Review Article
Oral Cancer-Nicotine and Alcohol
1Department of Chemistry, Sri J.N.M.PG College, Lucknow UP, India.
2Department of Chemistry, Dayanand Girls PG Kanpur, UP, India.
*Corresponding Author: D.K. Awasthi, Department of Chemistry, Sri J.N.M.PG College, Lucknow UP, India.
Citation: D.K. Awasthi, Dixit A. (2023). Oral Cancer – Nicotine and Alcohol. Clinical and Laboratory Research, BioRes Scientia Publishers. 1(2); DOI: 10.59657/clr.brs.23.007
Copyright: © 2023 D.K. Awasthi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 12, 2023 | Accepted: July 26, 2023 | Published: August 02, 2023
Abstract
Oral cancers include those that affect the lips, the front two-thirds of the tongue, the gums, cheeks, and roof and floor of the mouth. Oral cancers are on the rise, particularly among young people, so it is important to learn about early signs and symptoms and, even better, how to prevent them. More men than women are diagnosed with oral cancer, and its leading causes include tobacco and alcohol consumption. Sometimes the first sign of oral cancer is a small and seemingly harmless sore, so it’s important to visit your dentist or doctor to discuss any concerns you might have about your lips and mouth. Treatments for oral cancers include Surgery, radiation therapy and chemotherapy.
Keywords: oral cancer; tobacco; alcohol; radiation therapy
Introduction
Oral cancer includes cancer of the lips, tongue, cheeks, floor of mouth, hard palate, gums and minor salivary glands. Oral cancer usually occurs in people over the age of 45 but can develop at any age. Lip and oral cavity cancer occur when malignant (cancerous) cells form on the lips or within the oral cavity. As cancer progresses, it may invade deeper into other surrounding tissue. Cancers that develop in the lips or oral cavity are usually squamous cell carcinoma. Tumours of the minor salivary glands – like adenoid cystic carcinomas, mucoepidermoid carcinomas and sarcomas are rarer.
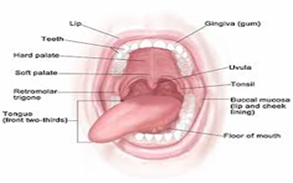
Figure 1: oral cavity.
The oral cavity includes the:
Retromolar trigone (the area behind the wisdom teeth), Hard palate (the roof of the mouth), Floor of the mouth (the space underneath the tongue), Buccal mucosa (the inner lining of the cheeks and lips), Front section of the tongue, Gums, lips and teeth.
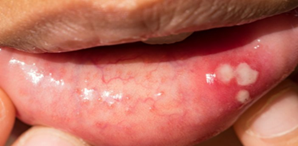
Figure 2: oral lip ulcer and white patches.
Red part of the lip
Tobacco use is known as a major risk factor for oral and many other cancers. All tobacco products, including cigarettes, cigars, pipe tobacco, chewing tobacco, and snuff, contain the Poisonous substances (toxins), Cancer-causing agents (carcinogens), Nicotine, an addictive substance All tobacco products, including cigarettes, cigars, pipes, and smokeless tobacco (chewing tobacco, snuff, or a type of chewing tobacco called betel quid) are linked to head and neck cancer (except for salivary gland cancers). Drinking any type of alcohol, such as beer, wine, or liquor, also raises the risk of getting cancers of the mouth, throat, and voice box.
About 70% of cancers in the oropharynx (which includes the tonsils, soft palate, and base of the tongue) are linked to human papillomavirus (HPV), a common sexually transmitted virus. Overexposure to ultraviolet (UV) rays from the sun, tanning beds, or sunlamps is a cause of cancer on the lips. Occupational exposures, or being exposed to certain substances while on the job, can increase the risk of getting cancers in the nasopharynx. Working in the construction, textile, ceramic, logging, and food processing industries can cause people to be exposed to substances like wood dust, formaldehyde, asbestos, nickel, and other chemicals. An infection with the Epstein-Barr virus, a cause of infectious mononucleosis and other illnesses, can raise the risk of cancers in the nose, behind the nose, and cancers of the salivary glands.
Figure 3: Smoking cigarettes
Cigarettes
Cigarettes, the most common form of tobacco used, causes about 90% of all lung cancers, according to the American Lung Association. Smokers are also at a 10 times higher risk for oral cancer compared to non-smokers. Smoking is linked to increased risk for more than 12 other types of cancer, too. In addition, cigarette smoking is linked to nearly 1 in 5 deaths in the U.S. Cigarettes contain more than 60 known cancer-causing agents.
Cigars and pipes
Cigars and pipes are often believed to be a less harmful way to smoke tobacco. However, even when not inhaling, cigar and pipe smokers are at increased risk for cancer of the oral cavity, oesophagus, voice box, and lungs. Pipe smokers also are at increased risk for lip cancers in areas where the pipestem rests. In addition, cigars take longer to burn and contain more tobacco than cigarettes, increasing the amount of second-hand smoke exposure.
Chewing tobacco and snuff
Spit tobacco, also known as chewing tobacco and snuff, are forms of tobacco that are put between the cheek and gum. Chewing tobacco can be in the form of leaf tobacco (which is packaged in pouches), or plug tobacco (which are packaged in "brick" form). Snuff is a powdered form of tobacco, usually sold in cans. The nicotine is released from the tobacco as the user "chews."
Although chewing tobacco and snuff are considered smokeless tobacco products, harmful chemicals including nicotine are ingested. More than 28 cancer-causing chemicals have been found in smokeless tobacco.
Chewing tobacco and snuff can cause cancer in the cheek, gums, and lips. Just as with a pipe, cancer often occurs where the tobacco is held in the mouth. Cancer caused by smokeless tobacco often begins as leucoplakia, with a whitish patch that develops inside the mouth or throat. Or the cancer may erythrolein. With this condition, a red, raised patch develops inside the mouth. It's also linked to oesophageal and pancreatic cancers.
