Review Article
Pathology of Bovine Skin Tumors and Its Health and Economic Impact on Cattle: A Review
1Working in Debre tabor university as veterinary pathology instructor, Ethiopia.
2Assistant Professor of Avian Medicine, scholar of Pan African University, AU, College Veterinary Medicine and Animal, Ethiopia.
*Corresponding Author: Mengesha Ayehu Getnet, Working in Debre tabor university as veterinary pathology instructor, Ethiopia.
Citation: Mengesha A. Getnet, Asnakew M. Berihun. (2024). Physical and Chemical Analysis of Soil Collected from Malakander Farm, Scientific Research and Reports, BioRes Scientia Publishers. 1(5):1-15. DOI: 10.59657/2996-8550.brs.24.033
Copyright: © 2024 Mengesha Ayehu Getnet, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 14, 2024 | Accepted: October 30, 2024 | Published: November 05, 2024
Abstract
A tumor is an abnormal mass of tissue that exceeds normal boundaries, resulting from uncoordinated and uncontrolled proliferations of cells. Tumor can affect different parts of the animal, such as skin, bones, glands, and different visceral organs. Among these, skin tumor is the most frequently diagnosed neoplastic disorder in domestic farm animal species such as bovine. The most common farm animal skin tumors include Bovine papilloma, squamous cell carcinoma, and bovine lymphosarcoma. Skin tumor pose significant health problem and have a negative economic impact on cattle production and its byproducts. Clinical features of skin tumors include hyperkeratosis, acanthosis, elongated rete-pegs, large nodular structure, exophytic and cauliflower like lesions, and friable lesions. Melanomas are a proliferative skin tumor characterized by spindle to round cell shape which containing abundant black pigment. More than 90 percent of skin tumors caused by prolonged exposure to ultraviolet radiation. Diagnosis of a skin tumor in cattle typically requires a skin biopsy and fine needle aspiration cytology. Histologically, a skin tumor cells show an increased nuclear to cytoplasmic ratio, Di cohesiveness of cells, cellular and nuclear pleomorphic. In addition to the health impact, skin tumors cause an economic loss due to reduced productivity, decrease reproduction, carcass condemnation, and skin and hide downgrading. Common treatments for skin tumors in cattle include chemotherapy, radiation and surgical removal. This review paper provides a brief overview of common skin tumors in cattle focusing on pathological features, economic implications and management options. Given skin tumor is an economic significant disease in Ethiopia it needs attentions from researchers, and concerning centers for control and preventions. Early diagnosis and management of the diseases is important.
Keywords: cattle; diagnosis; skin tumor; tumor; bovine
Introduction
Tumor is an abnormal growth of tissue due to uncontrolled proliferation of cells (Kabiraj et al., 2015) Based on its behavior, a tumor may be benign or malignant on stable (Constable et al., 2016). Benign tumor refers a clump of cells that do not spread throughout the body instead it invades only surrounding tissue of its origin (Hunt, 2017). It is the most common type of tumor in farm and pet animals (Sujatha, 2018). Whereas malignant tumor is more serious forms and has ability to spread at different level to other healthy tissues or organs (Shim and Meltzer, 2011) (Constable et al., 2016). However, it is noticeable that both forms of the tumor are increasingly become a major threat to the health and well-being of domestic animals (Rajmani et al., 2012).
Tumors can also be classified based on its developmental pathways such as cell origin and histogenesis into tumors of epithelial, mesenchymal, mixed and miscellaneous tumors. It affects different part of animal body including hard and soft tissue (Rosenberg et al., 2012). Skin, bones, glands, and different visceral organs are some of the animal body parts which are commonly affected by tumor (Neerja et al., 2018).Skin is the largest organ of the animal body composed of three distinct anatomical structures, namely, epidermis, dermis, and hypodermis. It is responsible for different physiological regulatory of the body system (Achalkar, 2019) as well as for a protection from the external environment and serve as a boundary for the internal body structure. Given that the skin is outermost layer of the body usually it is exposed to various types of diseases causing agents and physical injuries (Rosenberg et al., 2012). Tumor is one of the challenges which affect skin at different levels and reduces its function for animal and value for human consumptions (Mathewos et al., 2020). Various research findings indicated that skin tumors are the most frequent observed tumor in domestic animals, particularly in dogs, horses, cattle, and cats. This is due to the fact that skin is consistently exposed to the external environment and tumor inducing factors (Goldschmidt and Hendrick, 2002). Thus, prolonged exposure of skin to sunlight causes DNA damage and become unable to recover which leads to uncontrolled proliferation of skin cells (DeVita Jr and Rosenberg, 2012), (Bukhtoyarov and Samarin, 2015). Mostly, skin cancer occurs due to prolonged exposure to sunlight (DeVita Jr and Rosenberg, 2012) and development of tumors usually results when the DNA is damaged and the body is unable to recover this damage (Bukhtoyarov and Samarin, 2015)). The most commonly diagnosed skin tumors in domestic animals are equine sarcoid, squamous cell carcinoma (SCC), lymphosarcoma, melanoma, basal cell tumor, papilloma, astrocytoma, and canine transmissible venereal sarcoma. Nowadays, the occurrence of bovine skin cancer is increasing and it devalues the productivity of the animals and causes their death (Neerja et al., 2018 and Mathewos et al., 2020). Constable et al (2016) indicated that incidence of skin tumors is relatively increasing overtime in domestic animals, which causes major damages to the cattle’s skin and hide industry. Skin tumor causes live animal loss, decrease production and productivity of the cattle. Industry of skin and hide in Ethiopia is facing challenges related with skin and hide quality. The objective of this review is to summarize bovine skin tumor and its economic impact.
Common Skin Tumors Occur in cattle
Skin tumor is defined as abnormal skin cell growth and proliferation manifested on the sun-exposed skin surface of animal’s body(Hunt, 2017) (Rajmani et al., 2012). Currently, many researchers reported that, cattle ranked second after dogs for the incidence of all types of tumors that occur in domestic animals (Mathewos et al., 2020). The most common type of tumor affecting bovine species is skin tumors (Meuten, 2020). Many cattle are more likely to develop skin tumors in spite of the rate of occurrence of skin tumor may vary among different breeds, color animals and types of ago climate and cattle managements ((Hassanein and Mahmoud, 2009, Moharram et al., 2019)). As indicated in table1, the different types of cutaneous tumors affecting cattle include bovine papilloma, squamous cell carcinoma (SCC), bovine lymphosarcoma, and melanoma (Neerja et al., 2018, Mathewos et al., 2020, Hassanein and Mahmoud, 2009) indicated that bovine cutaneous papillomatosis and SCC are more prevalent form of cattle tumor. Skin tumor identified as a serious skin condition in cattle that led to the cattle health and welfare of the animals disastrous (Moharram et al., 2019, Feyisa, 2018).
