualize and quantify the expression of various biomarkers associated with inflammation, cartilage degradation, and bone remodeling in affected tissues. For example, studies have utilized IHC to investigate the localization and expression levels of pro-inflammatory cytokines, MMPs, and other mediators involved in the OA process [36,37]. By employing IHC techniques, researchers have been able to identify specific cellular populations, such as macrophages and T-cells, that contribute to the inflammatory milieu in OA-affected joints [38]. Additionally, the application of multiplex IHC in OA research has facilitated the simultaneous assessment of multiple markers, providing insights into the interplay between different cellular pathways and their contributions to disease progression [39,40]. This comprehensive approach not only enhances the understanding of OA pathogenesis but also aids in the identification of potential therapeutic targets and biomarkers for disease monitoring and progression [41,42]. Overall, the use of immunohistochemistry in KOA research underscores its significance in advancing our knowledge of this complex condition and improving clinical outcomes for patients.
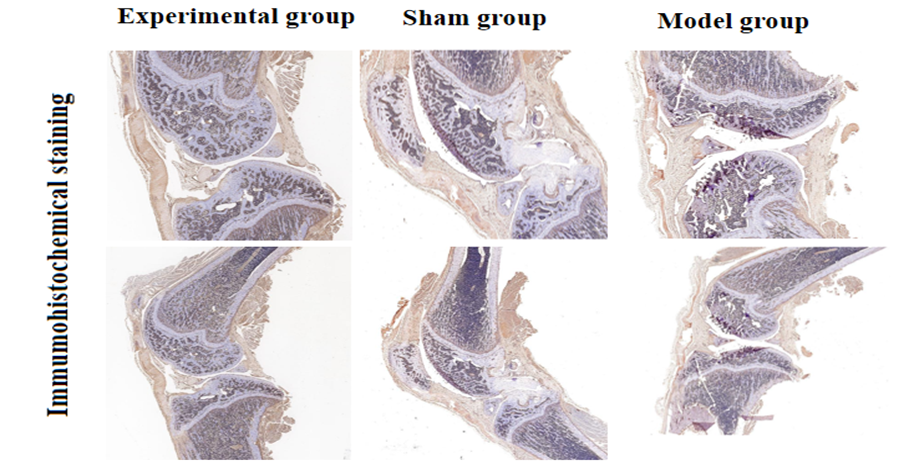
The immunohistochemical expression of COX-2 in rat KOA
To determine the immunohistochemical expression of COX-2 in KOA, 30 male SD rats aged 8.5 weeks were selected from the Animal Experiment Center of Guangxi Medical University (License No: SYXK Gui 2020-0004, Ethical Approval No: 202105004). The experimental group (10 rats) underwent no surgical intervention. Twenty SD male rats were anesthetized with 10% chloral hydrate (3 ml/kg) via intraperitoneal injection, and once satisfactory anesthesia was achieved, the rats were placed in a supine position and fixed to the surgical table, followed by routine disinfection and draping. The skin of the left and right hind limbs was shaved with a surgical razor, and the hind limbs were cleaned with gauze soaked in new chlorhexidine, followed by disinfection with iodine. In the model group (10 rats), a 2 cm incision was made parallel to the medial collateral ligament at the left and right knees, with the skin, muscle, and fascia separated in sequence, the joint capsule incised, and the patella displaced at a 90° flexion to open the joint cavity. The anterior cruciate ligament was located and cut with scissors, and a drawer test was performed to ensure complete transection of the anterior cruciate ligament. The joint cavity was flushed with 0.9% sodium chloride solution, and the joint capsule and skin were sutured. In the sham group (10 rats), only the joint capsule was incised without any further treatment, and then sutured. After all rats recovered, they were returned to their cages. One month later, the articular cartilage tissues from each group of rats were dewaxed and activated for enzyme assays, followed by routine paraffin embedding and sectioning.
Knee joint immunohistochemical staining: The paraffin sections were dewaxed to water, followed by antigen retrieval, and placed in a 3% hydrogen peroxide solution for 25 minutes at room temperature in the dark. The slides were washed 3-5 times in PBS (pH 7.4) on a decolorizing shaker, with each wash lasting 5 minutes, and then blocked with serum for 30 minutes. After removing the blocking solution, the prepared primary antibody was added to the sections, which were then incubated in a humid box at 4°C for 12 hours. The corresponding secondary antibody was applied at room temperature for 50 minutes. The slides were washed 3 times in PBS, with each wash lasting 5 minutes. DAB chromogenic solution was added, and the chromogenic time was controlled under a microscope; a brown-yellow color indicated a positive result, after which the slides were rinsed with running water to stop the reaction. The cell nuclei were stained with hematoxylin, dehydrated, and mounted. The expression of COX-2 related antibodies was detected. In this study, in the control group, COX-2 antibody showed positive expression in immunohistochemical staining. See Figure 1.
