Case Report
Paget’s Disease of The Nipple
- Hadhami Aidi *
- Ines Mekhinini
- Mariem Romdhani
- Sana Ghades
- Hammadi Jawaher
- Ben Ali Yasmine
- Ben Farhat Imen
- Knaz Samar
- Ridha Fatnassi
Department of Obstetrics and Gynecology, IBN El Jazzar University Hospital, Kairouan, Tunisia.
*Corresponding Author: Hadhami Aidi, Department of Obstetrics and Gynecology, IBN El Jazzar University Hospital, Kairouan, Tunisia.
Citation: Aidi H., Mekhinini I., Romdhani M., Ghades S., Jawaher H., et al. (2024). Paget’s Disease of The Nipple, Clinical Obstetrics and Gynecology Research, BioRes Scientia Publishers. 3(3):1-7. DOI: 10.59657/2992-9725.brs.24.020
Copyright: © 2024 Hadhami Aidi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 02, 2024 | Accepted: December 19, 2024 | Published: December 26, 2024
Abstract
Suspicion for Paget’s disease should always be raised by any persistent unilateral lesion of the nipple, and in such cases cytological scraping or nipple biopsy is indicated for diagnosis. Breast imaging should evaluate any underlying neoplasm of the breast-which is present in more than 80% of cases-and the presence of multifocal region, which has a high incidence rate. Preoperative MRI of the breast is helpful when conservative surgery is considered because of the very high percentage of cancers that remain occult at mammography and breast ultrasound. It should be noted, however, that erosive adenomatous nipple disease, a benign condition, may mimic Paget disease of the nipple. Diagnosis by biopsy is thus required. Therapy ranges from radical surgery to areolar-nipple complex excision (PAMectomy), possibly with subsequent radiotherapy. Such conservative management is, of course, dependent upon the absence of coexisting breast cancer. The prognosis of Paget’s disease of the nipple is related to whether there is an associated palpable mass, the stage and the invasiveness of the underlying cancer.
Keywords: paget’s disease of the nipple; breast cancer; eczema
Introduction
Paget’s disease of the breast is a very rare cancer that was first described in 1874 by Sir James Paget and corresponds to an intraepidermal adenocarcinoma of the nipple-areolar complex. It is almost invariably associated with galactophoric ductal adenocarcinoma. Diagnosis is confirmed through histopathological examination with immunohistochemistry. Paget cells express low molecular weight cytokeratins, notably cytokeratin 7 (CK7), with near-100% sensitivity, thus confirming their diagnosis.
In this respect, we report three cases, together with a review of the existing literature, to describe the clinical features and therapeutic management of the disease.
Case 1
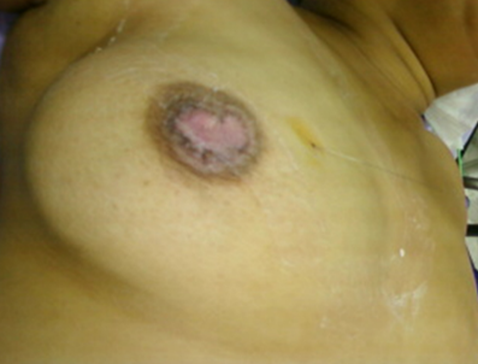
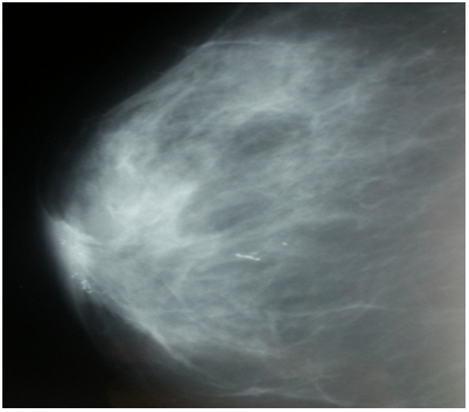
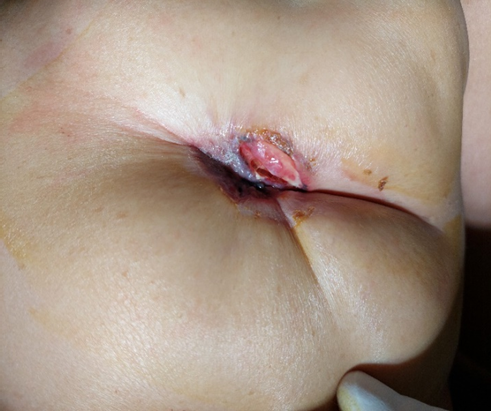
A 46-year-old woman, with a family history of breast cancer (sister affected) presented to our outpatient department with a nipple erosion. The lesion began as a scaly plaque involving the right nipple and the areola, evolving rapidly over the past month, prompting her consultation. Physical examination revealed an erythematous, ulcerated lesion with destruction of the right nipple (Figure 1). The remainder of the right breast exam showed no palpable nodules or nipple discharge. Axillary lymph nodes were clear. Breast ultrasound and mammography identified suspicious microcalcifications in the retroareolar region and another site at the junction of the internal quadrants on the right (Figure 2).