Table 1: Spontaneous skin tumors prevalence in farm animals
| Organ | Tumor Type | Animal | Number/Percent | Sex (M/F) | Age |
| Skin | Fibropapillomas | Cattle | 110 (54.4%) | 40M/70F | M2m-3y F 4m-6y |
| Skin | Equine Sarcoid | Equine | 65 (32.1%) | 39M/26F | M2m-6y F 2m-7y |
| cutis | Fibroma | Cattle | 14 3(8.4%) | 4M/10F | M2-4y F2-5y |
| Equine | 1M/2F | M5y and F 3y | |||
| Skin and sub cutis | SCC | Cattle | 31(1.9%) | 1M/2F | M3y F 2-3y F |
| Sheep | 1M | 3m | |||
| sub-cutis | Lymphosarcoma | Cattle | 11(0.9%) | 1F | 2y |
| Buffalo | 1F | 8m | |||
| Liver | Haemagiosarcoma | Cattle | 2 (0.9%) | 2M | 3y |
| sub cutis | Liposarcoma | Goat | 1 (0.4%) | 1F | 3y |
| Ovary | Malignant Teratoma | Cattle | 1 (0.4%) | 1F | 8m |
Note: M= male; F= female; m= months; y= years. Source: (Hassanein and Mahmoud, 2009).
Different types of skin tumor have different prevalence on the body skin of the cattle. All part of skins has not equally susceptible for different types of tumor disease (Moharram et al., 2019) as it showed on table 2.
Table 2: Tumor description on skin of cattle and buffaloes
| Tumors | Animals | Number of tumors | Location | Sex | Age | |
| F | M | |||||
| Cutaneous Papillomatosis | Cattle | 15 | head, around eyes, neck, back, shoulder, axilla and all over the body | 13 | 2 | 5m to 3y |
| Cutaneous Fibropapilloma | Cattle | 5 | Skin of head, around eyes, neck, back, shoulder, axilla and all over the body | 4 | 1 | 8m to 1y |
| Scc | Cattle | 25 | eye | 10 | 15 | 2-10y |
| 1 | Skin of muzzle | 1 | - | 9y | ||
| 8 | perineum | 8 | - | 7-9y | ||
| Scc | Buffalo | 1 | Skin of face | 1 | - | 3y |
| Bucal cavity | 1 | - | 8y | |||
| Epulis | Cattle | 2 | gum | 1 | 1 | 8m and 2y |
| Leiomyoma | Cattle | 2 | vagina | 2 | - | 4y |
| Fibroma | Cattle | 1 | Submandibular space | 1 | - | 6y |
| Liposarcoma | Cattle | 1 | Neck | 1 | - | 1y |
| Total | 66 | 7 | 19 | |||
SCC= Squamous cell carcinoma, Source(Moharram et al., 2019)
Bovine Papilloma
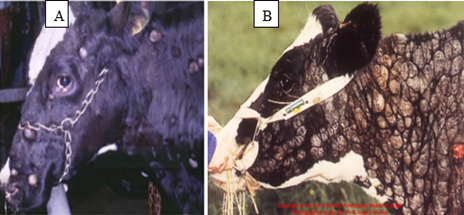
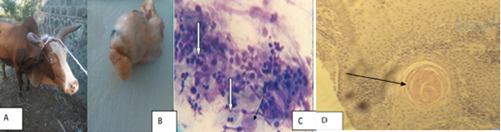
Bovine Papilloma is most commonly identified types of skin tumor in cattle and it is an exophytic growth of the epidermis of squamous cell due to bovine papilloma virus (BPV) (Constable et al., 2016). It is rarely regress without eliciting any serious clinical problems of the infected animal. However, occasionally it persists for long time and provides the focus for malignant transformation to SCC types of tumors (Moharram et al., 2019). Bovine papilloma (Bp) affects all the ages of the cattle but it commonly occurs in young animals (Feyisa, 2018). It spreads between animals through direct or indirect contact such as grooming materials and other fomite (Ugochukwu et al., 2019). Gross lesions: Bovine papilloma is found in a variety of locations throughout the body, such as on the head, neck, udder, around the eyes, shoulders, limbs, and ears It has peculiar features such as elevated and diffuse multi-nodular proliferations (Constable et al., 2016), lichenified appearance, thickened epidermis, pedunculated, firm, dense mass with rough, scaly, and dry surface; and cauliflower-like lesions (Mathewos et al., 2020) (Feyisa, 2018) (Figure 1). The color of Bovine papilloma affected part ranges from grayish-white to black (Moharram et al., 2019).
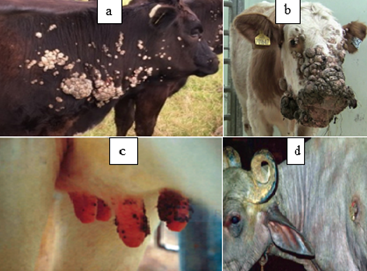
Figure 1: Gross appearance of Bovine papilloma. a = Bovine papilloma located on the neck and shoulder of cattle; b= Bovine papilloma presented on the feces of cattle (Rajmani et al., 2012) c= Bovine papilloma affected teat d=papillomatosis wart in buffalo (Kumar et al., 2015).
Histopathological features
Bovine papilloma shows prominent hyperkeratosis, acanthosis, and elongated rete-pegs surrounded by fibro-vascular stroma under light microscope (Goldschmidt and Hendrick, 2002, Feyisa, 2018). Hydropic degeneration of epidermal cells (Moharram et al., 2019) fibropapillomas ((Constable et al., 2016) (Meuten, 2020) and hypergranulosis of stratum granulosum are also showed on (Figure 3). Fibropapillomas demonstrate fibromas arise from stroma characterized by hyper-cellularity of fibroblasts forming whorls around blood vessels (Hunt, 2017).
