Case Report
Proportional Study on The Nutritional Values and Physicochemical Properties of Processed and Raw Juice Consumption in Arba Minch Town, Ethiopia
- Birhanu Zeleke Tilinti *
- Hirbo Geremew
- Kasahun Tsegaye Mekonnen
Department of Industrial Chemistry, Arba Minch, Ethiopia, Arba Minch University, College of Natural & Computational Sciences, Ethiopia.
*Corresponding Author: Birhanu Zeleke Tilinti,Department of Industrial Chemistry, Arba Minch, Ethiopia, Arba Minch University, College of Natural & Computational Sciences, Ethiopia.
Citation: Birhanu Z. Tilinti, Geremew H, Kasahun T. Mekonnen. (2024). Proportional Study on the Nutritional Values and Physicochemical Properties of Processed and Raw Juice Consumption in Arba Minch Town, Ethiopia. International Journal of Nutrition Research and Health, BioRes Scientia Publishers. 3(1):1-11. DOI: 10.59657/2871-6021.brs.24.030
Copyright: © 2024 Birhanu Zeleke Tilinti, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 24, 2024 | Accepted: September 10, 2024 | Published: October 12, 2024
Abstract
People worldwide are familiar with the health benefits of juices that are extracted from a variety of readily available fruits. The abundance, flavor, and health benefits of mangos make them valuable fruits. Mango extract is used to make mango juice. Thus, research on mango juice is required to assess its nutritional value using various nutrient parameters and comparative analyses. Because of the chemicals (such as sulfur dioxide and sodium benzoate) added to the ingredients and the high concentration of microorganisms (yeast, mold), processed mango juice may not always be safe. All age groups agree that mango juice is the most popular nonalcoholic beverage in the world. To determine the pH, moisture content, TSS, TTA, protein content, ash content, fat content, and vitamin C content of the raw and processed mango juices from various local companies sold in the Arba Minch markets, our research revealed the following values: 3.95, 84.20, 13.91, 0.13, 1.4, 0.61, and 0.51 and 28.87, 87.14, 12.19, 0.10, 0.99, 0.73, 0.51, and 39.18, respectively. The conventional physical and chemical methods determine all the parameters. This study showed that a significant number of various kinds of these nutritional components can be found in mango juice. The study's findings were contrasted with those of the original mango extract, which will help consumers evaluate the safety and nutritional value of mango juice. It was discovered that raw juice was more widely accepted than processed juice. The study suggested that kiwifruit juice be consumed raw by humans.
Keywords: contamination: proportional: shelf life: raw fruit and ripen
Introduction
Fruits and vegetables abound in Ethiopia and are diverse. The naturally occurring liquid found in fruit or vegetable tissue is called juice. Without heat or solvents, juice is made by mechanically pressing or macerating fruit or vegetable flesh [1, 2]. Fruit juices are an important source of vitamins and minerals and are commonly consumed as part of a regular diet in tropical countries. Because of its many health advantages, fruit juice consumption has increased over the past few decades, and fruit juice is frequently consumed in place of fresh fruit [3]. However, neither physicians nor dietitians typically consider the micronutrient composition of fruit juices when advising patients on a balanced diet or when considering supplements. This is mostly because there are few trustworthy published data points available on this topic. Mangos grow in Bangladesh. The nutritional value of mango makes it a significant fruit. Mango juice, for instance, has high levels of protein, trace metals, sugar, carbohydrates, and vitamin C. Mango juice may be among the most popular beverages consumed in the morning after breakfast. Fruit juices are commonly preserved and processed using canning, pasteurization, freezing, evaporation, and spray drying [4]. Different types of juice-making processes and operations reduce the value of nutritional parameters when extracts are used to prepare juices. In Bangladesh, mangoes are the most popular seasonal fruit. However, different kinds of chemicals are currently being mixed with mangoes to preserve them for a long time. The development of a health community may be seriously jeopardized by this adulteration and contamination, which can result in a host of illnesses, such as cancer, paralysis, mental retardation, and hypertension. However, as people become busier every day, there is a sharp rise in the demand for food and beverages that are ready to eat. Mango juices are therefore a safe substitute for raw fruits. The current study was carried out with careful consideration of all available data to compile fresh and current data regarding the nutritional makeup of mango juices [5]. Because of the chemicals (such as sulfur dioxide and sodium benzoate) added to the ingredients and the high concentration of microorganisms (yeast, mold), processed mango juice may not always be safe. Occasionally, manufacturers fail to maintain the right levels of aseptic conditions, pH, acidity, and total soluble solids. For these reasons, the product was unable to retain its deliciousness and nutritional value. Manufacturers use chemical preservatives, which can stop microbiological growth of any kind [6, 7]. Preservatives for processed fruit juice that are frequently used, such as sodium benzoate and sulfur dioxide (SO2), are harmful to human health and can cause severe damage to vegetative cells. The most efficient organisms for preventing food browning are bacteria, molds, and yeasts, which are inhibited by sulfites. They also lengthen the shelf life of juice products and inhibit microbial growth. Mango juice is commonly preserved using refrigeration and sterilization, which eliminate harmful microorganisms and maintain color, fragrance, and chemical composition. Mango juice is widely regarded as the most favored nonalcoholic beverage across all age brackets globally. Mango juice provides approximately 30 grams of sugary carbohydrates (sugars, such as glucose, fructose, maltose, and sucrose; dietary fibers) per cup, in addition to vitamins A and C. Although consuming moderate to high amounts of carbohydrates does not significantly alter blood sugar levels, it facilitates an easier metabolism that helps the body sustain hunger. The calories of the juice from nonfat sources make it an easily digestible and energy-boosting beverage [8, 9 and 10].