Figure 1: Erythematous, ulcerated lesion leading to destruction of the right nipple in a patient with high-grade intraductal carcinoma with Paget's disease of the nipple.
Figure 2: Breast ultrasound showing a suspicious area of microcalcifications in the right retroareolar region, with another focus at the junction of the right internal quadrants. This image is from a patient who consulted for a lesion that began as a scaly plaque involving the right nipple and areola, which had progressed rapidly in the month prior to consultation.
Guided excision and nipple biopsy were performed. Histopathological findings indicated high-grade ductal carcinoma in situ with Paget’s disease of the nipple; surgical margins were narrow. Further evaluation with abdominal ultrasound, chest radiography, bone scintigraphy, and CA15-3 levels was negative. A Patey mastectomy was performed with an uncomplicated postoperative course. Final histopathology revealed minimal residual high-grade ductal carcinoma in situ in the retroareolar area, associated with Paget’s disease of the nipple, and no nodal metastases in 12 sampled lymph nodes. Adjuvant treatment deemed unnecessary. The patient’s five-year follow-up shown a favorable outcome.
Case 2
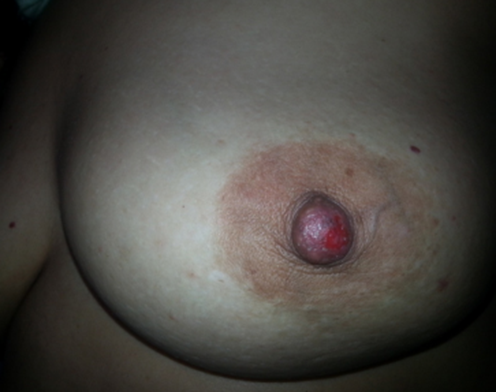
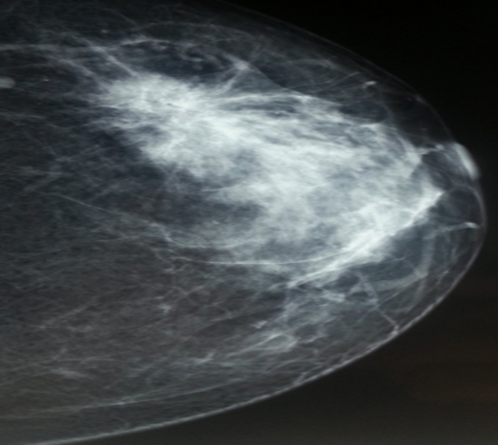
A 48-year-old woman, para 6, with no family history of breast cancer and a history of breastfeeding, came in with concern for a left breast lump detected by self-examination, accompanied by an ulcerative-crusted nipple lesion on the same side, persisting for four months. She had previously received topical corticosteroid treatment, but due to lack of improvement, she returned for further evaluation. Breast examination revealed a poorly defined, 4 cm mass in the upper quadrant of the left breast, mobile relative to both the skin and underlying tissue. An oozing ulcerative lesion was noted on the nipple (Figure 3) whole the Axillary lymph node was free of palpable adenopathy. Imaging results showed a stellate mass in the left breast, measuring 44 mm and classified as ACR4 (Figure 4).
Figure 3: Oozing ulcerative lesion on the Nipple of a patient with grade III Scarff-Bloom-Richardson invasive ductal carcinoma, concurrent d with Paget’s disease.
Figure 4: Left breast Mammogram of a patient who presented with a self-detected left breast nodule and an ulcerative-crusted nipple lesion persisting for four months. The image shows a 44 mm stellate lesion in the upper-outer quadrant of the left breast, classified as ACR4.
