Review Article
Pentalogy of Cantrell-Report of Three Cases and Review of the Literature
Pediatrician, Universidad Nacional Autónoma de México.
*Corresponding Author: E. Gálvez-Cuitiva, Pediatrician, Universidad Nacional Autónoma de México.
Citation: Cuitiva E. G, Castro P. N. (2024). Pentalogy of Cantrell-Report of Three Cases and Review of the Literature. Journal of Clinical Paediatrics and Child Health Care, BioRes Scientia Publishers. 1(1):1-6. DOI: 10.59657/2997-6111.brs.24.008
Copyright: © 2024 E. Gálvez-Cuitiva, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 08, 2024 | Accepted: April 29, 2024 | Published: May 05, 2024
Abstract
Introduction: The Pentalogy of Cantrell is a rare congenital disease, with an incidence of 1 per 65,000 live births, more frequently in women with a 1.35: 1 ratio. This is characterized by the presence of five anomalies: defects in the anterior part of the diaphragm, defects of the diaphragmatic pericardium, defects of the lower segment of the sternum, defects in the supra umbilical midline of the abdominal wall and intracardiac anomalies, mainly ectopia cordis. There is a classification that includes variables of the classical presentation, described by Tomaya.
Materials and methods: The search for cases diagnosed as Pentalogy of Cantrell was conducted between 2013 and 2019 in the city of Bogotá, Colombia. The information was collected retrospectively through the medical records.
Results: Three cases of Pentalogy of Cantrell are reported, with different anatomical variants. One case presented the five embryological defects, with normal karyotype study, with follow-up for two months without complications. Two other class 2 cases presented congenital intracardiac anomalies and supra umbilical abdominal wall defects, two of them associated with other congenital anomalies.
Conclusion: Additional research is required that thoroughly evaluate the etiological hypotheses, the associated factors, the clinical presentation and the survival of these patients.
Keywords: pentalogy of cantrell; congenital anomalies; congenital heart disease; abdominal wall defect
Introduction
Pentalogy of Cantrell is a rare congenital disease, described by Cantrell and associates in 1958 [1]. It was initially characterized by the presence of five anomalies: anterior diaphragmatic defect, diaphragmatic pericardium defect, lower sternal defect, midline supraumbilical abdominal wall defect, and intracardiac anomalies, mainly ectopia cordis. Tomaya presented a new classification in 1972, which allowed for the inclusion of various combinations of symptoms described in the classical definition [2]. Pentalogy of Cantrell has an incidence of approximately 1 per 65,000 live births, being slightly more frequent in women with a ratio of 1.35:1 [3].
As for its origin, it has been considered that a defect in embryo development occurs during the first 8 weeks of gestation, where a failure in the migration of the mesoderm folds to the midline causes diaphragmatic pericardial, anterior diaphragmatic and intracardiac defects [2,4,5]. There could also be an interruption in the development of the primary mesenchyme structures, resulting in sternal and abdominal wall defects [2,4]. A complementary genetic component and the negative influence of some factors on early embryogenesis, such as vitamin deficiencies or excesses, have also been presented as possible causes [2,5,6].
This article presents the description of three cases reported in Bogotá, Colombia. It also advocates for an active search of cases, that would help generate new data, and consequently, aid in the construction of knowledge.
Materials and methods
We searched the database of the national public health surveillance system (SIVIGILA) for cases diagnosed as Pentalogy of Cantrell in Bogotá, Colombia between 2013 and 2019. After obtaining informed consent by the patient's parents or guardians, the information was collected retrospectively through medical records and diagnostic studies. Table 1 provides a comparative overview of patient characteristics.
Table 1: Clinical features of reported cases. Source: prepared by the author
| Findings | Cases | ||
| 1 | 2 | 3 | |
| Sex of the newborn | Female | Male | Male |
| Maternal age (years) | 23 | 32 | 42 |
| Maternal Morbidity | Overweight | No | Unknown |
| Consanguineous parents | No | No | Unknown |
| Parity | 2 | 2 | 6 |
| Single newborn | Yes | Yes | Yes |
| (Product) healthy previous pregnancies | Yes | Yes | Unknown |
| Prenatal care checkups | Yes | No | Unknown |
| Prenatal ultrasound diagnosis | Yes | Yes | No |
| Gestational age at diagnosis (weeks) | 20 | 30 | 38 |
| Karyotype | Normal | Unknown | Normal |
| Gestational age at birth | 38 | 34 | 38 |
| Type of childbirth | C-section | Vaginal | Vaginal |
| Spontaneous neonatal adaptation | Yes | No | Yes |
| Breastfeeding | Yes | No | Yes |
| Surgical correction | Yes | No | Yes |
| Hospital discharge | Yes | No | Yes |
| Perinatal mortality | No | Yes | No |
| Clinical findings | |||
| Anterior diaphragmatic defect | Yes | Yes | No |
| Diaphragmatic pericardium defect | Yes | No | Yes |
| Lower sternal defect | Yes | Yes | Yes |
| Midline supraumbilical abdominal wall defect | Yes | Yes | Yes |
| Intracardiac anomalies |
|
|
|
|
|
| |
|
| ||
| |||
| Other findings | Absent |
|
|
|
| ||
|
| ||
|
| ||
| |||
Results
Patient 1
Female patient, with non-consanguineous parents, 23-year-old mother, product of a second gestation. Prenatal diagnosis at week 20, of ectopia cordis, omphalocele abdominal wall defect and intrauterine growth restriction.