Cigars became a trend in the 1990s, attracting the young and the old. Many people think cigars are less harmful to their health. But cigars actually pose the same risk for oral cancer as cigarettes do. Many cigar smokers don't inhale. But their risk for oral, throat, and oesophageal cancers is the same as for cigarette smokers. Consider these facts:
- Compared with non-smokers, regular cigar smokers are 4 to 10 times more likely to die from oral cancer, oesophageal cancer, and laryngeal cancer.
- Cigar smokers may spend an hour or more smoking 1 large cigar that can contain the same amount of nicotine as a full pack of cigarettes. And even unlit cigars, when held in the mouth for an extended period of time, promote nicotine absorption.
- Second-hand smoke from cigars contains toxins and cancer-causing agents (carcinogens) similar to second-hand cigarette smoke, but in higher concentrations.
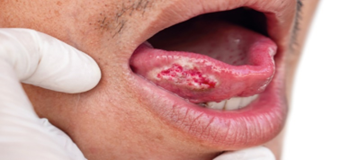
Figure 4: Toung cancer.
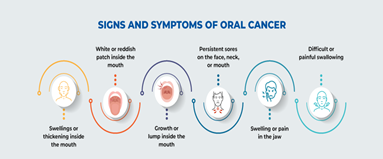
Other potential symptoms of oral cancer include [2]:
- White patch (called leucoplakia) or red patch (called erythroplakia) on the inside of the mouth
- Non-healing scab on the lip or mouth ulcer
- Bleeding from the mouth that is unrelated to an injury
- Pain and/or difficulty chewing
- Swollen glands (lymph nodes) or a mass in the neck
- Jaw pain or swelling
- Difficulty swallowing, chewing, speaking, or moving the tongue or jaw
- Numb tongue or area of the mouth
- Loose teeth or dentures
- Persistent bad breath
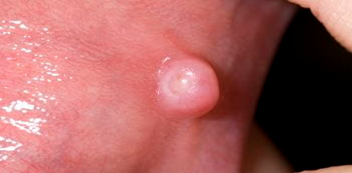
Figure 5: Oral Growth.
Causes
Perhaps the most significant risk factor for developing oral cancer is tobacco use.1 Smoking cigarettes, cigars, and pipes all increase your risk of cancer anywhere in the mouth or throat. Moreover, smokeless or oral tobacco products often called "dip" or "chew," heighten the risk for developing cancers of the cheek, gums, and inner part of the lips.
Other risk factors linked to the development of oral cancer include:
- Heavy alcohol consumption: Risk dramatically increases when a person both smokes and drinks heavily.
- Oropharynx cancer (occurs in tonsils, base of tongue, etc.) is the type of cancer commonly associated with HPV infection [4].
- Eating a diet deficient in fruits and vegetables.
- Being exposed to excess sunlight (increases risk for lip cancer).
- Having a weakened immune system.
- Having certain underlying health problems like graft-versus-host disease or a genetic syndrome like Fanconi anemia.
- Chewing betel quid, a stimulant drug that's ingested like chewing tobacco and often mixed with tobacco.
Oral cancer is more common in men, perhaps because men are more likely to use tobacco and alcohol than women.
Oral cancer is also more common in adults over age 55, although this is changing as cancers related to HPV infection are increasing in number.
Some of the tests involved in staging include [5]:
- HPV testing of the biopsy specimen.
- Magnetic resonance imaging (MRI).
- Computed tomography (CT) scan of the neck and chest.
- Positron emission tomography (PET) scan.
- Dental X-rays.
- Barium swallow (gastrointestinal series of X-rays of the esophagus and stomach).
The oral cavity includes the:
- Retromolar trigone (the area behind the wisdom teeth)
- Hard palate (the roof of the mouth)
- Floor of the mouth (the space underneath the tongue)
- Buccal mucosa (the inner lining of the cheeks and lips)
- Front section of the tongue
- Gums, lips and teeth
- Red part of the lip
Additional symptoms of lip and oral cavity cancer may include:
- Lump or thickening on the lips, gums, mouth and/or neck
- A white or red patch on the gums, tongue, tonsils or lining of the mouth
- Bleeding from the lip, mouth, chin or cheek
- Pain or numbness in the lip, mouth, chin or cheek
- Loose teeth or dentures that no longer fit well
- Trouble chewing, swallowing or moving the tongue or jaw
- Sore throat or feeling that something is caught in the throat
- Swelling of the jaw or neck
- Change in voice
- Weight loss
Stages of lip and oral cavity cancer
The staging system most often used for oral cavity or oropharyngeal cancers is the American Joint Committee on Cancer (AJCC) TNM system, which is based on three key pieces of information:
The extent of the tumour (T): How large is the main (primary) tumour and which, if any, tissues of the oral cavity or oropharynx have been affected?
The spread to nearby lymph nodes (N): Has cancer spread to nearby lymph nodes? If so, how many, are they on the same side where cancer started and how large are they?
The spread (metastasis) to distant sites (M): Has cancer spread to distant organs such as the lungs?
Numbers or letters after T, N and M provide more details about each of these factors. Higher numbers indicate that a patient’s cancer is more advanced. Once a person’s T, N and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
Cancer can be described as the uncontrolled growth of abnormal cells. Cancer that develops in the mouth or throat is termed as oral cancer. Tumours caused by oral cancer can appear on the lips, the floor of the mouth, cheeks, tongue, sinuses, hard and soft palate, and pharynx. Thus, in many cases, a dentist first detects oral cancer during routine dental check-ups. Early detection is key to the treatment of oral cancer. If left untreated, this form of cancer can be fatal.
Oral cancer is usually the result of a genetic mutation. This mutation makes normal cells grow abnormally fast and does not allow the cells to die. It is not clear what causes these mutations. As the cells keep growing, they form tumours. The tumours can then spread to other places in the mouth and throat as well as to other organs.
There are 5 stages of Oral cancer.
Stage 0: In this stage, the tumor affects only the top layer of cells and is often too small to be noticed.
Stage I: The tumour grows to about 2cm in size but is limited to one part of the mouth or throat.
Stage II: The tumour grows to a size of 2-4cm and may start affecting nearby parts of the mouth and throat.
Stage III: The tumour grows bigger than 4cm and may also be noticed in other parts of the mouth and throat.