The Core biopsy result confirmed a grade III invasive ductal carcinoma, as per the Scarff, Bloom, and Richardson (SBR) histologic grading system. Nipple scraping Cytology identified pagetoid cells. Staging work up-including abdominal ultrasound, chest X-ray, bone scintigraphy, and CA 15-3 levels-found no metastatic disease. A left mastectomy with ipsilateral axillary lymph node dissection was performed. Histopathology result confirmed a grade III SBR invasive ductal carcinoma with Paget’s disease of the nipple and involvement of 2 out of 11 lymph nodes and capsular rupture (Figure 5). The tumor was Hormone receptors positive and Her2 Neu-Negative.
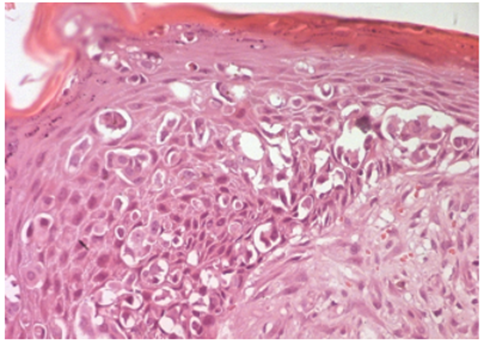
Figure 5: Histological analysis of the left mastectomy specimen from a patient with grade III Scarff-Bloom-Richardson invasive ductal carcinoma and concurrent Paget’s disease, showing pagetoid cells embedded within the squamous epithelium of the nipple. Hematoxylin-eosin stain, ×200.
The patient received a regimen of chemotherapy (eight cycles of 5-fluorouracil, epirubicin, and cyclophosphamide [FEC] 100), along with hormone therapy and locoregional radiotherapy. A favorable outcome with no recurrence or metastasis was seen during the two years follow up.
Case 3
Patient N.M., a 64-year-old female, nulligravid, with no family history of cancer, presented in our department due to left nipple retraction evolving over the past five years (Figure 6).
Figure 6: Retracted breast with crusted nipple in a patient with grade II invasive carcinoma of no special type and Paget’s disease of the nipple.
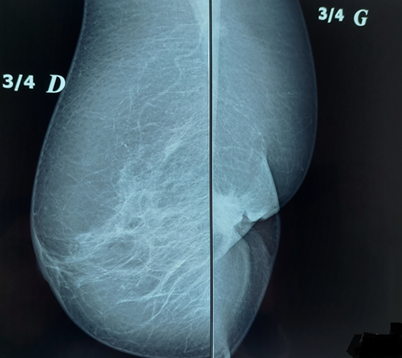
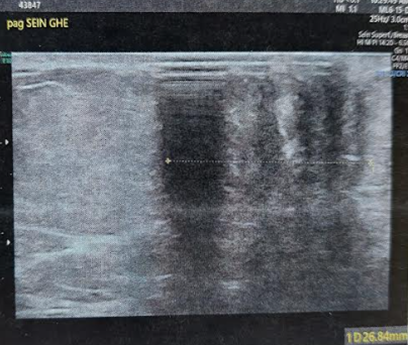
On senological examination, the left breast appeared retracted with a firm, ill defined, retroareolar nodule measuring 3 cm, fixed to deeper tissues. The nipple was barely visible and showed crusted lesions without discharge. The ipsilateral axilla was free of lymphadenopathy and the right breast examination was unremarkable. On mammography, the left breast, reduced in volume, revealed a large, spiculated retroareolar opacity of 35 mm and showing significant ultrasound beams attenuation on breast ultrasound with an associated broad area of architectural distortion (Figures 7 and 8). These findings were classified as ACR4B. Micro biopsy results of the lesion and the nipple was indicated an invasive breast carcinoma of no special type (NST), Scarff-Bloom-Richardson grade II, with a minimal mucinous component (10%) and pagetoid cells embedded the nipple.
Figure 7: Mammographic view of both breasts on an oblique 45° projection, demonstrating left breast retraction and a stellate retroareolar opacity.
Figure 8: Ultrasound appearance of the left retro nipple lesion, showing strong ultrasound attenuation.