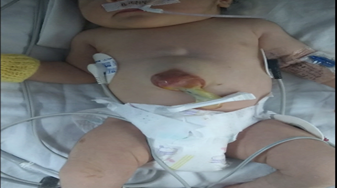
The newborn was delivered by scheduled cesarean section at 38 weeks of gestation, with a weight of 2450 g, length of 44 cm, head circumference of 32 cm, with an Apgar score of 8 at one minute after birth and 9 at five minutes after birth. The neonatal adaptation was spontaneous. The presence of ectopia cordis, absence of the lower third of the sternum and a supra-umbilical abdominal wall defect of 4x5 cm in diameter were confirmed, through which part of the liver and small intestine can be observed, without the manifestation of syndromic facial phenotype Figure 1.
Figure 1: Frontal view of the patient Supra-umbilical abdominal wall defect, through which part of the liver and small intestine can be observed. Source: the author
An ultrasound study was performed at 24 hours after birth showing mesocardia, agenesis of lower third of the sternum, persistent patent ductus arteriosus without hemodynamic impact, perimembranous ventricular septal defect with mechanisms of closure, patent foramen ovale, good ventricular function. Transfontanellar ultrasound 72 hours after birth reported normal and karyotype test using the G-tray technique indicated 46 XX.
The patient remained stable during her evolution; 7 days after birth, omphalocele correction was performed, without atretic segments, as well as diaphragmatic and pericardial reconstruction, without complications. Postoperative evolution with vasoactive support during 48 hours, sedoanalgesia during 72 hours, without the need for blood transfusion. Adequate therapeutic response so the patient is discharged from the facility 14 days after birth, without the need for complementary oxygen. Outpatient follow-up three months later, asymptomatic, normal transthoracic echocardiogram, with cerebral magnetic resonance within normal limits and neurodevelopment according to the Bayley III scale.
Patient 2
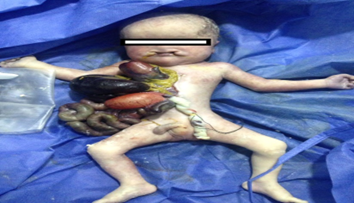
Male patient, with non-consanguineous parents, 32-year-old mother, product of a second gestation, no relevant obstetric history, insufficient prenatal care checkups. Diagnosed at 30 weeks of gestation with thoracic ectopia cordis and large abdominal wall defect by obstetric ultrasound. The expecting mother was admitted in advanced labor to the emergency department at 34 weeks of pregnancy. The patient was born with no vital signs and received unsuccessful cardiopulmonary resuscitation. Anthropometric measurements, weight 1220g, length 38cm, and head circumference 24cm. The physical examination showed epicanthal fold, low nasal bridge, short neck, bilateral hard and soft cleft palate Figure 2.
Figure 2: Frontal view of the patient Supra-umbilical abdominal wall defect, in which omphalocele and ectopia cordis can be observed. Source: the author
In addition, absence of two thirds of the lower sternum, thoracoabdominal wall defect, with exposure of the diaphragm, heart with 2 cm ventricular septal defect, patent ductus arteriosus, the liver, stomach, small and large intestine also occupied the chest cavity.
Patient 3
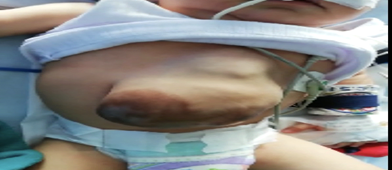
Male patient born in a rural area at 38 weeks of gestation, product of a sixth gestation, 42-year-old mother, was fed breast milk exclusively, and whose other perinatal antecedents are unknown. He was admitted at the age of 5 months with a length of 63 cm (WHO 15th percentile) and a weight of 7 kg. The physical examination showed a prominent forehead, sparse eyebrows, bilateral epicanthal fold, low-set ears, absence of the lower third of the sternum, with a supra-umbilical abdominal wall defect compatible with possible omphalocele and ectopy cardis Figure 3.