Stage IV: The tumour spread to other organs and lymph nodes.
Risk of developing Oral cancer if:
- you smoke cigarettes, cigars or tobacco pipes.
- you chew tobacco.
- you suffer from an HPV infection.
- you drink alcohol excessively.
- your face is exposed a lot to the sun.
- you have a weakened immune system.
- you do not get adequate nutrition.
- you have a history of oral cancer.
- someone in your family has Oral cancer.
Men have a higher risk of Oral cancer as compared to women.
- The symptoms of Oral cancer include:
- sore in the mouth or lip that does not heal
- growth or mass anywhere in the mouth or throat
- white or red patches in the mouth or throat
- loose teeth
- bleeding from the mouth
- trouble swallowing
- trouble wearing dentures
- chronic earache
- sore throat
- unexplained weight loss
- tongue pain
- stiffness in the jaw numbness in the face, lower lip or neck
Most cases of oral cancer are detected by dentists during routine check-ups. During a physical exam, the dentist will look for patches and growths in the mouth. If any suspicious lesions or tumours are found, a brush biopsy or tissue biopsy may be conducted. This may be followed by an X-ray, CT scan, MRI scan, PET scan, and endoscopy.
Treatment
If an abnormal area has been found in the oral cavity, a BIOPSY will determine whether it is cancer. Usually, you are referred to a head and neck surgeon, who removes part or all of the lump or abnormal-looking area. A pathologist examines the tissue under a microscope to check for cancer cells. Almost all oral cancers are squamous cell carcinomas, since squamous cells line the oral cavity.
Surgery
Surgery to remove the tumour in the mouth is the usual treatment for patients with oral cancer. If there is evidence that the cancer has spread or a concern that it has spread, the surgeon may also remove lymph nodes in the neck. If the disease has spread to muscles and other tissues in the neck, the operation may be more extensive.
Radiation Therapy
Radiation therapy, also called radiotherapy, is the use of high-energy rays to damage cancer cells and stop them from growing. Like surgery, radiation therapy is local therapy, affecting only the cells in the treated area. The energy may come from a large machine, or external radiation. Patients with large tumours may need both surgery and radiation therapy.
Chemotherapy
Chemotherapy is the use of drugs to kill cancer cells. Researchers are looking for effective drugs or drug combinations to treat oral cancer. They are also exploring ways to combine chemotherapy with other forms of cancer treatment to help destroy the tumour and prevent the disease from spreading.
Figure 6
Oral cancer does not go away on its own and should not be ignored. In its later stages, oral cancer can be fatal. In these stages, treatment cannot cure the disease and is aimed at only reducing the speed at which it progresses. Hence, it is important to seek treatment in the early stages of oral cancer. Complications that can arise from oral cancer include: changes in the appearance of the mouth, speech problems, difficulty eating and swallowing, depression and anxiety. The ideal treatment for a patient depends on many factors including the stage at which the cancer is diagnosed, the patient’s age, overall health, and personal preferences. In the case of small tumours restricted to one area, surgery may be advised. This is aimed at removing the tumours. Surgery may be followed by radiation to ensure that no part of a tumour has been left behind. If a tumour is large, radiation may precede surgery to allow a tumour to shrink. Chemotherapy is another form of treatment used for oral cancer. This may be combined with radiation. Targeted therapy may also be used to treat oral can.
Oral cancer is the 11th most common cancer in the world, accounting for an estimated 300,000 new cases and 145,000 deaths in 2012 and 702,000 prevalent cases over a period of five years (old and new cases) For this chapter, oral cancers include cancers of the mucosal lip, tongue, gum, floor of the mouth, palate, and mouth, corresponding to the International Classification of Diseases, 10th revision [ICD-10], codes C00, C02, C03, C04, C05, and C06, respectively. Two-thirds of the global incidence of oral cancer occurs in low- and middle-income countries (LMICs); half of those cases are in South Asia. India alone accounts for one-fifth of all oral cancer cases and one-fourth of all oral cancer deaths Oral Cancer in Men (All Ages): Global Incidence, Mortality, and Prevalence, World Health Organization Geographic Classification, 2012. Oral Cancer in Women (All Ages): Global Incidence, Mortality, and Prevalence, World Health Organization Geographic Classification, 2012.Tobacco use, in any form, and excessive alcohol use are the major risk factors for oral cancer. With dietary deficiencies, these factors cause more than 90 percent of oral cancers.
Conclusion
Oral cancer incidence and mortality are high in IndiaThe major causes of oral cancer worldwide remain tobacco in its many different forms, heavy consumption of alcohol, and, increasingly, infection with certain types of HPV. Although the relative contribution of risk factors varies from population to population, oral cancer is predominantly a disease of poor people. Prevention of this devastating disease can come from fundamental changes in socioeconomic status, as well as from actions to reduce the demand, production, marketing, and use of tobacco products and alcohol.
References
- Amagasa T, Yamashiro M, Uzawa N.(2011). Oral Premalignant Lesions:From a Clinical Perspective. International Journal of Clinical Oncology, 16(1):5-14.
Publisher | Google Scholor - Amarasinghe H K, Johnson N W, Lalloo R, Kumaraarachchi M, Warnakulasuriya S. (2010). Derivation and Validation of a Risk-Factor Model for Detection of Oral Potentially Malignant Disorders in Populations with High Prevalence. British Journal of Cancer, 103(3):303-309.
Publisher | Google Scholor - Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre J L., et al. (2004). Postoperative Irradiation with or Without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. New England Journal of Medicine, 350(19):1945-1952.
Publisher | Google Scholor - Bhurgri Y, Bhurgri A, Usman A, Pervez S, Kayani N. et al. (2006). Epidemiological Review of Head and Neck Cancers in Karachi. Asian Pacific Journal of Cancer Prevention, 7(2):195-200.
Publisher | Google Scholor - Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C. et al. (2011). Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): A Comprehensive Analysis by Tumour Site. Radiotherapy and Oncology, 100(1):33-40.