Nutritional composition of the fruit
Mango fruit is a good source of macronutrients such as protein, amino acids, carbohydrates, lipids, fatty acids, and organic acids. Mangos also contain micronutrients such as vitamins and minerals, as well as non-nutrients such as carotenoids, phenolic compounds, flavonoids, other polyphenols, and chlorophyll. Pulp has high energy content (250–795 kJ) per 100 g, making it a valuable fruit for human nutrition [11]. The water, non-nutritional and nutritional contents of mango fruits vary based on the cultivar and a number of pre- and post-harvest variables. For instance, the mature mango pulp of the Haden, Kent, Keitt, and/or Tommy Atkins varieties contains 83.4 g of water per 100 g of fresh fruit, whereas that of the cultivar Azucar from Colombia contains 79.3 g, according to the United States Department of Agriculture's (USDA) nutrient report data [12].
Table 1: Total Composition of Mango Fruit [13].
| Parameter | Content (g per 100 g of dry) |
| Water | 78.9-82.8 |
| Ashes | 0.34-0.52 |
| Total lipid | 0.30-0.53 |
| Total protein | 0.36-0.40 |
| Total carbohydrate | 16.20-17.18 |
| Total dietary fiber | 0.85-1.06 |
| Energy (kcal) | 62.1-190 |
Mango juice contains important nutrients that support healthy skin tissue, gene transcription, and eye function and growth. In his book "Plants against Cancer," Hartwell asserts that the numerous enzymes and phenols found in mangoes; including astragalin, methyl gallate, quercetin, is quercitrin, astragalin, and fisetin, have anticancer and healing properties.
Mango consumption has been shown to have a protective effect against gallbladder cancer. The use of three servings per day and the average breastfeeding frequency recommended by the FAO [14, 15]. Satisfy the vitamin and energy needs of children aged six to 24 months. Mangoes are also a great source of tryptophan, which is the building block for serotonin, the "happiness hormone" [16]. Iron and calcium are also found in good amounts in mango juice. Calcium promotes the development of strong teeth and bones, while iron aids in the removal of free radicals. Mango juice contains phytochemicals and antioxidants that are good for preventing many illnesses. Thus, assessing the nutritional value of raw or processed mango juice is the main goal of this research [7, 17].
Macronutrients in Fruits
Sugars (glucose, fructose, and sucrose) and other carbohydrates (starch and pectin) are abundant in ripened mango fruit. These are all important substances in terms of flavor and nutrition. Approximately 15% of the total sugar content is found in the flesh of ripe mangos. While sucrose is the main sugar in ripe mango fruit, fructose is the predominant monosaccharide during the proclamatory phase [18]. The protein content of mangos, similar to that of many other fruits, is lower than that of other macronutrients. For instance, mango pulp from Colombia contributes 0–0.6% of protein (Instituto Colombian de Bien star Familiar [ICBF]) [19], whereas mangos in Peru have 1.5–5.5% total protein; other cultivars, such as Java, have 1–2%, and some cultivars in India have low protein contents (0.5– 1%). Additionally, the amino acid composition differed among maturation levels and cultivars. In the ripe state, significant concentrations of the amino acid’s alanine, arginine, glycine, serine, leucine, and isoleucine are present, and trace amounts of the remaining amino acids are present [20]. Page 5/18 Mango pulp contains trace amounts of lipids, but the peel and seed are comparable to those of cocoa butter and have been identified as sources of fatty acids. The food and pharmaceutical industries can benefit from using these beneficial byproducts of mangos: fatty acids. The main fatty acids in mango kernels are linoleic, oleic, palmitic, and stearic acids. Lower concentrations of lignoceric, arachidic, linolenic, and behenic acids are found. A total of 11–38.8% 1,3-dipalmitoyl-2-oleoyl-glycerol (POP), 22.1–36.9% 1,3-distearoyl-2-oleoyl glycerol (SOS), and 15.4–16.2% 1-palmitoyl-3-stearoyl-2-oleoyl-glycerol (POS) were found in the mango seed blend [21, 22].