Figure 3: Frontal view of the patient Supra-umbilical abdominal wall defect, in which omphalocele and ectopia cordis can be observed. Source: the author
The patient also presented an interventricular septal defect without hemodynamic impact, the abdominal ultrasound showed evidence of herniation of thin and thick intestinal loops, and left lobe of the liver. Normal skull tomography study and 46 XY karyotype. Surgical correction of omphalocele-type abdominal defect, diaphragmatic muscles and pericardium was performed without alterations, surgery with no complications. The patient was discharged from the institution two weeks after the surgical procedure thanks to his satisfactory progress.
Discussion
Pentalogy of Cantrell is a rare syndrome [7,8], described for the first time by Cantrell et al. Its classical spectrum describes five anomalies [4]. Given that all cases do not follow a classic pattern [9], Toyama suggested a new classification based on the expression of symptoms: class 1 or definitive diagnosis presents with all five defects, class 2 or probable diagnosis presents with at least four defects and class 3 or incomplete varying combinations with expression of three defects, including lower sternal defect [10]. In this case report, we present two patients with characteristics that classify them as class 2 or probable diagnosis, in which intracardiac congenital anomalies and supra-umbilical defects of the abdominal wall were observed, and one case that exhibits all the characteristics described by Cantrell.
Although its etiology is unknown, some authors have postulated a genetic influence, such as the description of cases associated with Goltz-Gorlin syndrome, trisomies 21, 18 t 13, and Turner syndrome [11–13]. Steiner et al also reported a case of pentalogy of Cantrell associated with a duplication of the ALDH1A2 gene in the 15q21.3 locus, which encodes for the enzyme retinaldehyde dehydrogenase type 2, which is involved in the conversion of vitamin A [5]. In Colombia, limitations on genetic research restrict the availability of case information; however, genetic studies were conducted on two patients, class 1 and class 2, the second case involving perinatal mortality, where no abnormalities in the karyotype were found, similar to another case described in Colombia [14].
Although the Pentalogy of Cantrell is considered to be the result of a failure in the differentiation or migration of the mesenchymal or mesodermal structures during the embryonic phase of development [2], Saldarriaga et al. propose a new explanation for the failure in the formation of the thoracoabdominal wall, secondary to a cephalic fold defect; without the descent of the heart and the anterior part of the diaphragm to a central location in the thoracic cavity, which prevents the completion of transverse folding in the epigastric region, which would produce the defect in the thoracoabdominal wall, this would explain the occurrence of variables other than class 1 [14,15].
Although few cases have been reported in the literature and the prevalence of this disease is unknown, in Colombia, we found two case reports; the first occurred in the city of Cali and the second in Bogotá [14]. In Cali, Saldarriaga et al described a male newborn, delivered by vaginal birth, preterm 34 weeks of gestation, the son of non-consanguineous parents, 17-year-old mother, with ultrasound diagnosis at 25 weeks of gestation. This patient presented a supra-umbilical thoracoabdominal wall defect, through which the heart protruded without a pericardium, with a wide ventricular septal defect [14].
Another case in Bogotá, by Riaño et al, describes a female newborn, 37 weeks old, with a 25-year-old mother, product of a third gestation, fetal karyotype 45XX with Robertsonian translocation. Diagnosed with thoracic-abdominal ectopia cordis plus complex structural heart disease, by prenatal ultrasound. In addition, presence of anterior diaphragmatic defect and supraumbilical omphalocele greater than 8 cm, containing the liver inside. The newborn presented progressive hemodynamic deterioration until death [16].
Low survival rates have been reported by other authors, as described by Ghidni et al., who report 17 cases diagnosed before birth in 1988, of which six had early termination, four died at birth, one in the following week, four in the following two weeks, one in the fourth week, and one in the fourth month, their deaths were associated to respiratory and infectious deterioration [17]. Likewise, Vázquez et al. initially estimated a mortality rate of 52% in patients with Pentalogy of Cantrell. However, advances in pediatric surgery and intensive care have increased the survival rate to 61% [3.18]. Zhang et al propose that the prognosis depends largely on the presence of serious cardiac anomalies, linking ectopia cordis as a factor associated with poor prognosis [18]. In agreement, authors like Balderrábano et al. report 22 cases in a study, of which 13 presented ectopia cordis, with a mortality of 85% of the cases regardless of all the treatment, as opposed to 44% of those who did not present ectopia cordis and survived the surgical procedure [19].
It is also hypothesized that patients with incomplete symptoms may have better outcomes than those displaying the full range of Pentalogy of Cantrell, as reported by Mallula et al [20], but a true prognostic estimate cannot be achieved through the referenced studies.
Conclusion
The same pathology is reported in different cases, with an unclear embryological origin, which accounts for the wide heterogeneity of its symptoms, and therefore, in its final outcomes. For this reason, comprehensive prenatal care and evaluation during pregnancy is required in all cases of Pentalogy of Cantrell to assess the associated risks and the viability of the pregnancy. Further research is needed for an in-depth evaluation the etiological hypotheses, associated factors, clinical presentation, and patient survival.
