Research Article
Moisture Sorption Isotherm of Ighu (A Shredded Cassava Product) From Sandpaper Cassava Variety at Different Temperature
- Linus-Chibuezeh, Adindu
- Adindu-Linus, Chidiamara Onyinyechi
- Iwe, Maduebibisi Ofo
- Nwabueze, Titus Ugochukwu;
- Tasie, Uchechukwu Oscar
- Sopuru-Udogu
- Joy Chinemerem
Department of Food Science and Technology, MichaelOkpara University of Agriculture, PMB 7267, Umudike,Nigeria.
*Corresponding Author: Linus-Chibuezeh, Adindu,Department of Food Science and Technology, Michael Okpara University of Agriculture, PMB 7267, Umudike, Nigeria.
Citation: A. Linus-Chibuezeh *, C.O. Adindu-Linus, O. M. Iwe, T.U. Nwabueze, U.O. Tasie, et al. (2024). Moisture
sorption isotherm of Ighu (a shredded cassava product) from Sandpaper cassava variety at different temperature. International Journal of Nutrition Research and Health, BioRes Scientia Publishers. 3(1):1-8. DOI: 10.59657/2871- 6021.brs.24.031
Copyright: © 2024 Linus-Chibuezeh, Adindu, this is an open-access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 13, 2024 | Accepted: September 23, 2024 | Published: September 12, 2024
Abstract
This work examined the sorption characteristics of Ighu (dry shredded cassava product) from a local cassava variety called sandpaper using two indigenous methods of processing Ighu of people of Isuochi in Umunneochi LGA of Abia State and Udi of Enugu State. The difference between the methods is the characteristic feature of the shredder (called nko) which produced Ighu with average shred diameters of 4mm and 5mm respectively. Sulfuric acid solution of different concentrations was used to obtain water activity (0.09 to 0.9) and three storage temperatures (20oC, 30oC and 40oC) were used for the sorption studies in a thermostatic incubator. Duplicate samples (0.5g) were placed over the acid solution in a desiccator and weighed daily until constant weight (less than 0.05 g) was obtained in-between measurements and was used to estimate equilibrium moisture content (EMC). GAB and BET models were used to fit the sorption data while correlation coefficient and RMSE were used to access adequacy of the models. BET model was more suitable in describing the sorption with higher outputs in terms of monolayer moisture content, R2 and RMSE. However, estimated water activity from GAB model showed aw range of 0.08 to 0.1 indicating high stability to microbial infestation.
Keywords: sandpaper: water activity: monolayer moisture: equilibrium moisture content: ighu
Introduction
Ighu is one of the various edible products made from cassava tubers [1]. Unlike other products from cassava, Ighu is processed by boiling unpeeled cassava tubers for about 30-40 minutes depending on the quantity and rate of heat supply, cooling, peeling and shredding with a metallic shredder (nko) having many openings on it [2]. The thinly sliced shreds are sometimes spread in a rectangular shaped wooden basket (called nmimi in Igbo language) for several hours to further reduce the cyanogenic glycoside in the tuber. It is washed and dried on the wooden baskets on elevated platform referred to as “nlugbu”. It is also called jigbo, mpataka, abacha, eberebe jiapu, asharasha, jiapu mmiri, nsisa etc. in different Igbo dialects [3]. Food materials has been shown to be influenced by the temperature, relative humidity and moisture content of the surrounding environment, and thus the water activity (aW) of the material [4, 5]. Moisture sorption isotherms (important tools for predicting interactions between the water and the food components) describe the relationship between water activity and equilibrium moisture content of a product at a given temperature. It depends on several factors, such as physical structure, chemical composition and water affinity [5]. The moisture sorption behaviour of foods is described by several mathematical models, some of them are according to principles of the sorption mechanism and others categorized into empirical and semi-empirical models [6, 7, 8]. There is no unique sorption isotherm model for different foods, since the single components of food products have particular hydroscopic characteristics and may change the structure or composition of the food effect on the moisture sorption isotherm [9]. Moisture sorption isotherm is used to investigate structural features such as specific surface area, pore volume, pore size distribution and crystallinity of a food product [10]. Such data are used for selecting appropriate storage conditions and packaging systems that optimize retention of aroma, colour, texture, nutrients and biological stability [11]. Moisture sorption isotherms are characteristic of a food and vary with different foods, processing methods and temperature [12, 13, 14]. Moisture sorption isotherm equations give insight into the moisture binding characteristic of foods [15]. Therefore, it is useful to determine the sorptioncharacteristic of foods and the suitability of sorption models checked, such models allow the understanding of the different aspects of the food- water interaction [16]. The aim of this research is to evaluate the moisture sorption isotherm of Ighu (a shredder cassava product) at various temperatures and water activity levels using the BET and GAB models.
Materials And Methods
Material and Methods
Material
A popular cassava variety called Sandpaper usually used for processing Ighu among the natives of Umuaku Isuochi in Umunneochi LGA of Abia State Southeast of Nigeria was used in this work. The cassava stem was planted, monitored and harvested at
exactly eight (8) months after planting. While all reagents used are of analytical grade.
Methods
Processing of Ighu
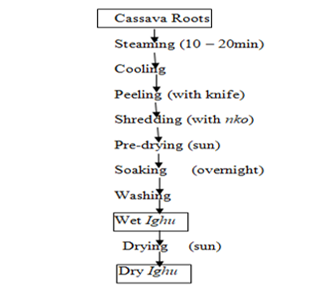
Processing of cassava tubers to Ighu was done according to the method described by [1] with slight modification (Figure 1). Similar processing methods are used by indigenous peoples of Isuochi in Abia State and Udi in Enugu State but with different shape and opening in the metallic material used for shredding the cassava tuber called nko (Plates 1 and 2) resulting to Ighu with average diameter of 2 mm and 4 mm respectively for Isouchi and Udi Ighu strands as shown in Plate 3.