The water-soluble and fat-soluble vitamin values from the Tommy Atkins, Keitt, Kent, and Haden cultivars were reported by the USDA's National Nutrient Database for Standard Reference. The predominance of vitamins C and A indicates that eating mango fruit on a regular basis can meet your body's needs for these nutrients [12].
Materials And Methods
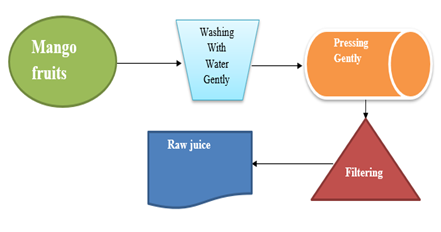
Simple flow of mango juice extraction
Study Areas and Description
The study was carried out in Arba Minch city, which is 1269 meters above sea level and is situated in the Gamo Zone, Southern Nations Nationality and Peoples Regions (SNNPR), 505 kilometers from Addis Ababa, the nation's capital. The study was carried out the floor of the southern part of Ethiopia's Central Rift Valley (CRV), between 6°1'60'' N latitude and 37°32'60'' E longitude.
Sample collection
Mango fruits were purchased from Arba Minch, the Sacha market, and the juice supermarket was the source of processed mango juice. The mature mango fruits were crushed using a crusher to obtain raw mango juice, as shown in Fig. 2 below.
Figure 1: Samples for the study
Physicochemical test methods
As soon as the experiment was finished, both the processed mango juice and the purchased mango fruits were refrigerated. This aids in preventing the temperature effect from ripening our sample too quickly [24].
Determination of pH
A previously standardized pH meter was used to measure the pH after 10 ml of each juice (separately) was poured into a beaker. Phosphate buffer (pH 4.0) was used to calibrate the pH meter [25].
Determination of Total Ash
According to the AOAC (2005), a sensitive balance was used to precisely weigh an empty crucible before 10 ml of sample was added to it. After the sample in the crucible was heated to 550°C for more than three hours, it was removed from the furnace and allowed to cool in a desiccator before being weighed [26].
Moisture Determination
Sensitive balancing was used to measure 10 milliliters of sample in a pristine crucible. The sample-filled crucible was put in an air-dry oven set to 105°C and left there for the night. After two hours, the crucible was placed back in the oven and weighed; this process was continued until a consistent weight was reached (27) and calculated as follows:
Moisture Content% = ((W2-W3)/(w2-W1) ×100)
Where: W1= weight of the empty crucible; W2= weight of crucible + wet sample; W3= weight of crucible +dry sample.
Table 2: Measured and determined values of moist true content
| Wc(w1) | Wc+s(w2) | Wds+c(w3) | Moisture content (%) | ||
| RJ | T1 | 29.61 | 43.58 | 31.86 | 83.89 |
| T2 | 25.13 | 38.82 | 27.25 | 84.51 | |
| PJ | T1 | 19.58 | 34.78 | 21.38 | 88.15 |
| T2 | 27.4 | 41.98 | 29.42 | 86.14 |
Were, WC weight of the empty crucible; Wc+s weight of crucible and sample; Wds+c weight of the dried sample and crucible; RJ Raw juice; PJ processed juice; T1 trial one; T2 trial two.
Total Soluble Solids
By evaporating a known weight of juice in an oven (Fisher Isotherm 175) at 105°C for three hours, the total solids content was ascertained. The total solids were determined by weighing the solids that remained after evaporation [28].