Publisher | Google Scholor - Bonifazi M, Malvezzi M, Bertuccio P, Edefonti V, Garavello W. et al. (2011). Age-Period-Cohort Analysis of Oral Cancer Mortality in Europe:The End of an Epidemic? Oral Oncology, 47(5):400-407.
Publisher | Google Scholor - Bray F, Ren J S, Masuyer E, Ferlay J. (2013). Estimates of Global Cancer Prevalence for 27 Sites in the Adult Population in (2008). International Journal of Cancer, 132(5):1133-1145.
Publisher | Google Scholor - Brocklehurst P, Kujan O, O’Malley L A, Ogden G, Shepherd S, Glenny A M. (2013). Screening Programmes for the Early Detection and Prevention of Oral Cancer. Cochrane Database of Systematic Reviews, 11:CD004150.
Publisher | Google Scholor - Brown L M, Check D P, Devesa S S. (2011). Oropharyngeal Cancer Incidence Trends:Diminishing Racial Disparities. Cancer Causes and Control, 22(5):753-763.
Publisher | Google Scholor - Chainani-Wu N, Epstein J, Touger-Decker R. (2011). Diet and Prevention of Oral Cancer:Strategies for Clinical Practice. Journal of the American Dental Association, 142(2):166-169.
Publisher | Google Scholor - Cooper J S, Pajak T F, Forastiere A A, Jacobs J, Campbell B H., et al. (2004). Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. New England Journal of Medicine, 350(19):1937-1944.
Publisher | Google Scholor - Dedhia R C, Smith K J, Johnson J T, Roberts M. (2011). The Cost-Effectiveness of Community-Based Screening for Oral Cancer in High-Risk Males in the United States:A Markov Decision Analysis Approach. Laryngoscope 121(5):952-960.
Publisher | Google Scholor - Deneo-Pellegrini H, Stefani E De, Boffetta P, Ronco A L, Acosta G. et al. (2012). Mate Consumption and Risk of Oral Cancer:Case-Control Study in Uruguay. Head & Neck 35(8):1091-1095.
Publisher | Google Scholor - Downer M C, Moles D R, Palmer S, Speight P M. (2004). A Systematic Review of Test Performance in Screening for Oral Cancer and Precancer. Oral Oncology, 40(3):264-273.
Publisher | Google Scholor - Edwards C P. (2013). Oral Cancer Screening for Asymptomatic Adults: Do the United States Preventive Services Task Force Draft Guidelines Miss the Proverbial Forest for the Trees? Oral Surgery, Oral Medicine. Oral Pathology and Oral Radiology, 116(2):131-134.
Publisher | Google Scholor - Elango K J, Anandkrishnan N, Suresh A, Iyer S K, Ramaiyer S K., et al. (2011). Mouth Self-Examination to Improve Oral Cancer Awareness and Early Detection in a High-Risk Population. Oral Oncology, 47(7):620-624.
Publisher | Google Scholor - El-Naaj I A, Leiser Y, Shveis M, Sabo E, Peled M. (2011). Incidence of Oral Cancer Occult Metastasis and Survival of T1-T2N0 Oral Cancer Patients. Journal of Oral & Maxillofacial Surgery, 69(10):2674-2679.
Publisher | Google Scholor - Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S. et al. (2013). GLOBOCAN 2012 V1.0, Cancer Incidence and Mortality Worldwide:IARC Cancerbase No. 11. Lyon, France:International Agency for Research on Cancer.
Publisher | Google Scholor - Fernandez G L, Sankaranarayanan R, Anta J J Lence, Rodriguez S A, Parkin D M. (1995). An Evaluation of the Oral Cancer Control Program in Cuba. Epidemiology, 6(4):428-431.
Publisher | Google Scholor - Forman D, Bray F, Brewster D H, Gombe C Mbalawa, Kohler B. et al. (2013). Cancer Incidence in Five Continents. (Electronic Version). Lyon, France:International Agency for Cancer.
Publisher | Google Scholor - Fujita M, Hirokawa Y, Kashiwado K, Akagi Y, Kashimoto K. et al. (1999). Interstitial Brachytherapy for Stage I and II Squamous Cell Carcinoma of the Oral Tongue:Factors Influencing Local Control and Soft Tissue Complications. International Journal of Radiation Oncology Biology Physics 44(4):767-775.
Publisher | Google Scholor - Gandini S, Negri E, Boffetta P, La V C, Boyle P. (2012). Mouthwash and Oral Cancer Risk Quantitative Meta-Analysis of Epidemiologic Studies. Annals of Agricultural and Environmental Medicine 19(2):173-180.
Publisher | Google Scholor - Gupta B, Ariyawardana A, Johnson N W. (2013). Oral Cancer in India Continues in Epidemic Proportions:Evidence Base and Policy Initiatives. International Dental Journal 63(1):12-25.
Publisher | Google Scholor - Gupta P C, Ray C S, Sinha D N, Singh P K. (2011). Smokeless Tobacco:A Major Public Health Problem in the SEA Region:A Review. Indian Journal of Public Health 55(3):199-209.
Publisher | Google Scholor - Harty L C, Caporaso N E, Hayes R B, Winn D M, Bravo-Otero E. et al. (1997). Alcohol Dehydrogenase 3 Genotype and Risk of Oral Cavity and Pharyngeal Cancers. Journal of the National Cancer Institute 89(22):1698-1705.
Publisher | Google Scholor - Heck J E, Berthiller J, Vaccarella S, Winn D M, Smith E M., et al. (2010). Sexual Behaviours and the Risk of Head and Neck Cancers:A Pooled Analysis in the International Head and Neck Cancer Epidemiology(INHANCE) Consortium. International Journal of Epidemiology 39(1):166-181.
Publisher | Google Scholor - Hicks W L Jr, Loree T R, Garcia R I, Maamoun S, Marshall D. et al. (1997). Squamous Cell Carcinoma of the Floor of Mouth: A 20-Year Review. Head & Neck 19(5):400-05.
Publisher | Google Scholor - Hirsch J M, Wallstrom M, Carlsson A P, Sand L. (2012). Oral Cancer in Swedish Snuff Dippers. Anticancer Research, 32(8):3327-3330.