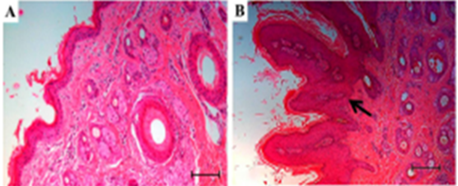
Figure 2: Histopathological analysis of papilloma, using H&E staining; A. Normal skin with epidermal attachments( 5X).; B. Papilloma showing acanthosis (arrow), (10X) (Araldi et al., 2015).
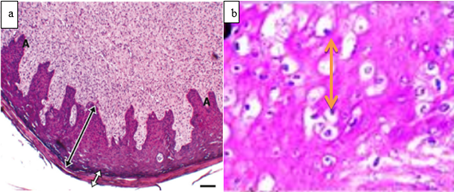
Figure 3: Histopathological presentation of bovine papilloma. a. acanthosis (A), hyperkeratosis (black dual-head arrow) and stratum spinosum layer thickening (white dual head arrow) B. Hydropic degeneration of epidermal cells demonstrated (Timurkan and Alcigir, 2017).
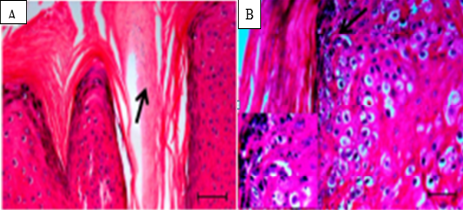
Figure 4: Histopathology of cutaneous papilloma. A. Parakeratotic hyperkeratosis indicating a high mitogenic activity (10X)., B. Hypergranulosis (arrow), ( 10X and 40X) (Araldi et al., 2015).
Squamous-Cell Carcinoma
Bovine SCC is a cancer that develops in the squamous cells of the skin, which are keratinocytes (Moharram et al., 2019). It is the second most common type of bovine tumor next to papilloma. Bovine SCC grows rapidly on different body parts, particularly common on bovine eyelids, following prolonged skin exposure to sunlight (Qadir, 2016). In addition, mechanical irritations, injuries, and burns can also lead to SCC (Moharram et al., 2019). It generally develops in cattle above the age of seven and rarely in cattle younger than three years old (Qadir, 2016). Bovine SCC is more prevalent in cattle with white hair and light-colored skin especially Holsteins and Ayrshires reed of animal (Moharram et al., 2019). In addition to UV (ultraviolet) light , bovine ocular SCC is caused by viruses though the specific causative agent is not known (Rosenberg et al., 2012, Nasir and Campo, 2008).
Macroscopic appearance
SCC appears as large nodular and cauliflower like lesions, exophytic, ulcerated and friable lesions (Qadir, 2016). As indicated in figure 5, Bovine SCC nodular, hemorrhagic accompanied with or without purulent discharge and metastasize to retro-pharyngeal lymph node are also appeared (Mathewos et al., 2020).
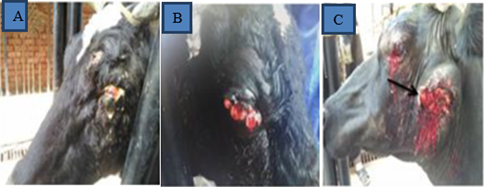
Figure 5: Gross pictures of cattle affected with ocular SCC. A shows Bovine SCC on the eyelid, B. SCC developed with haemoregic on the eyelid and C. the eyelid and the proximal part of mandible is affected and ulcer (Moharram et al., 2019).
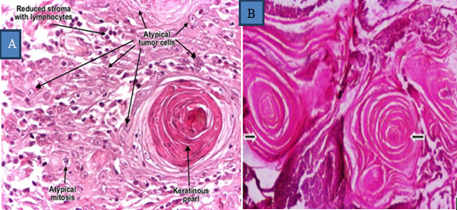
Microscopic appearance: It reveals proliferation of anastomosing nests, sheets and strands of atypical keratinocytes originating in the epidermis and infilt-rateing into the dermis(Hunt, 2017) (Goldschmidt and Hendrick, 2002), The neoplastic cells differentiated with a formation of distinct keratin pearls which are characteristic of SCC (Qadir, 2016). On higher magnification, keratin tono-filaments are visible as intracytoplasmic eosinophilic fibrillar material (Moharram et al., 2019). Mitotic figures, pleomorphism, hyperchromatism of the neoplastic cells, enlarged and prominent nucleoli and vacuolation of neoplastic cells are evident. Nuclear to cytoplasm ratio is markedly increased (Timurkan and Alcigir, 2017) Fig 6).
Figure 6: Microscopic appearance of SCC. Both A and B Tumor mass showing round masses of keratin pearls in SCC (arrow) Haematoxylin (H) &Eosin (E) x40) (Habte, 2022).
Bovine Lymphosarcoma
Cutaneous lymphosarcoma is another common type of tumor in cattle (Mathewos et al., 2020). Lymphoma is a lymph cancer that develops from lymphocytes (Doyle and Gordon, 1993). It is a diffuse malignant lymphoma that arises in the skin, lymph nodes, or other lymphoid tissue (Nagy, 2006). Lymphoma can be classified as primarily cutaneous when the lymphatic proliferation is limited to the skin with no involvement of the bone marrow, lymph nodes, or viscera at the time of the diagnosis (Sokołowska-Wojdyło et al., 2015). Lymphosarcoma in cattle may be sporadic, which is seen in calves and young herds and has no known cause, or it may result from infection with the bovine leukemia virus (BLV), often referred to as an enzootic bovine leucosis, which is seen in adults (Misdorp, 2004). BLV is spread through contaminated blood containing infected lymphocytes. Cutaneous lymphosarcoma is most common in cattle aged 1–3 years (Nagy, 2006) and is extremely rare in sheep, goats, and swine (DeVita Jr and Rosenberg, 2012). It is present in all breeds, but it is most common in Holsteins (Shim and Meltzer, 2011).