%Total solids = W2/W1 ×100 = 100% moisture
Where, W1 = the initial weight, W2 = Dried weight
Table 3: Measured and calculated total soluble solids
| WC | WC+S | WDC+S | W1 | W2 | TSS | |||
| RJ | T1 | 29.62 | 51.58 | 32.14 | 29.59 | 21.96 | 2.89 | 13.16 |
| T2 | 25.25 | 51.82 | 29.12 | 25.22 | 26.57 | 3.9 | 14.67 | |
| PJ | T1 | 19.62 | 45.25 | 22.64 | 19.58 | 25.66 | 3.06 | 11.92 |
| T2 | 27.4 | 57.42 | 31.18 | 27.4 | 30.3 | 3.78 | 12.47 |
Determination of Ascorbic Acid
(Vitamin C) Contents of 30 g of the sample were combined with a suitable 0.4% oxalic acid solution. (4 g/liter), and white filter paper was used for filtering. A generous amount of 250 ml of 0.4 oxalic acid was added. After 20 milliliters of the filtrate were pipetted into a conical flask, a known strength of 2-6-dichlorophenol indophenol was added, and the mixture was titrated until a light pink hue emerged. The dye strength was ascertained by adding 5 milliliters of 10% oxalic acid (50 mg/100 ml) to a standard ascorbic acid solution (0.05/250 ml) and titrating the solution with 2–6 dichlorophenol indophenol (0.2 g/500 ml) until a faint pink color was achieved, which was then expressed in milligrams per hundred grams [29].
Ascorbic Acid =(Titer(ml)×dyestrength×100%)/factor
Fat Content Determination
This was done utilizing the AOAC (2005) method. Five grams of oven-dried juice concentrate was added, and the cleaned and dried thimble was weighed again (W2). Petroleum ether (40–60°C) was added to a round bottom flask to fill it to ¾ of its capacity. The sample was placed in the thimble and placed into the Soxhlet apparatus, and extraction under reflux was carried out with petroleum ether for six hours. The Soxhlet extractor was fixed with a reflux condenser to adjust the heat source so that the solvent boiled gently. Next, the extractor barrel was emptied, the condenser and thimble were removed, and the object was placed in an oven set at 100°C for one hour. It was then allowed to cool in a desiccator before being weighed once more (W3) [26].
Determination of Crude Protein Content Using the Kjeldahl method
The crude protein content was calculated by multiplying the nitrogen content by 6.25. After weighing 5.5 milliliters of the sample in a Kjeldahl flask, 1 gram of a catalyst mixture containing K2SO4 and CuSO4 and 13 milliliters of concentrated H2SO4 were added. Following two to three hours of boiling at maximum heat, the flask was digested and distilled using 40% NaOH. The ammonia was then transferred to a 100 ml conical flask that contained 4% boric acid. Following a 0.1 NHCl titration of the distillate samples, the crude protein percentage was computed (AOAC, 2005) [26].
Crude protein content = NxTx.10 ml×14×100×6.2
1000, where N = the normality of HCl for sample titration and T = the titration. 10 ml = weight of sample. 1000: Number of milligrams in one gram. 14: Equivalent weight of nitrogen. 6.25: Protein conversion factor.
Determination of Ascorbic Acid (vitamin C) Contents
Thirty grams of the sample blended with a reasonable amount of 0.4% oxalic acid. (4 g/liter) and filtered through man (No. 1) filter paper. The volume was increased to 250 ml with 0.4% oxalic acid. Twenty milliliters of filtrate were pipetted into a conical flask and titrated with known strength 2-6-dichlorophenol indophenol until a faint pink color appeared. The dye strength was determined by adding 5 ml of 10% oxalic acid (50 mg/00 ml) and standard 10% ascorbic acid (0.05/250 ml) titrated with 2-6-dichloerophenol indophenol (2 g/500 ml) until a faint pink was detected (AOAC, 2005).
Ascorbic Acid = (Titer (ml)×dye strength ×100%)/factor
Table 4: Determination of ascorbic acid or vitamin C
| Juice Type | Titration starts(ml) | Titration ends(ml) |
| For Raw juice | 0 | 28 |
| For Processed juice | 14 | 52 |
| For dye strength | 26 | 92 |
Were, Factor=0.0064 Ascorbic acid for raw juice=0.028*0.066*100%/0.0064=28.87%; For processed juice =0.038*0.066*100%/0.0064=39.18%
The fat content of the sample was determined as free or total fat [26]. Free fat was extracted from the lyophilized sample by Soxhlet using ether as a solvent. The total fat content was determined by the acid-hydrolysis method [27]. Samples (1.5 g) were digested with dilute hydrochloric acid (5 ml) for approximately 45 minutes in a water bath. The mixture obtained was then extracted with a combination of methanol (2.5 ml), diethyl ether (7.5 ml) and petroleum ether (7.5 ml). Thereafter, the mixture was centrifuged, the ether–fat layer was decanted and evaporated, and the fat content was measured [25].