Figure 1: Immunohistochemical manifestations of osteoarthritis pathology in SD rats.
Conclusion
In conclusion, the role of COX-2 in KOA is a pivotal element that warrants further investigation. COX-2 is not only an enzyme involved in the inflammatory response but also a key player in the pain pathways associated with OA. The modulation of COX-2 activity could potentially provide therapeutic benefits, as evidenced by the varying responses observed in different studies. As such, it is crucial to balance the emerging viewpoints surrounding COX-2 inhibitors, considering both their analgesic properties and their potential adverse effects. The intricate relationship between cytokines and the inflammatory response in KOA further complicates the therapeutic landscape. Cytokines such as IL-1β and TNF-α are known to exacerbate joint inflammation and degrade cartilage. However, there is a growing body of evidence suggesting that the inhibition of specific cytokine pathways may alleviate symptoms and slow disease progression. This highlights the need for a nuanced understanding of the inflammatory milieu in OA, as targeting one cytokine may not yield the desired results without considering the broader context of cytokine interactions. Moreover, the exploration of signaling pathways as potential therapeutic targets presents an exciting avenue for future research. Pathways such as NF-κB, MAPK, and JAK/STAT are integral to the inflammatory process in OA. Identifying specific inhibitors that can selectively modulate these pathways could lead to innovative treatment strategies that minimize side effects while effectively managing symptoms. Looking ahead, future research should focus on the integration of findings from diverse studies to establish a more comprehensive understanding of OA pathology. Multi-target approaches that consider the interplay between COX-2, cytokines, and signaling pathways could enhance therapeutic outcomes.
Declarations
Ethics approval and consent to participate
This paper and accompanying images have been published with the consent of the Hospital and Animal Ethics.
Consent for publication
The publication of this paper has been approved by Guangxi Bone Injury Hospital.
Availability of data and materials
The data and materials are authentic and available.
Competing interests
None.
Funding
None.
Authors' contributions
Study concept/design: all.
Data collection: all.
Writing the paper: all.
Critical revision: all.
Acknowledgements
Thanks to Guangxi Bone Injury Hospital for providing the research platform.
Authors' information
Guangxi Bone and Injury Hospital, limb trauma Department, Department director, deputy chief physician.
References
- Hoveidaei, A. H., Nakhostin-Ansari, A., Chalian, M., et al. (2023). Burden of knee osteoarthritis in the Middle East and North Africa (MENA): An epidemiological analysis from 1990 to 2019. Archives of Orthopaedic and Trauma Surgery, 143(10):6323-6333.
Publisher | Google Scholor - Spitaels, D., Mamouris, P., Vaes, B., et al. (2020). Epidemiology of knee osteoarthritis in general practice: A registry-based study. BMJ Open, 10(1):e031734.
Publisher | Google Scholor - Driban, J. B., Harkey, M. S., Barbe, M. F., et al. (2020). Risk factors and the natural history of accelerated knee osteoarthritis: A narrative review. BMC Musculoskeletal Disorders, 21(1):332.
Publisher | Google Scholor - Ahmadi, M., Bekeschus, S., Weltmann, K. D., von Woedtke, T., & Wende, K. (2022). Non-steroidal anti-inflammatory drugs: Recent advances in the use of synthetic COX-2 inhibitors. RSC Medicinal Chemistry, 13(5):471-496.
Publisher | Google Scholor - Fischer, A. G., Titchenal, M. R., Migliore, E., Asay, J. L., Erhart-Hledik, J. C., & Andriacchi, T. P. (2024). Elevated proinflammatory cytokines in response to mechanical stimulus are associated with reduced knee loading 2 years after anterior cruciate ligament reconstruction. Clinical Biomechanics, 116:106286.
Publisher | Google Scholor - Clark, G. P. (2023). Treatment options for symptomatic knee osteoarthritis in adults. JAAPA, 36(11):1-6.
Publisher | Google Scholor - Bafrani, H. H., Ahmadi, M., Jahantigh, D., & Karimian, M. (2019). Association analysis of the common varieties of IL17A and IL17F genes with the risk of knee osteoarthritis. Journal of Cellular Biochemistry, 120(10):18020-18030.
Publisher | Google Scholor - Zhou, S., Maleitzke, T., Geissler, S., et al. (2022). Source and hub of inflammation: The infrapatellar fat pad and its interactions with articular tissues during knee osteoarthritis. Journal of Orthopaedic Research, 40(7):1492-1504.