Gross lesion appearance
Cutaneous lymphosarcoma presents as cutaneous plaques, 1–5 cm in diameter, that appear as yellow-tan discrete nodular masses or a diffuse tissue infiltrate on the neck, back, rump, and thighs (Mathewos et al., 2020). This form of lymphosarcoma may undergo spontaneous remission; however, relapses may occur (Nagy, 2006). Additionally, local lymph nodes may grow (enlarge), with a color range from white to tan ((Misdorp, 2004, Neerja et al., 2018). Those tumors are often multifocal and commonly involve the neck and trunk surface, with variable-sized firm nodules and lesions that resemble an urticarial reaction The overlying skin surface may be showing normal, variable alopecia, hyperkeratosis, or ulceration as indicated in Figure7 (Sokołowska-Wojdyło et al., 2015).
Figure 7: Gross appearance of cutaneous lymphosarcoma. A= scattered lymphosarcoma on the skin and B= over congested lymphosarcoma on the skin (Mathewos et al., 2020).
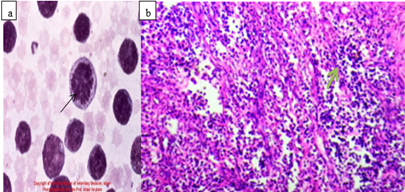
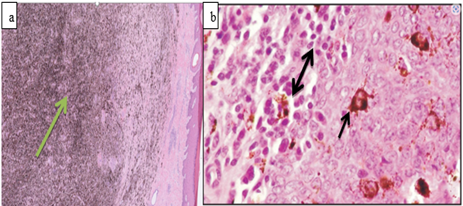
Microscopic appearance: Histological and cytological preparations showed sheets of a relatively homogeneous population of neoplastic lymphocytes. Monomorphic lymphocytic cells packed closely together make up the tumor masses (Mathewos et al., 2020) extensive dermal invasion of lymphoblast (Nagy, 2006, Sokołowska-Wojdyło et al., 2015) as showed Figure 8.
Figure 8: Microscopic appearances of lymphosarcoma; Cytological features of Lymphosarcoma (bovine leukemia) from FNAC; and tumor mass showing pleomorphic small to medium and round to oval neoplastic lymphocytes separated by a delicate collagenous fibrous tissue in lymphoma, H &E ;x40), b (Habte, 2022).
Melanoma
Melanoma is a benign form of tumor of melanocytes or pigment-generating cells (Burden, 2011). The majority of melanoma cases are on the skin surface of head, neck, trunk, or legs although they can very rarely develop in the mouth, intestines, or eye (uveal melanoma) (Hunt, 2017). Despite this, there are rare reports of locally invasive tumors as well as metastasis to distant sites. The main cause of melanoma is UV light exposure with low levels of the skin pigment melanin (Qadir, 2016). Gross lesion appearance: Melanoma tumor size in cattle varies widely from less than 5 cm to up to 25 cm (Constable et al., 2016). It appears as single or multiple, raised, firm and black (Mathewos et al., 2020). The mass is composed of a proliferation of spindle to round shaped cells frequently containing abundant black pigment (Qadir, 2016). On cross-section of the tissue, it showed as pigmented brown or black and oily (Figure 9).
Figure 9: Skin melanoma the arrow depicts the melanoma on the neck of cattle (Doyle and Gordon, 1993).
Histopathological appearance: neoplastic melanocytes are stacked in sheets in a pattern that resembles a band like pattern (Goldschmidt and Hendrick, 2002). Melanocytic tumors reveal the characteristic melanin-containing neoplastic cells often mixed with heavily pigmented melanophages and arranged in nests and clusters (Doyle and Gordon, 1993). Cellular pleomorphism and mitotic activity are variable indicated on (Figure 10). Melanoma, dendritic cell type, the dermal and/or hypodermal melanoma with dendritic and spiral cells is formed by cells with a dense, disorderly or band-like arrangement, with extremely high melanin content. Cellular details can only be seen after discoloring. Cells have polyhedral or round shapes, being present in numerous small dendritic cells. Mitoses are not numerous, but necrotic foci are frequently found, figure b (Baba and Câtoi, 2007) as showed on Figure 11.
Figure 10: Microscopic appearance of melanoma; Photomicrograph depicting a proliferation of spindle to round shaped cells containing abundant black glandular pigment.
Figure 11: Histopathological features of melanoma. The arrow depicts hyper chromatic melanocytes along the basal and suprabasal layer of the epidermis surrounded by a clear space (H&E; x100) (Šitum et al., 2014).
Occurrence Of Bovine Skin Tumors in Ethiopia
In Ethiopia, various types of tumors from different origins are a common problem in domestic animals (Feyisa, 2018)which include epithelial and mesenchymal origin tumors ((Mathewos et al., 2020). The most common tumor that occurs in cattle among those of epithelial origin is a squamous cell papilloma indicated in (Table 3). Similarly, among tumors of mesenchymal origin, a cutaneous fibrosarcoma tumor is observed on the dewlap of the bull. Cutaneous fibrosarcoma is a type of cancer that affects a specific cell known as fibroblasts (Constable et al., 2016). Epithelial-originated tumors are the most frequently occurring tumor type in Ethiopia (Mathewos et al., 2020). A study from Wolayita Sodo in Ethiopia on the cytopathological characteristics of the papilloma indicated that young animals are more susceptible to bovine papillomatosis than mature animals (Feyisa, 2018). This resulted in early age culling or slaughtering of animals which in turn attributed for significant economic implications as indicated Fig 12.
Table 3: Frequencies of tumors of epithelial origin among domestic animals
| No. | Tumor Type | No. of Cattle affected | Percent | No. of Dog affected | Percent | No. of Pig affected | Percent |
| 1 | Squamous cell papilloma | 20 | 33.3 | - | - | - | - |
| 2 | Ocular SCC | 1 | 1.7 | - | - | - | - |
| 3 | Basal Cell Carcinoma | - | - | - | 1 | 1.7 | |
| 4 | Mammary Gland Tumor | - | - | 4 | 6.7 | - | - |
| Papillary Mammary Adenocarcinoma | - | - | 1 | 1.7 | - | - | |
| Carcinoma Mixed Type | - | - | 2 | 3.3 | - | - | |
| Mammary Adenocarcinoma | - | 1 | 1.7 | - | |||
| 5 | Sertoli Cell Tumor | - | - | 1 | 1.7 | - | - |
| 6 | Atypical Anal Sac Adenocarcinoma | - | - | 1 | 1.7 | - | - |
| Total | 21 | 35 | 6 | 10.1 | 1 | 1.7 |
Source: (Feyisa, 2018, Mathewos et al., 2020)
Figure 12: Gross appearance of papilloma on different body part of cattle (Mathewos et al., 2020). Then A-G shows different part of skin affected by skin tumor.