Figure 3: Fat content determination using rotary evaporator
This was carried out using the method of AOAC (2005). Clean and dry thimble was weighed. (W1), and 5 g of oven-dried juice concentrate was added and reweighed (W2). The round bottom flask was filled with petroleum ether (40-60°C) up to ¾ of the flask temperature. The Soxhlet extractor was fixed with a reflux condenser to adjust the heat source so that the solvent boiled gently, the sample was put in the thimble and inserted into the Soxhlet apparatus, and extraction under reflux was carried out with petroleum ether for 6 h. After that, the barrel of the extractor was emptied, the condenser and the thimble were removed, the sample was placed in the oven at 100°C for 1h and later cooled in the desiccator and weighed again (W3)
Table 5: Determination of Fat Content
| W1(g) | W2(g) | W3(g) | |
| For raw juice | 3.01 | 8.02 | 7.98 |
| For processed juice | 3.18 | 8.99 | 8.96 |
Determination of Total Ash
According to (AOAC, 2005), an empty crucible was accurately weighed, and then 10 ml of sample was weighed using a sensitive balance. The sample in the crucible was placed in a furnace at 550°C for more than 3 hours until white to gray ash was obtained. Then, the crucible was removed from the furnace to a desiccator to cool and then weighed.
Figure 4: Dry Ash of Juice from Furnace
Table 6: Measured and calculated values of ash content
| WC(w1) | WS(w3) | WCA(w2) | ASH (%) | ||
| RJ | T1 | 29.61 | 28.72 | 27.72 | 0.57 |
| T2 | 25.13 | 13.69 | 25.22 | 0.65 | |
| PJ | T1 | 19.59 | 15.19 | 19.68 | 0.59 |
| T2 | 27.4 | 14.58 | 27.52 | 0.82 |
Were, Wk. weight of empty crucible, Was weight of sample; WCAP weight of crucible with ash, RJ raw juice PJ processed juice, T1 trial one, T2 trial two
Determination of Ascorbic Acid (vitamin C) Contents
Thirty grams of the sample was blended with a reasonable amount of 0.4% oxalic acid. (4 g/liter) and filtered through man (No. 1) filter paper. The volume was increased to 250 ml with 0.4% oxalic acid. Twenty milliliters of filtrate were pipetted into a conical flask and titrated with known strength 2-6-dichlorophenol indophenol until a faint pink color appeared. The dye strength was determined by adding 5 ml of 10% oxalic acid (50 mg/00 ml) and standard 10% ascorbic acid (0.05/250 ml) titrated with 2-6-dichloerophenol indophenol (0.2 g/500 ml) until a faint pink was detected (AOAC, 2005). Ascorbic acid = Titer (ml) X dye strength X 100%/factor.
Table 7: Determination of ascorbic acid or vitamin
| Juice type | Titration starts(ml) | Titration ends(ml) |
| For Raw juice | 0 | 28 |
| For Processed juice | 14 | 52 |
| For dye strength | 26 | 92 |
Were, Factor = 0.0064; Ascorbic acid; For raw juice =0.028*0.066*100%/0.0064; =28.87%; For processed juice =0.038*0.066*100%/0.0064 = 39.18%
Evaluation of Sensory Properties
Fifteen untrained panelists assessed randomly coded juice samples to gauge consumer acceptance of the two juice varieties. The panels received four samples simultaneously. A 6-point hedonic scale—6 = like extremely, 5 = like very much, 4 = like moderately, 3 = dislike moderately, 2 = dislike very much, and 1 = dislike extremely—was used to assess the appearance, aroma, taste, and overall preference [30].
Statistical analyses
The acquired data were calculated using the mean ± standard deviation. To ascertain the significance difference between the means at the significance level of p < 0 xss=removed>
Result
Results of the nutritional values
Table 7. Below are the findings of the nutritional values of the raw and processed juice. The obtained results were compared to the reference values.