Publisher | Google Scholor - Liu, Z., Sun, J., Liang, T., & Huang, X. (2021). Increased expression of cyclooxygenase-2 in synovium tissues and synovial fluid from patients with knee osteoarthritis is associated with downregulated microRNA-758-3p expression. Experimental and Therapeutic Medicine, 22(3):1001.
Publisher | Google Scholor - Ke, C., Li, H., Yang, D., et al. (2022). Melatonin attenuates the progression of osteoarthritis in rats by inhibiting inflammation and related oxidative stress on the surface of knee cartilage. Orthopaedic Surgery, 14(9):2230-2237.
Publisher | Google Scholor - Cho, S. K., Choi, S., Kim, H., et al. (2022). COX-2 inhibitor use as an early treatment option for knee osteoarthritis patients in Korea: A population-based cross-sectional study. Journal of Korean Medical Science, 37(18), e148.
Publisher | Google Scholor - Timur, U. T., Caron, M. M. J., Jeuken, R. M., et al. (2020). Chondroprotective actions of selective COX-2 inhibitors in vivo: A systematic review. International Journal of Molecular Sciences, 21(18):6962.
Publisher | Google Scholor - Li, W., Hu, S., Chen, X., & Shi, J. (2021). The antioxidant resveratrol protects against chondrocyte apoptosis by regulating the COX-2/NF-κB pathway in created temporomandibular osteoarthritis. Biomedical Research International, 2021:9978651.
Publisher | Google Scholor - Zhao, X., Zhang, Q., & Bu, Z. (2023). Study on the protective effect of platelet-rich plasma combined with injection of pain points around knee joint on knee osteoarthritis and its molecular mechanism. Alternative Therapies in Health and Medicine.
Publisher | Google Scholor - Wang, M. N., Liu, L., Zhao, L. P., et al. (2020). Zhongguo Gu Shang, 33(4):388-392.
Publisher | Google Scholor - Udomsinprasert, W., Jittikoon, J., & Honsawek, S. (2019). Interleukin-34 as a promising clinical biomarker and therapeutic target for inflammatory arthritis. Cytokine Growth Factor Reviews, 47:43-53.
Publisher | Google Scholor - Udomsinprasert, W., Ungsudechachai, T., Wunthong, S., Yuttanarad, S., Jittikoon, J., & Honsawek, S. (2023). Effect of galectin-3 on synovial inflammation in knee osteoarthritis via stimulating phosphatidylinositol-3-kinase/Akt pathway. International Immunopharmacology, 122:110673.
Publisher | Google Scholor - Rolle, N. A., Jan, I., Sibbitt, W. L. Jr, et al. (2019). Extractable synovial fluid in inflammatory and non-inflammatory arthritis of the knee. Clinical Rheumatology, 38(8) :2255-2263.
Publisher | Google Scholor - Liu, J., Hou, W., Zong, Z., et al. (2024). Supplementation of nicotinamide mononucleotide diminishes COX-2 associated inflammatory responses in macrophages by activating kynurenine/AhR signaling. Free Radical Biology and Medicine, 214:69-79.
Publisher | Google Scholor - Jordan, P. M., & Werz, O. (2022). Specialized pro-resolving mediators: Biosynthesis and biological role in bacterial infections. FEBS Journal, 289(14):4212-4227.
Publisher | Google Scholor - Sahu, A., Raza, K., Pradhan, D., Jain, A. K., & Verma, S. (2023). Cyclooxygenase-2 as a therapeutic target against human breast cancer: A comprehensive review. Wiley Interdisciplinary Reviews: Mechanisms of Disease, 15(3):e1596.
Publisher | Google Scholor - Nagaraju, G. P., & El-Rayes, B. F. (2019). Cyclooxygenase-2 in gastrointestinal malignancies. Cancer, 125(8):1221-1227.
Publisher | Google Scholor - Najafi, S. M. A. (2020). The canonical Wnt signaling (Wnt/β-catenin pathway): A potential target for cancer prevention and therapy. Iranian Biomedical Journal, 24(5):269-280.
Publisher | Google Scholor - Kontomanolis, E. N., Koutras, A., Fasoulakis, Z., et al. (2022). A brief overview of oncogenes and signal transduction pathways in gynecological cancer. Cancer Diagnosis and Prognosis, 2(2):134-143.
Publisher | Google Scholor - Hosokawa, Y., Hosokawa, I., Shimoyama, M., et al. (2022). The anti-inflammatory effects of iberin on TNF-α-stimulated human oral epithelial cells: In vitro research. Biomedicines, 10(12):3155.