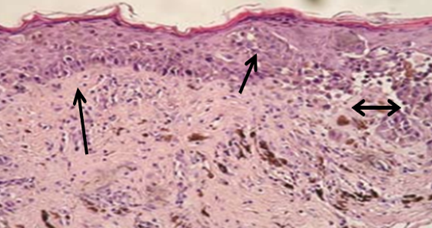
The peri-ocular SCC is located on the third eyelid of bull (Mathewos et al., 2020) and grossly identified by its raised with irregular surfaces, some portion friable, and soft (some portion) and hard (most part) in consistency, ovoid in shape and white to gray in color (Feyisa, 2018, Moharram et al., 2019) indicated on Figure 13.
Figure 13: Gross and microscopic appearance of peri-ocular SCC. A and B represents the macroscopic features, C and D shows the microscopic appearance of peri-ocular SCC from the third eyelid of the bull (Mathewos et al., 2020). The arrow indicates the tumor and carcinogenic cell of histopathology
Cause And Risk Factor of Skin Tumor
Most tumors are linked with problems in gene expression. However, the specific causes for various skin tumors remains unknown (Hunt, 2017). Several decades investigations indicated that different factors are induces the development of skin tumor. The actual causes of most skin malignancies, which have been connected to a problem with gene expression, are still unknown despite decades of research and advancements in early identification and therapy(Achalkar, 2019, Hunt, 2017). These factors include excessive exposure to UV light, exposure to chemical toxins (e.g., aflatoxins and reactive oxygen radicals) (Anthony, 2018), oncogenic viruses (DeVita Jr and Rosenberg, 2012), and spontaneous changes in DNA replication. More than 90 percent of skin tumors are associated with UV radiation (Gallagher et al., 2010, Feyisa, 2018, Mathewos et al., 2020). Furthermore, skin color, species, and lack of pigment on the skin Centers for Disease Control and Prevention (2019), age, breed, sex of individuals, immune system disorders, and other inherited disease that causes sensitivity to the sun are all contributing factors to DNA mutations thereby developing either benign or malignant form of tumor. The cattle's white color increases the severity of a skin tumor. Additionally, exotic cattle are more prone to tumors than indigenous breeds (Ramos-Vara and Miller, 2014).
Pathogenesis And Development of Skin Tumor
Development of Skin Tumor
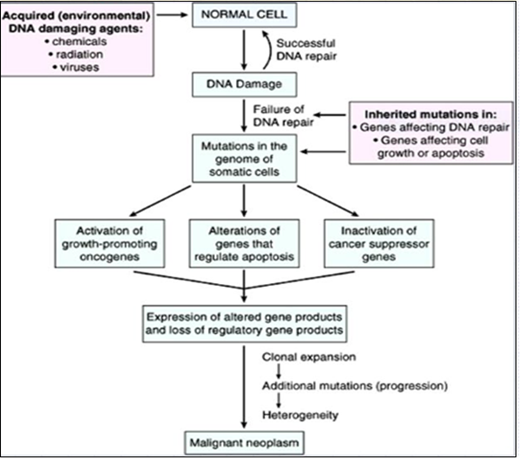
The mechanisms that lead to the formation and development of skin tumors are similar to other cancer types (Ok et al., 2018). Damage a cell's genetic machinery is the widely acknowledged etiology for all types of cancer (Gallagher et al., 2010). In general, the development of tumor lesion is a complex phenomenon and usually it is unpredictable or uncertain (Shim and Meltzer, 2011). However, three phases are recognized in many tumors, namely, mutation (altered DNA replication), promotion, and irreversible tumor growth (Hunt, 2017). The physiological process of tissue repair sometimes leads to abnormal process when multiple foci of micro damage occur in the organism (Ruddon and Holland, 2003) (Rosenberg et al., 2012, Bukhtoyarov and Samarin, 2015). The pathophysiological mechanism of multiple permanent (long-lasting) tissue micro damages with sympathetic dominance to sustain cell proliferation while inhibiting anti-tumor immunity (Hunt, 2017). Cancer reparative trap is the resistant pathophysiological condition of the organism that contributes to the appearance, development, and generalization of cancer indicated on Figure 14 (Bukhtoyarov and Samarin, 2015).
Figure 14: Pathogenesis of tumor (cancer) (Bukhtoyarov and Samarin, 2015)
Skin Tumor Metastasis
Metastasis refers to the development of secondary cancer in locations other than the primary tumor (Shim and Meltzer, 2011)). The cells continue to proliferate at new areas, eventually forming further tumors made up of cells that resemble the tissue of origin. The spread of cancerous cells from a primary tumor to the skin is referred to as skin metastasis (Shim and Meltzer, 2011, Hassanein and Mahmoud, 2009). More genetic modifications provide advantageous properties, such as the ability to attach cells, degrade the matrix, induce normal cell killing/lytic factors secretion, increase cell motility, and spread across the tissue, as well as demonstrate growth autonomy, resistance to anti-growth signals, and apoptosis evasion (immortality) (Hassanein and Mahmoud, 2009) and angiogenesis aids in further cancer growth and spreading in different tissues (Kumar et al., 2015). Several classes of molecules are involved in metastatic cascade; including molecules that govern invasion (degradative enzymes and motility factors) and adhesion (integrins, selectins, and cadherins) ((Ok et al., 2018, Solomon and Authority, 2003, Marcus et al., 2014).
Diagnosis of skin Tumor
Diagnosis of skin tumor can be performed both clinically and Histopathological. Clinically, skin tumor can be identified through palpation and percussion of a raised and swollen, firm, nodular mass, or any non-healing growth or lesion, as well as growth that is profusely bleeding, should be suspected of being a tumor (cancer) (Misdorp, 2004)Histopathological diagnosis of skin tumor involves tissue biopsy (histopathology) and FNAC ((Marcus et al., 2014)).