Table 8: Tabulated Physicochemical and Nutritional Content
| NO. | Properties | Mean value | ||
| For raw Juice (%) | For processed Juice (%) | Standard value (%) | ||
| 1 | PH | 3.95 ± 0.08 | 3.94 ± 0.05 | 3.91 |
| 2 | TSS | 13.91 ± 0.37 | 12.19 ± 0.26 | 12.89 |
| 3 | Moisture content | 84.20 ± 0.41 | 87.14 ± 0.5 | 86.47 |
| 4 | Ash content | 0.61 ± 0.1 | 0.73 ± 0.3 | 0.71 |
| 5 | TTA | 0.13 ± 0.03 | 0.10 ± 0.04 | 0.19 |
| 6 | Vitamin C | 28.87 ± 1.4 | 39.18 ± 1.2 | 33 |
| 7 | Fat content | 0.79 ± 0.06 | 0.51 ± 0.03 | 0.8 |
| 8 | Protein content | 1.4 ± 0.08 | 0.99 ± 0.05 | 1.08 |
NB: Values are expressed as the mean ± SD
Discussion
The purpose of this study was to assess the nutritional value and physical and chemical characteristics of juices to determine their quality. For this experiment, processed juice and mango fruit juices were collected. Every result is given as a percentage (%) for each 100-gram sample that was examined. According to the study, mango juices and fruits from town markets in Arba Minch are good sources of various nutritional elements. The combination of the protein, fat, ash, and moisture content of the samples is correlated with their vitamin C content. While TSS and fat contents were lower in the raw juice than in the processed juice due to its fresher nature the raw juice had higher pH, ash, moisture protein, and vitamin C values than did the processed juice. Both the raw and processed mango juice had protein contents of 1.4 and 0.95, respectively. This suggested that raw juice has greater protein content than processed juice. Table 2 shows that the percentage of TSS in the raw juice was 13.95%, while the percentage in the processed juice was 12.19%. These outcomes roughly correspond to those discovered by (31). The brix is linked to other soluble solids, such as vitamins and minerals, and includes sugar and carbohydrates. In the present study, the total solids content and moisture content (84.20% and 87.14%, respectively) for both the raw and processed juices were inversely related. The total solids content decreases when the moisture content increases, and vice versa [32]. The pH values of the raw and processed mango juice samples were 3.95 and 3.94, respectively (Table 2). Mango juices typically have a pH of 2.8 to 5.4, with the presence of naturally occurring acids contributing to their low pH [33]. Mango juices have very little fat. According to the analysis, the fat content of the mango was between 0.79% and 0.51%, while the standard value was 0.80%. All fruit that are used to extract juice must, in general, undergo thorough washing, brushing, and rinsing with potable water. All juice makers who participated in this study, however, were not trained in personal hygiene or juice processing. All accountable authorities handling food and public health matters in our nation, Ethiopia, are faced with this challenge. The results of the sensory analyses revealed a significant impact of the gelatin concentration (0.2–0.4%) on the sensory qualities (aroma, taste, flavors, and appearance) of both juices. Heating, however, was found to have a negative impact on the sensory scores of processed juices. A juice concentration greater than 0.4% changed the flavor and taste, and the raw juice also seemed to have a more viscous consistency. The firmest structure was produced by adding the most extra juice, but the resulting structure was extremely dense and compact. The panelists found all four mango juice samples to be acceptable based on the results of the hedonic test for sensory qualities. To determine whether there were any statistically significant differences between the various types of processed fruit juice samples, data on average color, texture, flavor, aroma, and overall score were analyzed. The color sensory scores closely corresponded with previous reports [34, 35]. In terms of the overall acceptance of banana and papaya juice, this study is better than those reported in [36], while the overall acceptance of processed juice in the present study is lower than that reported in a previous study.
Conclusion
Based on the analysis's findings, it is feasible to conclude that using mango juice instead of persimmon to make a mixed juice with orange and pineapple is a fascinating alternative. The juice of these fruits can have more beneficial sensory and nutritional properties when combined than when consumed separately. Mango fruit juices, which are composed of orange juices that are sweeter, more acidic and have a softer, less intense red color, are preferred by consumers. The results of the analysis revealed that all of the nutritional and physicochemical characteristics of mango juice can be fixed and optimized, paving the way for future work to scale up to a large factory in Ethiopia's SNNP.
Recommendations
It is possible to advise the community and researchers at Arba Minch University to establish a large-scale industry that will allow them to produce mango juice on a large scale. Furthermore, fruits from the Arba Minch region are abundant and can be used to make juice both alone and in combination with other ingredients. For this reason, it is preferable to summarize the results of the mixed juice for future Page 11/18 researchers. Ethiopia currently has only one complex share company that manufactures single-juice products, but since raw materials are readily available and inexpensive in the SNNP, it is possible to start a business that produces both mixed and single-juice products by involving relevant field specialists. Ultimately, it is better to advise the Chemistry Department that since our capacity is too small, advisors should be assigned on time, and research projects take longer than anticipated; we are unable to fully correct all of our characterizations in eight courses that run concurrently with research. Taking this into account, each recent graduate's student should be assessed, time management should be performed, and the number of courses for upcoming sessions should be determined.