Publisher | Google Scholor - Howlader N, Ries L A, Mariotto A B, Reichman M E, Ruhl J. et al. (2010). Improved Estimates of Cancer-Specific Survival Rates from Population-Based Data. Journal of the National Cancer Institute, 102(20):1584-1598.
Publisher | Google Scholor - Hsue S S, Wang W C, Chen C H, Lin C C, Chen Y K, Lin L M. (2007). Malignant Transformation in 1458 Patients with Potentially Malignant Oral Mucosal Disorders: A Follow-Up Study Based in a Taiwanese Hospital. Journal of Oral Pathology & Medicine, 36(1):25-29.
Publisher | Google Scholor - IARC (International Agency for Research on Cancer). (2004) a. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 83: Tobacco Smoke and Involuntary Smoking. Lyon, France: ARC.
Publisher | Google Scholor - IARC (International Agency for Research on Cancer). (2004) b. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Betel-Quid and Areca-Nut Derived Nitrosamines. Lyon, France: ARC (85).
Publisher | Google Scholor - IARC (International Agency for Research on Cancer) (2007). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines. Lyon, France: ARC, (89).
Publisher | Google Scholor - IARC (International Agency for Research on Cancer) (2010). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Alcohol Consumption and Ethyl Carbamate. Lyon, France: ARC, (96).
Publisher | Google Scholor - Javed F, Chotai M, Mehmood A, Almas K. (2010). Oral Mucosal Disorders Associated with Habitual Gutka Usage: A Review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 109(6):857-864.
Publisher | Google Scholor - Johnson N W, Warnakulasuriya S, Gupta P C, Dimba E, Chindia M. et al. (2011). Global Oral Health Inequalities in Incidence and Outcomes for Oral Cancer: Causes and Solutions. Advances in Dental Research, 23(2):237-246.
Publisher | Google Scholor - Lambert R, Sauvaget C, Camargo C M De, Sankaranarayanan R. (2011). Epidemiology of Cancer from the Oral Cavity and Oropharynx. European Journal of Gastroenterology & Hepatology, 23(8):633-641.
Publisher | Google Scholor - Licitra L, Grandi C, Guzzo M, Mariani L, Lo V S., et al. (2003). Primary Chemotherapy in Resectable Oral Cavity Squamous Cell Cancer: A Randomized Controlled Trial. Journal of Clinical Oncology, 21(2):327-333.
Publisher | Google Scholor - Lu D, Yu X, Du Y. (2011). Meta-Analyses of the Effect of Cytochrome P450 2E1 Gene Polymorphism on the Risk of Head and Neck Cancer. Molecular Biology Reports, 38(4):2409-2416.
Publisher | Google Scholor - Lucenteforte E, Garavello W, Bosetti C, La V C. 2009. Dietary Factors and Oral and Pharyngeal Cancer Risk. Oral Oncology, 45(6):461-467.
Publisher | Google Scholor - Lundahl R E, Foote R L, Bonner J A, Suman V J, Lewis J E., et al. (1998). Combined Neck Dissection and Postoperative Radiation Therapy in the Management of the High-Risk Neck:A Matched-Pair Analysis. International Journal of Radiation Oncology Biology Physics, 40(3):529-534.
Publisher | Google Scholor - Marsiglia H, Haie-Meder C, Sasso G, Mamelle G, Gerbaulet A. (2002). Brachytherapy for T1-T2 Floor-of-the-Mouth Cancers: The Gustave-Roussy Institute Experience. International Journal of Radiation Oncology Biology Physics 52(5):1257-1263.
Publisher | Google Scholor - Mathew B, Sankaranarayanan R, Sunil Kumar K B, Kuruvila B, Pisani P. et al. (1997). Reproducibility and Validity of Oral Visual Inspection by Trained Health Workers in the Detection of Oral Precancer and Cancer. British Journal of Cancer 76(3):390-394.
Publisher | Google Scholor - Mathew B, Sankaranarayanan R, Wesley R, Nair M K. (1995). Evaluation of Mouth Self-Examination in the Control of Oral Cancer. British Journal of Cancer, 71(2):397-399.
Publisher | Google Scholor - Mehta F S, Gupta P C, Bhonsle R B, Murti P R, Daftary D K, Pindborg J J. 1986. Detection of Oral Cancer Using Basic Health Workers in an Area of High Oral Cancer Incidence in India. Cancer Detection and Prevention, 9(3-4):219-225.
Publisher | Google Scholor - Napier S S, Speight P M. (2008). Natural History of Potentially Malignant Oral Lesions and Conditions: An Overview of the Literature. Journal of Oral Pathology & Medicine, 37(1):1-10.
Publisher | Google Scholor - Patel S G, Shah J P. (2005). TNM Staging of Cancers of the Head and Neck: Striving for Uniformity among Diversity. CA: A Cancer Journal for Clinicians, 55(4):242-58.
Publisher | Google Scholor - Patton L L, Epstein J B, Kerr A R. (2008). Adjunctive Techniques for Oral Cancer Examination and Lesion Diagnosis: A Systematic Review of the Literature. Journal of the American Dental Association, 139(7):896-905.
Publisher | Google Scholor - Pavia M, Pileggi C, Nobile C G, Angelillo I F. (2006). Association between Fruit and Vegetable Consumption and Oral Cancer:A Meta-Analysis of Observational Studies. American Journal of Clinical Nutrition 83(5):1126-1134.
Publisher | Google Scholor - Piemonte E D, Lazos J P, Brunotto M. (2010). Relationship between Chronic Trauma of the Oral Mucosa, Oral Potentially Malignant Disorders and Oral Cancer. Journal of Oral Pathology & Medicine 39(7):513-517.
Publisher | Google Scholor - Prabhu S, Wilson D. (2013). Human Papillomavirus and Oral Disease: Emerging Evidence:A Review. Australian Dental Journal, 58(1):2-10.
Publisher | Google Scholor - Pulte D, Brenner H. (2010). Changes in Survival in Head and Neck Cancers in the Late 20th and Early 21st Century: A Period Analysis. Oncologist, 15(9):994-1001.