The tumor was classified as luminal B molecular subtype with a Ki67 proliferation index estimated at 30
Discussion
In 1874, Sir James Paget first described Paget’s disease as an eczematoid nipple lesion linked to underlying breast cancer [1]. This rare condition represents only 1-4% of breast cancers, with less than 5% of male breast cancers, where it is often associated with Klinefelter syndrome. It predominantly affects postmenopausal women, with a mean age of 62.6 years [2]. To date, no specific clinical or epidemiological factors have been identified as predisposing factors to this disease [2]. While isolated in 1.4-13.3% of cases, Paget disease is associated with underlying breast carcinoma in 82-100% of cases [3]. In 13.3-52% of cases, it involves ductal carcinoma in situ (DCIS) [4]; while 30-60% present with invasive ductal carcinoma [3]. Two main theories on histogenesis have been proposed with the more accepted epidemiologic theory suggesting a migration of Paget cells from an underlying carcinoma [5]. An alternative theory posits the malignant transformation of nipple keratinocytes independently of any associated breast pathology [6].
Clinical Presentation
Typically, Paget’s disease manifests unilaterally. In its Early stages, Paget disease is marked by itching, followed by erythema and a shiny appearance of the nipple. In intermediate stages, the nipple becomes thickened, rough, and scaly. In late stages, it shows oozing ulceration and crusting with sharp borders. Nipple retraction and loss of nipple structure gradually develop, with centrifugal spread from nipple to areola. Bloody or serous discharge occurs in 33-60% of cases, while a palpable mass accompanies nipple changes in 33-50% of cases, with 20-67% of these masses being distant from the nipple [3]. Transient improvements and pigmented forms resembling melanoma have also been documented. Diagnosis of Paget’s disease is established through cytological scraping of the nipple or an areola-nipple biopsy (punch biopsy). Paget cells, typically 1.5 to 2 times larger than neighboring keratinocytes, have clear cytoplasm, irregular hyperchromatic nuclei, and mitoses visible in both CM and standard histology. In confocal microscopy (CM), these cells appear as dark intraepidermal zones or as a “target” structure, with a central hyperreflective core surrounded by a dark halo [7,8]. Typically expressing CK7, Paget cells reach close to 100% diagnostic sensitivity, although rare cases lack CK7 expression [9].
The primary differential diagnosis includes erosive Adenoma of the Nipple (EAN), a benign tumor a benign tumor of the lactiferous ducts, and nipple eczema, generally linked to atopy. Clinically, it presents itself as a unilateral nipple thickening, ulceration, and in 60% of the cases as a bloody discharge, with a possible palpable nodule beneath the nipple. On histopathology samples reveal a benign mixed adenomatous and papillary proliferation in the nipple’s lactiferous duct [10]. Nipple Eczema, primarily associated with atopic conditions, affects the areola- nipple region bilaterally and is accompanied with an intense pruritus but no nipple deformation. Treatment relies on intermittent topical corticosteroids application [11]. Mammography detects abnormalities in 35-78% of cases [4-12], with the most frequent finding being microcalcifications (44%) [4], which are often punctate or linear within the nipple-areolar complex and may cluster patches. Other findings, each present in approximately 20% of cases, [4] include masses, thicked peri-nipple skin, macrocalcifications, and focal density asymmetry. Clinical examination and mammography/ultrasound have limited sensitivity for detecting carcinoma associated with Paget’s disease. Studies have shown that in cases of Paget’s disease without a palpable mass or mammographic abnormalities, histology still detects approximately 43% of occult cancers, 33-75% of non-invasive cancers, and 5-9% of invasive cancers [12].
Paget’s disease frequently displays multifocality and multicentricity, with multifocality rates reported between 20-41% [4]. Breast MRI is therefore recommended for patients with Paget’s disease and a Bi-RADS 1 or 2 mammogram, or uncertain tumor extent. MRI can reveal abnormal enhancement or nipple-areolar thickening and is particularly valuable for identifying associated in situ or invasive cancers [13]. Historically, mastectomy has long been the standard of care, mainly due to potential multifocality. However, some teams now propose a conservative approach called pamectomy, combined with radiotherapy to the entire breast in selected patients without a palpable mass or mammographic abnormalities. Studies have demonstrated that long-term survival in these patients is comparable to that of those who underwent mastectomy [2]. With the high rate of occult cancers in Paget’s disease, sentinel lymph node biopsy is advised for all patients [14].