Declarations
Declarations Ethics Approval
Ethical approval clearance was obtained from the institutional review boards. Permission was also sought from each zone during field work.
Consent to Participate
Not applicable. Informed consent was obtained from all subjects involved in the study, and this manuscript has not been published elsewhere and is not under consideration for publication in other journals.
Funding
No external funding was obtained for the study.
Authors’ Contributions
Birhanu Zeleke Talent conceived the study, analyzed the data, and interpreted the results. Birhanu Zeleke Talent, Harbo Geremew and Kasahun Tsegaye analyzed the data, interpreted the results and finalized the manuscript in the present form.
Acknowledgments
The authors acknowledge the Industrial Chemistry Departments of Arba Minch University for supporting the laboratory. The author thanks the organizing staff Research and Publication Directorate and especially the Department of Industrial Chemistry for their encouragement.
Conflict of interest
There are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morton J. Mango. (1987). Fruits of warm climates. New crop Resource Online Program. Center for New Crops & Plant Pro Purdue University, 221.
Publisher | Google Scholor - Ko Gibney, M. J., Lanham-New, S. A., Cassidy, A., and Vaster, H. H. (2009). Introduction to human Nutrition, second edition. New, A.Godoy.
Publisher | Google Scholor - H. T. and Rodriguez-Amaya, D. B. (1987). Changes in individual carotenoids on processing and Storage of mango (mangiferindica) slices and puree. International Journal of Food Science and Technology, 22(5):451-460.
Publisher | Google Scholor - Reddy L. V. A, Joshi VK. (2012). Utilization of tropical fruits for wine production with specia Emphasis on mango (Mangifera Indica L.). New York, 30:679.
Publisher | Google Scholor - USDA National Nutrient Database for Standard Reference. (2016). SR-28, Full Report (All Nutrients): 09176, Mangos, raw. National Agricultural Library. USDA.
Publisher | Google Scholor - FAO statistics. (2007). Food and Agriculture Organization of UN Rome, Italy.
Publisher | Google Scholor - Carrillo L. A, Ramirez-B. F, Valdez-Torres J. B., Rojas-V. R, Yahia E. M. (2000). Ripening and quality Changes in mango fruit as affected by coating with an edible film. Journal of Food Quality, 23:479-486.
Publisher | Google Scholor - Gizachew G, Gezahegn G, Seifu F. (2016). Chemical composition of mango (Mangiferaindica L) fruit as Influence by postharvest treatments in Arba Minch, Southern Ethiopia. Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT), 10(11):70-77.
Publisher | Google Scholor - Sheehan V. M, Ross P, Fitzgerald G. F. (2000). assessing the acid tolerance and the technological Robustness of probiotic cultures for fortification in fruit juices. Innovative Food science and Emerging Technologies, 8(2):279-284.
Publisher | Google Scholor - Saeed A, Shahid M, Haider S. J, Ijaz A, Shaukat U. (2013). Quality evaluation of different brands of Tetra Pak mango juices available in market, Pak. J. Food Sci; Devika BD. Mango's Wide influence in Indian culture, 22(2):96-100.
Publisher | Google Scholor - Kumar BV, Mannepula S, Vijaya S. R. (2013). Physico-chemical analysis of fresh and probioticated Fruit juices with Lactobacillus Casei. Int Journal ApplSciBiotechnol, 1(3):127-131.
Publisher | Google Scholor - USDA (United States Department of Agriculture). Nutritional Database for Standards, 28:314.2011.
Publisher | Google Scholor - Slavin J. Fiber and Prebiotics. (2013). Mechanisms and Health Benefits. Nutrients, 5:1417-1435.
Publisher | Google Scholor - Joanne L, Slavin, B. L. (2012). Health benefits of fruits and vegetables. American Society for Nutrition. Nutr, 3:506-516.
Publisher | Google Scholor - Farid S. M, Enani M. A. (2010). Levels of trace elements in commercial fruit juices in, Saudi Arabia. Medical Journal of Islamic World Academy of Sciences, 18(1):31-38.
Publisher | Google Scholor - Bernardes, S. A. P., do Nascimento, J. R. O., Lajolo, F. M., and Cordenunsi, B. R. (2008). Starch Mobilization and sucrose accumulation in the pulp of Keitt mangoes during postharvest ripening. J. Food Biochem, 32:384-395.
Publisher | Google Scholor - Instituto Colombiano de Bienestar Familiar (ICBF). (2015). Table de composición de alimento.Bogotá SAS, Editor. Bogotá: Universidad Nacional deColombia, 1-321.