Histopathological examination
Histopathology is a method by which a diagnosis can be made to understand abnormal cellular characteristics such as anaplasia, invasion, extreme mitosis, metastasis, and loss of polarity, which indicate malignancy ((Gallagher et al., 2010); (Timurkan and Alcigir, 2017). It used to differentiate benign from malignant tumors. A skin or tissue biopsy is utilized to perform cytological and histological studies and typically regarded as the gold standard for making a final diagnosis. High cellularity, cellular enlargement, an increased nuclear/cytoplasmic ratio, nuclear hyperchromatic, cell Di cohesiveness, and prominent and large nucleoli (Thomas et al., 2016)are all characteristics of a cancer cell. Moreover, cellular and nuclear pleomorphism and background tumor necrosis are some of the histological characteristics of tumor cells (Goldschmidt and Hendrick, 2002).
Application of Immunohistochemistry for tumor diagnosis
It is the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues (Wu, 2015). After tissue sections are incubated with the prospective antibodies, positive reactions (tumor antigen-antibody binding) are identified through the application of detection system (Kabiraj et al., 2015, Ramos-Vara and Miller, 2014). Immunohistochemistry is used to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of biological tissue (Pohanka, 2009). Tumor markers are biochemical indicators to identify the presence of tumors. These include cell surface proteins, cytoplasmic proteins, enzymes, or hormones (Gireesh, 2019; Pohanka, 2009). This system tends to be very sensitive because it allows for the attachment of a relatively large number of enzyme molecules, such as peroxidase, at the antigen site (Kabiraj et al., 2015) Specific molecular markers are characteristic of particular cellular events such as proliferation or cell death (apoptosis) example cells (keratinocytes), antibodies tumors and markers (Rajmani et al., 2012). Visualizing an antibody-antigen interaction can be accomplished in a number of ways, mainly either of the following: chromogenic immunohistochemistry, wherein an antibody is conjugated to an enzyme, such as peroxidase (the combination being termed immunoperoxidase), that can catalyze a color-producing reaction (Ramos-Vara and Miller, 2014), usually diaminobenzidine (brown) or aminoethylcarbazole (red), with which the enzyme reacts (Kabiraj et al., 2015). Immunofluorescence, which is the antibody, is tagged to a fluorophore, such as fluorescence (Ramos-Vara and Miller, 2014).
Cytological examination
Cytological examination of tumor can be done by using the fine needle aspiration cytological (FNAC) technique. Cytology is an important tool that can help the veterinarian to distinguish a tumor from inflammatory lesions ((Timurkan and Alcigir, 2017, Rajmani et al., 2012). It involves cell sampling by inserting a thin hollow needle into the tissue mass, staining and then examined under a microscope (Kumar et al., 2015, Thomas et al., 2016). Other techniques may include dermoscopy, various imaging modalities such as X-ray, computed tomography scan, positron emission tomography, ultrasound, and magnetic resonance imaging can be possibly used for the diagnosis of tumor (Ramos-Vara and Miller, 2014); (Rosenberg et al., 2012) and tests such as urine and blood tests (Timurkan and Alcigir, 2017).
Economic Impact of Skin Tumor
Cattle provide 2.38 million hides annually (Thomas et al., 2016)and contributed for GDP of a country. In Ethiopia, skin and hide accounts for 14–16% of all export revenue (Nigussu, 2014). However, the quality of raw materials continues to be challenges which hamper the leather industries substantially. One of the main causes of poor quality of skin is due to skin tumor and are responsible for multidimensional significant economic losses (Kumar et al., 2015). Skin and hide market share value has declined overtime as a result of a decline in the price of leather on the global market arises from a decline in the quality of the skin (Thomas et al., 2016), (Solomon and Authority, 2003). The economic loss due to skin tumor is associated with morbidity and mortality of domestic animals (Mathewos et al., 2020). Furthermore, morbidity of animals resulted in a drop in body weight, reduced productivity, and reproduction, calving/milking difficulty, carcass condemnation, treatment cost, affects skin quality and reduces its value, suckling problems, and mortality (Kumar et al., 2015). Peri-ocular SCC is causes blindness in cattle which leads to early age culling of animals (Mathewos et al., 2020) and responsible for about 12% of carcass condemnation (Rajmani et al., 2012). Another common skin tumor is lymphosarcoma that caused extensive organ condemnation (Chen et al., 2016) and accounts for 13.5% of beef cattle condemnations and 26.9% of dairy carcass condemnations (Mauldin, 2019). The suggested estimated annual financial loss from the condemnation of carcasses affected by all types of tumors is approximately 2.2 million dollars (Mathewos et al., 2020). This highlights the economic impact of skin disease. Skin tumors in the cattle industry cause an increased culling rate due to metastases to other critical organs, such as the lungs, liver, and draining lymph nodes, which in turn cause severe complications (Kumar et al., 2015, Mauldin, 2019). Management of skin cancer yielded data from over 880,000 healthcare providers who received 77 billion dollars in Medicare payments (Chen et al., 2016).
Management And Medication of Skin Tumor
Prevention and management of tumor is challenging due to the presence of numerous potential environmental influences that result in normal DNA replication alteration (Mauldin, 2019). However, early diagnosis and treatment are remains an important approach for effective management before it undergoes metastasis (Meuten, 2020). In addition, strengthening national policies and programs to raise awareness and reduce exposure to risk factors such as prolonged UV radiation exposure is important (Achalkar, 2019). The type of management and/or treatment methods for skin tumors may depend on the stage of cancer, the type of tumor, and the likelihood of cancer spreading or regressing (healing) (Thomas et al., 2016). The most common management methods used for skin tumors are radiation therapy, chemotherapy, and surgical removal. Electroporation, gene therapy, Cryotherapy (using liquid nitrogen or carbon dioxide to freeze and destroy cancer cells) (Timurkan and Alcigir, 2017), and immunotherapy via administering inactivated tumor cells (Bukhtoyarov and Samarin, 2015). If the tumor is localized and limited to a specific place, surgery is more important; if it is widely spread, chemotherapy and radiotherapy are more important (Kumar et al., 2015).