References
- Asai H, Shingu Y, Ito N, Et al. (2020). Norwood Operation of a Neonate with Pentalogy of Cantrell. Ann. Thorac. Surg,109(2):135-136.
Publisher | Google Scholor - Jnah A, Newberry D, England A. (2015). Pentalogy of Cantrell: Case Report with Review of the Literature. Adv Neonatal Care, 15(4):261-268
Publisher | Google Scholor - Vazquez J, Muehler E, Daebritz S, et al. (1998). Cantrell’s syndrome: a challenge to the surgeon. Ann Thorac Surg, 65(4):1178-1185
Publisher | Google Scholor - Hubbard R, Hayes S, Gillis H. (2018). Management Challenges in an Infant with Pentalogy of Cantrell, Giant Anterior Encephalocele, and Craniofacial Anomalies: A Case Report. A&A Practice,11(9):238-240.
Publisher | Google Scholor - Steiner M, Vengoechea J, Collins R. (2013). Duplication of the ALDH1A2 gene in association with pentalogy of Cantrell: a case report. J Med Case Rep, 7:287.
Publisher | Google Scholor - Boughman JA. (1992). Pentalogy of Cantrell and associated midline anomalies: a possible ventral midline developmental field. Am J Med Genet. 42(1):90-95
Publisher | Google Scholor - Engels AC, Debeer A, Russo FM, et al. (2017). Pericardio-Amniotic Shunting for Incomplete Pentalogy of Cantrell. Fetal Diagnosis and Therapy, 41(2):152-156.
Publisher | Google Scholor - Patel N, Sollinger C, D’Angio CT, et al. (2017). Congenital Diaphragmatic Hernia with Liver Herniation into the Pericardial Sac in a 30-Week Gestation Infant. Pediatr. Dev. Pathol, 20(5):421-425.
Publisher | Google Scholor - Kunapinun N, Treetipsatit J. (2017). Discordant anomalies with combined features of pentalogy of Cantrell and OEIS complex: a case report in monochorionic twins. Fetal Pediatr Pathol, 36(5):357-363.
Publisher | Google Scholor - Kaul B, Sheikh F, Zamora I, Mehollin-Ray A, Cassady C, et al. (2015). 5, 4, 3, 2, 1: embryologic variants of pentalogy of Cantrell.J Surg Res, 199(1):141-148
Publisher | Google Scholor - Smigiel J, Lombardi M, Jaworski W, Slezak R, Patkowski D, Hennekam R. (2011). Co-occurrence of severe Goltz–Gorlin syndrome and pentalogy of Cantrell—Case report and review of the literature. Am J Med Genet, 155:1102-1105
Publisher | Google Scholor - Onderoglu L, Baykal C, Tulunay G, Talim B, Kale G. (2003). Prenatal diagnosis of Cantrell’s pentalogy: a case report. Turk J Pediatr, 45:357-358
Publisher | Google Scholor - Madi JM, Festugatto JR, Rizzon M, et al. (2019). Ectopia Cordis Associated with Pentalogy of Cantrell-A Case Report. Rev Bras Ginecol Obstet, 41(5),352-356.
Publisher | Google Scholor - Saldarriaga W. (2014). Pentalogía de Cantrell. Reporte de caso [case report]. Salud Uninorte, 30(3):505-512
Publisher | Google Scholor - Salinas V, De La O-Espinoza E, Salinas R. (2017). Severe Intrauterine Amputations in One Dichorionic Twin with Pentalogy of Cantrell: Further Evidence and Consideration for Mechanical Teratogenesis. Pediatr Dev Pathol, 20(5):440-443
Publisher | Google Scholor - Riaño C, Otoya J, Gentile J, Mosquera W, Socarrás J, Castro J, et all. (2010). Pentalogía de Cantrell (ectopia cordis): reporte de un caso [case report]. Rev Colomb Cardiol, 17:286-290
Publisher | Google Scholor - Ghidini A, Sirtori M, Romero R, Hobbins JC. (1988). Prenatal diagnosis of pentalogy of Cantrell. J Ultrasound Med, 7(10):567-572
Publisher | Google Scholor - Zhang X, Xing Q, Sun J, Hou X, Kuang M, Zhang G. (2014). Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg, 49(8):1335-1340
Publisher | Google Scholor - Balderrábano N, Vizcaíno A, Sandoval E, et al. (2011). Pentalogy of Cantrell: forty-two years of experience in the Hospital Infantil de Mexico Federico Gomez. World J Pediatr Congenit Heart Surg, 2(2):211-218
Publisher | Google Scholor - Mallula K, Sosnowski C, Awad S. (2013). Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol, 34(7):1703-1710
Publisher | Google Scholor