Publisher | Google Scholor - Jahurul, M. H. A., Zaidul, I. S. M., Norulaini, N. N. A., Sahena, F., Jaffri, J. Met et al. (2013). Supercritical carbon dioxide extraction and studies of mango seed kernel for cocoa butter analogy Fats. CyTA-J. Food, 12:97-103.
Publisher | Google Scholor - Byers, T. and Guerrero, N. (1995). Epidemiologic evidence for vitamin C and vitamin E in cancer Prevention. The American Journal of Clinical Nutrition, 62:1385S-1392S.
Publisher | Google Scholor - FAO (Food and Agriculture Organization of the United States). (2001). 13/18.
Publisher | Google Scholor - Frei, B., Stocker, R., England, L, AmLee, S. K. and Kader, A. A. (2000). Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology,.Ascorbate: the most effective antioxidant in human blood plasma. Journal of Advances Experimental Medicine and Biology. 20:207-220
Publisher | Google Scholor - Nishikimi, M., Fukuyama, R., Minoshima, S., Shimizu, N. and Yagi, K. Cloning. (2015). chromosomal mapping of the human nonfunctional gene for L-gulono- -lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. The Journal of Biological Chemistry, 269(18):13685-13688,
Publisher | Google Scholor - Saleem D., M., Oak, P., Chidley, H., Deshpande, A., Giri, A., and Gupta, V. (2016). “Nutrient and flavor content of mango (Mangiferaindica L.) Cultivars: an appurtenance to the list of staple foods,” in Nutritional composition of fruit cultivars. (Switzerland), 445-468.
Publisher | Google Scholor - T H, Amare Aregahegn, Tariku Bekele, Alle Madhusudhan, Investigation of the Levels of Selected Metals in Edible and Medicinal Fruits Grown in Dilla, Ethiopia, Research Journal of Chemical and Environmental Sciences Res J. Chemical Environ Scince, 3, 221-345.
Publisher | Google Scholor - Marianela, F., and Coauthors. (2016). Past and present biophysical redundancy of countries as a Buffer to changes in food supply. Environ. Res. Lett, 11:55008.
Publisher | Google Scholor - Official Methods of Analysis 17th Ed. AOAC INTERNATIONAL, Gaithersburg.
Publisher | Google Scholor - Swapan C. A., Mominu I. and Aminul K. M. I. (2017). Nutritional Benefits and Pharmaceutical Potentialities of Chili: A Review. Journal of Fundamental and Applied Agriculture, 2(2):227-232,
Publisher | Google Scholor - Ghaly, A. E., Ramakrishnan, V. V., Brooks, M. S., Budge, S. M., & Dave, D. (2013). Fish Processing Wastes as a Potential Source of Proteins. Amino Acids and Oils: A Critical Review. J Microb Biochem Technol, 5(4)/;107-129,
Publisher | Google Scholor - Moser, U., & Bendich, A. (2000). In Handbook of Vitamins, 2nd Ed., L.J. Machlin (Ed.), Marcel Dekker, New York, NY, 195-232.
Publisher | Google Scholor - Ndife J, Awogbenja D and Zakari U. (2013). Comparative evaluation of the nutritional and sensory Quality of different brands of orange juice in Nigerian Market. African J. Food Sci., 7(12):479-484
Publisher | Google Scholor - Smirnoff, N. and Wheeler, G. L. (2000). Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Biochemistry and Molecular Biology, 35(4):291,
Publisher | Google Scholor - Sudhakar, P., Latha, P., and Reddy, P. V. (2016). Plant pigments, in Phenotyping crop plants for physiological and biochemical traits (New York, FL: CRC Press), 127.
Publisher | Google Scholor - WHO (World Health Organization). (2003). Diet, nutrition and the prevention of chronic diseases: report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series, No. 916. Geneva.
Publisher | Google Scholor - Zhang, Y. (2013). Biological Role of Ascorbate in Plants. In: Ascorbic Acid in Plants. Chapter, 2:7.
Publisher | Google Scholor - Sadaf A. and PW R. (2017). Sensory and Nutritional study of locally available fresh and processed Fruit and Vegetable juices in Allahabad City. The Pharma Innovation Journal, 6(7):380-386.
Publisher | Google Scholor - Jahurul, M. H. A., Zaidul, I. S. M., Norulaini, N. N. A., Sahena, F., Jaffri, J. M., and Omar, A. K. (2014). Supercritical carbon dioxide extraction and studies of mango seed kernel for cocoa butter analogy fats. CyTA-Journal of Food Science, 12:97-103.
Publisher | Google Scholor