Chemotherapy and Vaccination
Chemotherapy can be administered subcutaneously, orally, or intravenously. The topical therapy drugs used for non-melanoma skin cancer (SCC and papilloma) are imiquimod and 5-fluorouracil (Timurkan and Alcigir, 2017). Systemic chemotherapy uses anticancer (cytotoxic) drugs that travel through the bloodstream or metastasis to destroy cancer cells (Ugochukwu et al., 2019, Thomas et al., 2016). Some common chemotherapy drugs used to kill cancer cells such as melanoma or slow their growth are cisplatin and paclitaxel (Taxol) (Kumar et al., 2015). Intravenous infusions with magnesium chloride, which have been reported to be effective in bovine cutaneous papillomatosis when the lesions are few, are recommended, together with treatment with Ivermectin (Ugochukwu et al., 2019). Drug therapy aids in the destruction of cancer cells on the skin or in the body, slows or stops the growth and spread of cancer (Šitum et al., 2014)shrinks a tumor before other treatments such as surgery or radiation therapy, destroys cancer cells left behind after surgery and relieves or controls the symptoms of advanced non-melanoma skin cancer (Solomon and Authority, 2003).
Vaccination
Vaccination remains the best prevention method against papillomatosis. BPV vaccine strategies are based on the use of recombinant BPV L1 protein vaccine and intradermal administration of autogenously produced wart vaccine in bovine papillomatosis (Ugochukwu et al., 2019). Due to a lack of vaccinations, the only attempts to reduce the risk of enzootic bovine lymphosarcoma are by controlling the spread of the bovine leukemia virus and, for sporadic cases, reducing the risk factors. There is no curative treatment for viral infection or for lymphosarcoma in cattle (Habte, 2022).
Radiation Therapy
Radiation therapy involves the exposure of the cancerous part of the body to high doses of radiation which can destroy rapidly growing cells and shrink tumors, which are targeted to the specific part of the body where the cancer is developing (Hennequin et al., 2016, Low and Sahi, 2012). Radiation can be applied after surgery has done as an adjuvant to kill any small areas of remaining cancer cells that may not have been visible during surgery (Hennequin et al., 2016).
Conclusion
Skin tumor is the most frequently diagnosed neoplastic disorder in bovines. Bovine papilloma and squamous cell carcinoma are the most commonly diagnosed and economically important tumors in cattle. Although the specific causative agent remains unknown, most skin tumors are associated with prolonged exposure to ultraviolet radiation. Skin tumors result in sizable economic losses due to reduced productivity, reduced reproduction, calving and milking difficulties, treatment costs, suckling problems, early culling, carcass condemnation, and management costs. Given skin tumor is an economic significant disease in Ethiopia it needs attentions from researchers, and concerning centers for control and preventions. Early diagnosis and management of the diseases is important.
Declarations
Ethical consideration
The current review has been approved for its ethical soundness for the time from November 2023 to March 2024 by the Institutional Ethical Review Board (IRB) of the College of Veterinary Medicine and Animal Sciences, University of Gondar, Ethiopia. And it has been given at reference (Reference No: CVMAS.Sc.16.282027).
Author’s Contribution
Dr. Mengesha Ayehu Getnet has written the draft of the paper; Dr. Asnakew Mulaw Berihun supervises the drafts, and he has commented on the work of the reviewed manuscript. All authors checked and approved the final draft of the manuscript. Therefore, all authors have participated accordingly.
Consent for publication
All the participants in this research have played a great role in the preparation of this paper, and we have all observed the final product of the finished paper, evaluated any corrections and updates, and finally, assured that the paper is very good. And the paper should have a great role in the cattle production system, accompanied by cancer prevention and control system. So, at the end of the day, all authors decided, as we have great consent for the publication of this paper. I confirm the corresponding author has read the journal policies and submit this manuscript in accordance with those policies.
Data availability
The data to this review is not available right now because we have not used any data to prepare this review.
Competing interests
This manuscript has not been published elsewhere, nor are they under consideration by another publisher. I declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the review and/or discussion reported in this paper. So that and we declare that there is no conflict of interest.
Funding
Not applicable
Acknowledgments
Not applicable.
References
- Achalkar, G. V. (2019). Clinicopathological evaluation of non-neoplastic and neoplastic skin lesions: A study of 100 cases. Indian J Pathol Oncol, 6:118-122.
Publisher | Google Scholor - Araldi, R., Melo, T., Neves, A., Spadacci-Morena, D., Magnelli, R., Modolo, D., De-Sá-Júnior, P., Mazucchelli-De-Souza, J., Carvalho, R. & Beçak, W. (2015). Hyperproliferative action of bovine papillomavirus: genetic and histopathological aspects. Genetics and Molecular Research, 14:12942-12954.
Publisher | Google Scholor - Baba, A. I. & Câtoi, C. (2007). Epithelial and melanocytic tumors of the skin. Comparative Oncology. The Publishing House of the Romanian Academy.
Publisher | Google Scholor - Bukhtoyarov, O. V. & Samarin, D. M. 2015. Pathogenesis of cancer: Cancer reparative trap. Journal of Cancer Therapy, 6:399-412.
Publisher | Google Scholor - Burden, K. 2011. Melanomas and their effect on the grey horse. Young Scientists Journal, 4:75.
Publisher | Google Scholor - Chen, J. T., Kempton, S. J. & Rao, V. K. (2016). The economics of skin cancer: an analysis of Medicare payment data. Plastic and Reconstructive Surgery–Global Open, (4):e868.
Publisher | Google Scholor - Constable, P. D., Hinchcliff, K. W., Done, S. H. & Grünberg, W. (2016). Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats, Elsevier Health Sciences.
Publisher | Google Scholor - Devita Jr, V. T. & Rosenberg, S. A. (2012). Two hundred years of cancer research. New England Journal of Medicine, 366:2207-2214.
Publisher | Google Scholor - Doyle, K. & Gordon, H. (1993). Merck veterinary manual. Australian Veterinary Journal, 70:278-278.
Publisher | Google Scholor - Feyisa, A. (2018). Cutaneous bovine papillomatosis (warts) treatment outcome using ivermectin: a case of crossbred heifer and calf. Journal of Veterinary Science and Technology, 9, 2.
Publisher | Google Scholor - Gallagher, R. P., Lee, T. K., Bajdik, C. D. & Borugian, M. (2010). Ultraviolet radiation. Chronic diseases and injuries in Canada, 29.
Publisher | Google Scholor - Goldschmidt, M. & Hendrick, M. (2002). Tumors of the skin and soft tissues.
Publisher | Google Scholor - Habte, D. (2022). Subcutaneous Benign Tumour; A Case of an Ox and Its Management Outcome; A Case Report. Journal of Cancer Research Reviews & Reports, 4:1-3.
Publisher | Google Scholor - Hassanein, K. M. & Mahmoud, A. (2009). Pathological studies on tumor incidence in farm animals. Alex. J. Vet. Sci, 28:105-117.