Publisher | Google Scholor - Radoi L, Paget-Bailly S, Cyr D, Papadopoulos A, Guida F. et al. (2013). Tobacco Smoking, Alcohol Drinking and Risk of Oral Cavity Cancer by Subsite: Results of a French Population-Based Case-Control Study, the ICARE Study. European Journal of Cancer Prevention 22(3):268-276.
Publisher | Google Scholor - Ramadas K, Lucas E, Thomas G, Mathew B, Balan A. et al. (2008). A Digital Manual for the Early Diagnosis of Oral Neoplasia. Lyon, France: International Agency for Research on Cancer.
Publisher | Google Scholor - Rethman M P, Carpenter W, Cohen E E, Epstein J, Evans C A., et al. (2010). Evidence-Based Clinical Recommendations Regarding Screening for Oral Squamous Cell Carcinomas. Journal of the American Dental Association, 141(5):509-520.
Publisher | Google Scholor - Richards D. (2010). Does Toluidine Blue Detect More Oral Cancer? Evidence-Based Dental Practice, 11(4):104-105.
Publisher | Google Scholor - Ries L A G, Melbert D, Krapcho M, Stinchcomb D G, Howlader N. et al. (2008). Cancer Statistics Review, 1975-(2005). Bethesda, Denationalize Cancer Institute.
Publisher | Google Scholor - Saba N F, Goodman M, Ward K, Flowers C, Ramalingam S. et al. (2011). Gender and Ethnic Disparities in Incidence and Survival of Squamous Cell Carcinoma of the Oral Tongue, Base of Tongue, and Tonsils: A Surveillance, Epidemiology and End Results Program-Based Analysis. Oncology, 81(1):12-20.
Publisher | Google Scholor - Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Thomas G. et al. (2013). Long Term Effect of Visual Screening on Oral Cancer Incidence and Mortality in a Randomized Trial in Kerala, India. Oral Oncology, 49(4):314-321.
Publisher | Google Scholor - Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S. et al. (2005). Effect of Screening on Oral Cancer Mortality in Kerala, India:A Cluster-Randomised Controlled Trial. The Lancet, 365(9475):1927-1933.
Publisher | Google Scholor - Sankaranarayanan R, Swaminathan R. (2011). Cancer Survival in Africa, Asia, the Caribbean and Central America. Scientific Publications No. 162. Lyon, France: International Agency for Research on Cancer.
Publisher | Google Scholor - Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia K S., et al. (2010). Cancer Survival in Africa, Asia, and Central America: A Population-Based Study. The Lancet Oncology, 11(2):165-173.
Publisher | Google Scholor - Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F. et al. (2009). EUROCARE-4. Survival of Cancer Patients Diagnosed in 1995-(1999). Results and Commentary. European Journal of Cancer, 45(6):931-991.
Publisher | Google Scholor - Satyanarayana L, Asthana S. (2008). Life Time Risk for Development of Ten Major Cancers in India and Its Trends over the Years 1982 to 2000. Indian Journal of Medical Sciences, 62(2):35-44.
Publisher | Google Scholor - Scott S E, Rizvi K, Grunfeld E A, Mcgurk M. (2010). Pilot Study to Estimate the Accuracy of Mouth Self-Examination in an At-Risk Group. Head & Neck, 32(10):1393-1401.
Publisher | Google Scholor - Sinha D N, Palipudi K M, Rolle I, Asma S, Rinchen S. (2011). Tobacco Use among Youth and Adults in Member Countries of South-East Asia Region: Review of Findings from Surveys under the Global Tobacco Surveillance System. Indian Journal of Public Health, 55(3):169-176.
Publisher | Google Scholor - Sobin L H, Wittekind C. (2002). UICC TNM Classification of Malignant Tumours. Geneva: Union for International Cancer Control.
Publisher | Google Scholor - Somatunga L C, Sinha D N, Sumanasekera P, Galapatti K, Rinchen S. et al. (2012). Smokeless Tobacco Use in Sri Lanka. Indian Journal of Cancer, 49(4):357-363.
Publisher | Google Scholor - Speight P M, Palmer S, Moles D R, Downer M C, Smith D H. et al. (2006). The Cost-Effectiveness of Screening for Oral Cancer in Primary Care. Health Technology Assessment 10(14):1-144, Iii-Iv.
Publisher | Google Scholor - Su W W, Yen A M, Chiu S Y, Chen T H. (2010). A Community-Based RCT for Oral Cancer Screening with Toluidine Blue. Journal of Dental Research, 89(9):933-937.
Publisher | Google Scholor - Subramanian S, Sankaranarayanan R, Bapat B, Somanathan T, Thomas G. et al. 2009. Cost-Effectiveness of Oral Cancer Screening: Results from a Cluster Randomized Controlled Trial in India. Bulletin of the World Health Organization, 87(3):200-206.
Publisher | Google Scholor - Tseng C H. (2013). Oral Cancer in Taiwan: Is Diabetes a Risk Factor? Clinical Oral Investigations, 17(5):1357-1364.
Publisher | Google Scholor - Meij Van Der E H, Bezemer P D, Waal I Van Der. (2002). Cost-Effectiveness of Screening for the Possible Development of Cancer in Patients with Oral Lichen Planus. Community Dentistry and Oral Epidemiology, 30(5):342-351.
Publisher | Google Scholor - Vijayakumar M, Burrah R, Sabitha K S, Nadimul H, Rajani B C. (2011). To Operate or Not to Operate: N0 Neck in Early Cancer of the Tongue? A Prospective Study. Indian Journal of Surgical Oncology, 2(3):172-175.
Publisher | Google Scholor - Walsh T, Liu J L, Brocklehurst P, Glenny A M, Lingen M. et al. (2013). Clinical Assessment to Screen for Oral Cavity Cancer and Potentially Malignant Disorders in Apparently Healthy Adults. Cochrane Database of Systematic Reviews, 11:CD010173.
Publisher | Google Scholor - Warnakulasuriya K A, Ekanayake A N, Sivayoham S, Stjernsward J, Pindborg J J., et al. (1984). Utilization of Primary Health Care Workers for Early Detection of Oral Cancer and Precancer Cases in Sri Lanka. Bulletin of the World Health Organization, 62(2):243-250.