Conclusion
Adjuvant therapies, such as chemotherapy and hormone therapy, are offered based on the same criteria used for associated breast carcinomas. The prognosis for Paget’s disease depends mainly on the invasiveness of any associated carcinoma, with invasive forms linked to a more aggressive disease course. Paget’s disease generally portends a more aggressive course when associated with underlying malignancy.
Declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors are sincerely grateful to the patients for giving the permission to share this informative report.
Data Availability
No data is associated with this article.
References
- Paget, J. (1874). On Disease of Mammary Areola Preceding Cancer of The Mammary Gland. St Barth Hosp Rep, 10:87-89.
Publisher | Google Scholor - Cy, C. (2006). Paget's Disease of The Breast: Changing Patterns of Incidence, Clinical Presentation, And Treatment in the US. Cancer, 107:1448-1458.
Publisher | Google Scholor - Ashikari, R., Park, K., Huvos, A. G., Urban, J. A. (1970). Paget's Disease of The Breast. Cancer, 26(3):680-685.
Publisher | Google Scholor - Kothari, A. S., Beechey-Newman, N., Hamed, H., Fentiman, I. S., D'Arrigo, C., et al. (2002). Paget Disease of The Nipple: A Multifocal Manifestation of Higher-Risk Disease. Cancer, 95(1):1-7.
Publisher | Google Scholor - Yim, J. H., Wick, M. R., Philpott, G. W., Norton, J. A., Doherty, G. M. (1997). Underlying Pathology in Mammary Paget's Disease. Annals of Surgical Oncology, 4:287-292.
Publisher | Google Scholor - Muir, R. (1935). The Pathogenesis of Paget's Disease of The Nipple and Associated Lesions. British Journal of Surgery, 22(88):728-737.
Publisher | Google Scholor - Guitera, P., Scolyer, R. A., Gill, M., Akita, H., Arima, M., et al. (2013). Reflectance Confocal Microscopy for Diagnosis of Mammary and Extramammary Paget’s Disease. Journal of the European Academy of Dermatology and Venereology, 27(1):e24-e29.
Publisher | Google Scholor - Pan, Z. Y., Liang, J., Zhang, Q. A., Lin, J. R., Zheng, Z. Z. (2012). In Vivo Reflectance Confocal Microscopy of Extramammary Paget Disease: Diagnostic Evaluation and Surgical Management. Journal of the American Academy of Dermatology, 66(2):e47-e53.
Publisher | Google Scholor - Lundquist, K., Kohler, S., Rouse, R. V. (1999). Intraepidermal Cytokeratin 7 Expression Is Not Restricted to Paget Cells but Is Also Seen in Toker Cells and Merkel Cells. The American Journal of Surgical Pathology, 23(2):212-219.
Publisher | Google Scholor - Da Costa, D., Taddese, A., Cure, M. L., Gerson, D., Poppiti Jr, R., et al. (2007). Common and Unusual Diseases of The Nipple-Areolar Complex. Radiographics, 27(suppl_1):S65-S77.
Publisher | Google Scholor - Fowble, B., Solin, L. J., Schultz, D. J., Weiss, M. C. (1992). Breast Recurrence and Survival Related to Primary Tumor Location in Patients Undergoing Conservative Surgery and Radiation for Early-Stage Breast Cancer. International Journal of Radiation Oncology* Biology* Physics, 23(5):933-939.
Publisher | Google Scholor - Caliskan, M., Gatti, G., Sosnovskikh, I., Rotmensz, N., Botteri, E., et al. (2008). Paget’s Disease of The Breast: The Experience of The European Institute of Oncology and Review of The Literature. Breast Cancer Research and Treatment, 112:513-521.
Publisher | Google Scholor - Frei, K. A., Bonel, H. M., Pelte, M. F., Hylton, N. M., Kinkel, K. (2005). Paget Disease of The Breast: Findings at Magnetic Resonance Imaging and Histopathologic Correlation. Investigative Radiology, 40(6):363-367.
Publisher | Google Scholor - Sukumvanich, P., Bentrem, D. J., Iii, H. C., Brogi, E., Fey, J. V., et al. (2007). The Role of Sentinel Lymph Node Biopsy in Paget’s Disease of The Breast. Annals of Surgical Oncology, 14:1020-1023.
Publisher | Google Scholor