Publisher | Google Scholor - Hennequin, C., Rio, E. & Mahé, M.-A. (2016). Radiothérapie des cancers cutanés. Cancer/Radiothérapie, 20: S249-S255.
Publisher | Google Scholor - Hunt, J. L. (2017). Neoplasia and pre-neoplasia in head and neck mucosal sites. Pathology, 49:S9.
Publisher | Google Scholor - Kabiraj, A., Gupta, J., Khaitan, T. & Bhattacharya, P. T. (2015). Principle and techniques of immunohistochemistry—a review. Int J Biol Med Res, 6:5204-5210.
Publisher | Google Scholor - Kumar, R., Srivastava, R. & Srivastava, S. (2015). Detection and classification of cancer from microscopic biopsy images using clinically significant and biologically interpretable features. Journal of medical engineering.
Publisher | Google Scholor - Low, G. & Sahi, K. (2012). Clinical and imaging overview of functional adrenal neoplasms. International journal of urology, 19:697-708.
Publisher | Google Scholor - Marcus, A., Gowen, B. G., Thompson, T. W., Iannello, A., Ardolino, M., Deng, W., Wang, L., Shifrin, N. & Raulet, D. H. (2014). Recognition of tumors by the innate immune system and natural killer cells. Advances in immunology, 122:91-128.
Publisher | Google Scholor - Mathewos, M., Demissie, T., Fesseha, H. & Yirgalem, M. (2020). Histological, cytological characteristics and treatment options on common skin tumors of domestic animals: A review. Int J Recent Biotechnol, 8:1-24.
Publisher | Google Scholor - Mauldin, E. A. (2019). Book Review: Surgical Pathology of Tumors of Domestic Animals: Volume 1. Epithelial Tumors of the Skin. SAGE Publications Sage CA: Los Angeles, CA.
Publisher | Google Scholor - Meuten, D. J. (2020). Tumors in domestic animals, John Wiley & Sons.
Publisher | Google Scholor - Misdorp, W. (2004). Mast cells and canine mast cell tumours. A review. Veterinary quarterly, 26:156-169.
Publisher | Google Scholor - MOHARRAM, I., AWADIN, W., SALEM, M. & MOSBAH, E. (2019). A survey of tumors affecting cattle, buffaloes and sheep, in El-Dakahlyia Governorate. Mansoura Veterinary Medical Journal, 20:37-45.
Publisher | Google Scholor - Nagy, D. W. (2006). Decreasing perinatal bovine leukosis virus infection in calves. University of Missouri--Columbia.
Publisher | Google Scholor - NASIR, L. & CAMPO, M. S. (2008). Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids. Veterinary dermatology, 19:243-254.
Publisher | Google Scholor - Neerja, T., Sujata, P., Bhushan, P. & Umesh, S. (2018). Abstracts-USICON 2018. Indian Journal of Urology, 34: S7-S72.
Publisher | Google Scholor - Nigussu, F. (2014). Characterization of sheep and goat skin lesions caused by different agents and impact on the respective leathers at tanneries. Msc thesis Addis Ababa University, Ethiopia.
Publisher | Google Scholor - OK, C., WODA, B. & KURIAN, E. (2018). The pathology of cancer. University of Massachusetts Medical School: Worcester, MA, USA.
Publisher | Google Scholor - QADIR, M. I. (2016). Skin cancer: Etiology and management. Pakistan journal of pharmaceutical sciences, 29.
Publisher | Google Scholor - RAJMANI, R., MISHRA, M., SINGH, P. K., SAHOO, A. P., TIWARI, A. K., DOLEY, J. & KUMAR, G. R. )2012). Common neoplasms in animals—An overview. Journal of Animal Research, 2:127-137.
Publisher | Google Scholor - Ramos-Vara, J. & Miller, M. (2014). When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry—the red, brown, and blue technique. Veterinary pathology, 51:42-87.
Publisher | Google Scholor - ROSENBERG, L., KRIEGER, Y., SILBERSTEIN, E., ARNON, O., SINELNIKOV, I. A., BOGDANOV-BEREZOVSKY, A. & SINGER, A. J. (2012). Selectivity of a bromelain based enzymatic debridement agent: a porcine study. Burns, 38:1035-1040.
Publisher | Google Scholor - Ruddon, R. & Holland, F. (2003). What makes a cancer cell a cancer cell. Hoolland-Frei Cancer Medicine.
Publisher | Google Scholor - Shim, H. & Meltzer, C. C. (2011). Introduction: molecular imaging in oncology. Seminars in Oncology, 1-2.
Publisher | Google Scholor - Šitum, M., Buljan, M., Kolić, M. & Vučić, M. (2014). Melanoma–clinical, dermatoscopical, and histopathological morphological characteristics. Acta Dermatovenerologica Croatica, 22:2-2.
Publisher | Google Scholor - Sokołowska-Wojdyło, M., Olek-Hrab, K. & Ruckemann-Dziurdzińska, K. (2015). Primary cutaneous lymphomas: diagnosis and treatment. Advances in Dermatology and Allergology/Postępy Dermatologii i Alergologii, 32:368-383.
Publisher | Google Scholor - Solomon, A. & Authority, E. L. M. (2003). Livestock marketing in Ethiopia: a review of structure, performance, and development initiatives.
Publisher | Google Scholor - Thomas, W. E., Reed, M. W. & Wyatt, M. G. (2016). Oxford textbook of fundamentals of surgery, Oxford University Press.
Publisher | Google Scholor - Timurkan, M. O. & Alcigir, M. E. 2017. Phylogenetic analysis of a partial L1 gene from bovine papillomavirus type 1 isolated from naturally occurring papilloma cases in the northwestern region of Turkey. Onderstepoort Journal of Veterinary Research, 84:1-6.
Publisher | Google Scholor - Ugochukwu, I. C. I., Aneke, C. I., Idoko, I. S., Sani, N. A., Amoche, A. J., Mshiela, W. P., Ede, R. E., Ibrahim, N. D. G., Njoku, C. I. O. & Sackey, A. K. B. (2019). Bovine papilloma: aetiology, pathology, immunology, disease status, diagnosis, control, prevention and treatment: a review. Comparative Clinical Pathology, 28:737-745.
Publisher | Google Scholor