Publisher | Google Scholor - Warnakulasuriya S, Johnson N W, Waal I Van Der. (2007). Nomenclature and Classification of Potentially Malignant Disorders of the Oral Mucosa. Journal of Oral Pathology & Medicine, 36(10):575-580.
Publisher | Google Scholor - Warnakulasuriya K A, Nanayakkara B G. 1991. Reproducibility of an Oral Cancer and Precancer Detection Program Using a Primary Health Care Model in Sri Lanka. Cancer Detection and Prevention, 15(5):331-334.
Publisher | Google Scholor - Wendt C D, Peters L J, Delclos L, Ang K K, Morrison W H., et al. 1990. Primary Radiotherapy in the Treatment of Stage I and II Oral Tongue Cancers: Importance of the Proportion of Therapy Delivered with Interstitial Therapy. International Journal of Radiation Oncology Biology Physics, 18(6):1287-1292.
Publisher | Google Scholor - Woolgar J A. (2006). Histopathological Prognosticators in Oral and Oropharyngeal Squamous Cell Carcinoma. Oral Oncology, 42(3):229-239.
Publisher | Google Scholor - World Cancer Research Fund and AICR (American Institute for Cancer Research). (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR.
Publisher | Google Scholor - Wrangle J M, Khuri F R. (2007). Chemoprevention of Squamous Cell Carcinoma of the Head and Neck. Current Opinion in Oncology, 19(3):180-187.
Publisher | Google Scholor - Yako-Suketomo H, Matsuda T. (2010). Comparison of Time Trends in Lip, Oral Cavity and Pharynx Cancer Mortality (1990-2006) between Countries Based on the WHO Mortality Database. Japanese Journal of Clinical Oncology, 40(11):1118-1119.
Publisher | Google Scholor - Zwetyenga N, Majoufre-Lefebvre C, Siberchicot F, Demeaux H, Pinsolle J. (2003). [Squamous-cell carcinoma of the tongue: treatment results and prognosis]. Revue de stomatologie et de chirurgie maxillo-faciale, 104(1):10-17.
Publisher | Google Scholor - Scattoloni J. (2004). Screening for Oral Cancer: A Brief Evidence Update for the U.S. Preventive Services Task Force. Rockville, Mediagenic for Healthcare Research and Quality, AHRQ Pub No. 05-0564-B.
Publisher | Google Scholor - (2014). U.S. Preventive Services Task Force. Screening for Oral Cancer: Recommendation Statement. Rockville, MD:Agency for Healthcare Research and Quality; AHRQ Pub No. 05-0564-A.
Publisher | Google Scholor - World Health Organization. (2000). International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization.
Publisher | Google Scholor - van der Waal R, van der Waal I. (2007). Oral non-squamous malignant tumors; diagnosis and treatment. Med Oral Patol Oral Cir Bucal., 12(7):e486-e491.
Publisher | Google Scholor - Brocklehurst P, Kujan O, Glenny AM, Oliver R, Sloan P, Ogden G, et al. (2010). Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev., (11):CD004150.
Publisher | Google Scholor - Warnakulasuriya S, Johnson NW, van der Waal I. (2007). Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 36(10):575-580.
Publisher | Google Scholor - Kademani D. Oral cancer. (2007). Mayo Clin Proc., 82(7):882-887.
Publisher | Google Scholor - Petti S. (2003). Pooled estimate of world leukoplakia prevalence:a systematic review. Oral Oncol, 39(8):770-780.
Publisher | Google Scholor - Napier SS, Speight PM. (2008). Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med., 37(1):1-10.
Publisher | Google Scholor - Scully C, Bagan J. (2009). Oral squamous cell carcinoma overview. Oral Oncol. 45(4-5):301-308.
Publisher | Google Scholor - American Cancer Society. Cancer Facts & Figures (2011). Atlanta:American Cancer Society.
Publisher | Google Scholor - Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44(1):10-22.
Publisher | Google Scholor - National Cancer Institute. SEER*StatDatabase: Incidence - SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2010 Sub(2000-2008). Bethesda, MD: National Cancer Institute.
Publisher | Google Scholor - National Cancer Institute. (2011). SEER*StatDatabase: Mortality - All COD, Aggregated with State, Total U.S. (1969-2008). Bethesda, MD: National Cancer Institute.
Publisher | Google Scholor - Warnakulasuriya S. (2009). Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309-316.
Publisher | Google Scholor - Petti S. (2009). Lifestyle risk factors for oral cancer. Oral Oncol., 45(4-5):340-350.
Publisher | Google Scholor - Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C et al. (2007). Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer:pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst., 99(10):777-789.
Publisher | Google Scholor - Patton LL. (2003). The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncol., 39(7):708-723.
Publisher | Google Scholor - Patton LL, Epstein JB, Kerr AR. (2008). Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc, 139(7):896-905.
Publisher | Google Scholor - Schlecht HP. (2012). Oral human papillomavirus infection: hazard of intimacy. JAMA. 307(7):724-725.
Publisher | Google Scholor - Brown LM, Check DP, Devesa SS. (2011). Oropharyngeal cancer incidence trends:diminishing racial disparities. Cancer Causes Control, 22(5):753-763.
Publisher | Google Scholor - Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. (2011). Human papillomaviruses in oral carcinoma and oral potentially malignant disorders:a systematic review. Oral Dis., 17(S1):58-72.
Publisher | Google Scholor - Kreimer AR, Clifford GM, Boyle P, Franceschi S. (2005). Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev., 14(2):467-475.
Publisher | Google Scholor - Shillitoe EJ. (2005). The role of viruses in squamous cell carcinoma of the oropharyngeal mucosa. Oral Oncol, 45(4-5):351-355.
Publisher | Google Scholor - ClinicalTrials.gov. (2011). Epstein-Barr Detection in Squamous Cell Carcinoma and Healthy Oral Mucosa. Bethesda, MD: National Institutes of Health. NCT01039272.
Publisher | Google Scholor - Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. (2010). Prevalence of oral HPV infection in the United States, JAMA. 307(7):693-703.