Publisher | Google Scholor - Okamoto, R., Hosokawa, Y., Hosokawa, I., Ozaki, K., & Hosaka, K. (2024). Cardamonin inhibits the expression of inflammatory mediators in TNF-α-stimulated human periodontal ligament cells. Immunopharmacology and Immunotoxicology, 46(4):521-528.
Publisher | Google Scholor - Eo, S. H., & Kim, S. J. (2019). Resveratrol-mediated inhibition of cyclooxygenase-2 in melanocytes suppresses melanogenesis through extracellular signal-regulated kinase 1/2 and phosphoinositide 3-kinase/Akt signalling. European Journal of Pharmacology, 860:172586.
Publisher | Google Scholor - Li, B., Wang, M., Chen, S., et al. (2022). Baicalin mitigates neuroinflammation through the TLR4/MyD88/NF-κB and MAPK pathways in LPS-stimulated BV-2 microglia. Biomedical Research International, 2022:3263446.
Publisher | Google Scholor - Liu, X., Su, J., Wang, G., et al. (2021). Discovery of phenolic glycoside from Hyssopus cuspidatus attenuates LPS-induced inflammatory responses by inhibition of iNOS and COX-2 expression through suppression of NF-κB activation. International Journal of Molecular Sciences, 22(22):12128.
Publisher | Google Scholor - Han, M., Yu, H., Yang, K., et al. (2021). A network pharmacology-based approach to investigating the mechanisms of Fushen granule effects on intestinal barrier injury in chronic renal failure. Evidence-Based Complementary and Alternative Medicine, 2021:2097569.
Publisher | Google Scholor - Okamoto, R., Hosokawa, Y., Hosokawa, I., Ozaki, K., & Hosaka, K. (2024). Cardamonin decreases inflammatory mediator expression in IL-1β-stimulated human periodontal ligament cells. Molecular Biology Reports, 51(1):222.
Publisher | Google Scholor - McGinnis, L. M., Ibarra-Lopez, V., Rost, S., & Ziai, J. (2021). Clinical and research applications of multiplexed immunohistochemistry and in situ hybridization. Journal of Pathology, 254(4):405-417.
Publisher | Google Scholor - Sato, E. (2021). Gan To Kagaku Ryoho, 48(3):320-324.
Publisher | Google Scholor - Maiques, O., Georgouli, M., & Sanz-Moreno, V. (2019). Recent advances in tissue imaging for cancer research. F1000Research, 8, F1000 Faculty Rev-1980.
Publisher | Google Scholor - Schlecht, A., Boneva, S., Salie, H., et al. (2021). Imaging mass cytometry for high-dimensional tissue profiling in the eye. BMC Ophthalmology, 21(1):338.
Publisher | Google Scholor - Fang, L., Lin, L., Lv, Y., et al. (2021). The mechanism of aerobic exercise combined with glucosamine therapy and circUNK in improving knee osteoarthritis in rabbits. Life Sciences, 275:119375.
Publisher | Google Scholor - Wang, J., Su, S., Dong, C., et al. (2024). Human adipose-derived stem cells upregulate IGF-1 and alleviate osteoarthritis in a two-stage rabbit osteoarthritis model. Current Stem Cell Research & Therapy.
Publisher | Google Scholor - Zhang, W., Zhang, L., Yang, S., Wen, B., Chen, J., & Chang, J. (2023). Electroacupuncture ameliorates knee osteoarthritis in rats via inhibiting NLRP3 inflammasome and reducing pyroptosis. Molecular Pain, 19:17448069221147792.
Publisher | Google Scholor - Shin, S. M., Cai, Y., Itson-Zoske, B., et al. (2020). Enhanced T-type calcium channel 3.2 activity in sensory neurons contributes to neuropathic-like pain of monosodium iodoacetate-induced knee osteoarthritis. Molecular Pain, 16 :1744806920963807.
Publisher | Google Scholor - Chiu, P. E., Fu, Z., Tsai, Y. C., et al. (2024). Fu's subcutaneous needling promotes axonal regeneration and remyelination by inhibiting inflammation and endoplasmic reticulum stress. Translational Research.
Publisher | Google Scholor - Moon, S. W., Park, E. H., Park, J. S., et al. (2020). Pain-relieving effect of 4.4 MHz of pulsed radiofrequency on acute knee arthritis in rats. Pain Medicine, 21(8):1572-1580.
Publisher | Google Scholor - Cheng, J. H., Wang, C. J., Chou, W. Y., Hsu, S. L., Chen, J. H., & Hsu, T. C. (2019). Comparison efficacy of ESWT and Wharton's jelly mesenchymal stem cell in early osteoarthritis of rat knee. American Journal of Translational Research, 11(2):586-598.
Publisher | Google Scholor