Publisher | Google Scholor - National Institute of Dental and Craniofacial Research. (2011). Detecting Oral Cancer: A Guide for Health Care Professionals. Bethesda, MD: National Institutes of Health.
Publisher | Google Scholor - Patton LL, Epstein JB, Kerr AR. (2008). Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc., 139(7):896-905.
Publisher | Google Scholor - Rethman MP, Carpenter W, Cohen EE, Epstein J, Evans CA, Flaitz CM, et al. (2010). Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J Am Dent Assoc., 141(5):509-520.
Publisher | Google Scholor - Centers for Disease Control and Prevention. (2009). QuickStats: percentage of adults aged ≥18 years who have ever had an oral cancer examination, by smoking status and age group—National Health Interview Survey, United States. MMWR Morb Mortal Wkly Rep., 58(36):1013.
Publisher | Google Scholor - U.S. Department of Health and Human Services. (2011). Healthy People 2020 Topics and Objectives: Oral Health. Washington, DC: U.S. Department of Health and Human Services.
Publisher | Google Scholor - Sankaranarayanan R, Mathew B, Jacob BJ, Thomas G, Somanathan T, et al. (2000). Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. Cancer, 88(3):664-673.
Publisher | Google Scholor - Ramadas K, Sankaranarayanan R, Jacob BJ, Thomas G, Somanathan T, Mahé C, et al. (2003). Interim results from a cluster randomized controlled oral cancer screening trial in Kerala, India. Oral Oncol., 39(6):580-588.
Publisher | Google Scholor - U.S. Preventive Services Task Force. (2011). Procedure Manual. Rockville, MD: U.S. Preventive Services Task Force.
Publisher | Google Scholor - National Institute for Health and Clinical Excellence. (2012). The Guidelines Manual 2009. London: National Institute for Health and Clinical Excellence.
Publisher | Google Scholor - Oxman AD, Guyatt GH. (1991). Validation of an index of the quality of review articles. J Clin Epidemiol, 44(11):1271127-1271188.
Publisher | Google Scholor - Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, et al. (2005). Effect of screening on oral cancer mortality in Kerala, India:a cluster-randomised controlled trial. Lancet, 365(9475):1927-1933.
Publisher | Google Scholor - Downer MC, Evans AW, Hughes Hallet CM, Jullien JA, Speight PM. et al. Evaluation of screening for oral cancer and precancer in a company headquarters. Community Dent Oral Epidemiol, 23(2):84-88.
Publisher | Google Scholor - Jullien JA, Downer MC, Zakrzewska JM, Speight PM. (1995). Evaluation of a screening test for the early detection of oral cancer and precancer. Community Dent Health., 12(1):3-7.
Publisher | Google Scholor - Mathew B, Sankaranarayanan R, Sunilkumar KB, Kuruvila B, Pisani P. (1997). Reproducibility and validity of oral visual inspection by trained health workers in the detection of oral precancer and cancer. Br J Cancer., 76(3):390-394.
Publisher | Google Scholor - Mehta FS, Gupta PC, Bhonsle RB, Murti PR, Daftary DK, Pindborg JJ. (1986). Detection of oral cancer using basic health workers in an area of high oral cancer incidence in India. Cancer Detect Prev, 9(3-4):219-225.
Publisher | Google Scholor - Downer MC, Moles DR, Palmer S, Speight PM. (2004). A systematic review of test performance in screening for oral cancer and precancer. Oral Oncol., 40(3):264-273.
Publisher | Google Scholor - Kujan O, Glenny AM, Oliver RJ, Thakker N, Sloan P. (2006). Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev., (3):CD004150.
Publisher | Google Scholor - Elango KJ, Anandkrishnan N, Suresh A, Iyer SK, Ramaiyer SK. et al. (2011). Mouth self-examination to improve oral cancer awareness and early detection in a high-risk population. Oral Oncol., 47(7):620-624.
Publisher | Google Scholor - Scott SE, Rizvi K, Grunfeld EA, McGurk M. (2010).Pilot study to estimate the accuracy of mouth self-examination in an at-risk group. Head Neck, 32(10):1393-1401.
Publisher | Google Scholor - Su WW, Yen AM, Chiu SY, Chen TH. (2011). A community-based RCT for oral cancer screening with toluidine blue. J Dent Res, 89(9):933-937.
Publisher | Google Scholor - Lagakos SW. (2006).The challenge of subgroup analyses—reporting without distorting. N Engl J Med., 355(2):113-117.
Publisher | Google Scholor - Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. (2007). Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med., 357(21):2189-2194.
Publisher | Google Scholor - Warnakulasuriya KA, Nanayakkara BG. Reproducibility of an oral cancer and precancer detection program using a primary health care model in Sri Lanka. Cancer Detect Prev. 1991;15(5):331-334.
Publisher | Google Scholor - Warnakulasuriya S, Pindborg JJ. (1990). Reliability of oral precancer screening by primary health care workers in Sri Lanka. Community Dent Health, 7(1):73-79.
Publisher | Google Scholor - Subramanian S, Sankaranarayanan R, Bapat B, Somanathan T, Thomas G, et al. (2009). Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bull World Health Organ, 87(3):200-206.
Publisher | Google Scholor - Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, et al. (2006). The cost-effectiveness of screening for oral cancer in primary care. Health Technol Assess. 10(4):1-144.
Publisher | Google Scholor - Dedhia RC, Smith KJ, Johnson JT, Roberts M. (2011). The cost-effectiveness of community-based screening for oral cancer in high-risk males in the United States: a Markov decision analysis approach. Laryngoscope, 121(5):952-960.
Publisher | Google Scholor - Koepsell TD, Weiss NS. (2003). Epidemiologic Methods: Studying the Occurrence of Illness. New York: Oxford University Press; Screening, 442-463.
Publisher | Google Scholor - Lang TA, Secic M. (2006). How to Report Statistics in Medicine: Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia: American College of Physicians.
Publisher | Google Scholor - HealthPartners Dental Group and Clinics. (2007). Oral Cancer Guideline. Minneapolis, MN: HealthPartners.
Publisher | Google Scholor