Figure 1: Flow chart for Ighu processing
Source: Nwagbara and iwe (2008)
Plate 1: Nko from udi enugu state
Plate 2: Nko from isuochi Abia state
Plate 3: Dried Ighu Samples from Isuochi and Udi methods
Moisture sorption isotherm experiment
Moisture sorption isotherm experiment
Sulphuric acid solution of different concentrations (15 to 65%) representing a range of water activity (0.10 to 0.90) as shown in Table 2 were used for the adsorption isotherm determination of the Ighu samples. About 100 ml of the acid was introduced into 500 ml airtight glass jars and wire gauze was forced into each of the containers over the saturated solutions to form support for the samples. Triplicate samples, each (0.5 g) of sample was weighed in crown cork and placed on the wire gauze above the saturated solutions for experiment. The containers were tightly covered with lids and allowed to equilibrate in thermostatically controlled incubators set at different temperatures of 20oC, 30oC and 40oC. The samples were removed and weighed every 12 hr. intervals until difference between consecutive readings were less than 0.5 % of sample weight. The total time for removal and putting back in the airtight containers was about 1-2 minutes to reduced atmospheric adsorption occurring as recommended by the co- operative project cost 90 [17]. Equilibrium moisture contents were determined by drying of the saturated samples in an oven for 4 hours at 105 oC and subsequent cooling in a desiccator. Moisture content was calculated and expressed in dry-weight basis method (gH2O/100 solid) using the equation
 - [1]
- [1]
Modelling of sorption data
EMCs, water activities, and temperature data were fitted into two sorption kinetic models of Brunauer-Emmet-Teller (BET) and Guggenheim-Anderson-de Boer (GAB). These models were chosen based on versatility, relatively simple mathematical computation, and broad usage in modeling in experimental sorption data of many carbohydrate foods. The GAB method has been used to describe sorption isotherms of agro-foods within the water activity range of 0.00–0.95 as noted by [18]. while BET is known to be used for water activities below 0.5 (aw <0>
Table 1: Adsorption models for EMC
| Model | Equation | Equation numbers |
| BET |  | 2 |
| GAB |  = = + + | 3 |
Where: AW=water activity; C,G and K=model constants of sorption; Source:Rangel-Marron et al. (2011)
Surface area of adsorption (So)
The monolayer moisture values from BET model were used to evaluate the surface area of adsorption of the sorbent using Equation 4:
 [4]
[4]
Where No is the Avogadro's number (6.023×1023molecules/mole), A is the apparent surface area of one water molecule (1.05×10-19 m2), Ms is the molar mass of water (18 g/mol), Mo is the monolayer moisture content (gH2O/g solid), and so is the apparent area of sorption.
Table 2: Water activity of sulphuric acid at selected temperature
| Treatment | Quantity of H2SO4 to water | 20 oC | 30 oC | 40 oC |
| 1 | 15% | 0.8237 | 0.9245 | 0.9253 |
| 2 | 25% | 0.8218 | 0.8252 | 0.8285 |
| 3 | 30% | 0.7491 | 0.7549 | 0.7604 |
| 4 | 35% | 0.6607 | 0.6693 | 0.6773 |
| 5 | 40% | 0.5599 | 0.5711 | 0.5816 |
| 6 | 45% | 0.4524 | 0.4653 | 0.4775 |
| 7 | 50% | 0.3482 | 0.3674 | 0.3702 |
| 8 | 55% | 0.2440 | 0.2563 | 0.2658 |
| 9 | 65% | 0.0895 | 0.0972 | 0.1052 |
Result and Discussion
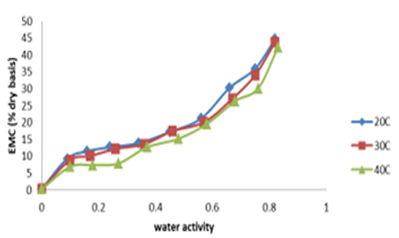
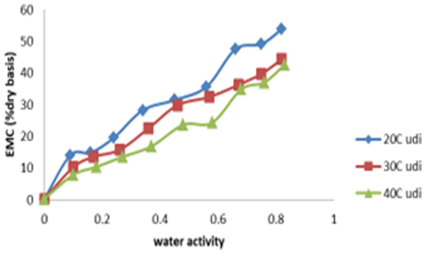
Effect of water activity on equilibrium moisture content (EMC) of Ighu at different temperatures. The result of effect of water activity on equilirium moisture content (EMC) of Ighu processed using different indigenous methods at different temperature is shown in Figures 2 and 3.
The figures depict the moisture adsorption isotherm and behaviour of the Ighu samples as affected by changing levels of water activity and storage temperatures. An increasing EMC with increasing water activity levels were observed for the samples irrespectiviely of the processing method used. The higher EMC recorded for Udi processing method could be attributed the higher shredd diameter (average of 5mm) Ighu strands which was due to the bigger shredding aparture of the metallic device (nko) used for the processing. The bigger strands of the sample resulted to increased absorption of moisture at the storage temperatures observed (20, 30 and 40oC). Figure 2 showed an overlapping isotherm at temperature of 20oC and 30oC for Ighu processed using Isuochi indigeneous method while a clear line was observed at higher temperature of 40oC. The increasing EMC of the cassava shreds with increasing aW at selected temperatures irrespective of the processing method could also be due to increase in water vapor pressure present in food with that of the surrounding which corresponds with findings of in adsorption study using mushroom, for Gongronema latifolium leaf grits and for awara (tofu-like product from soybean [19,20,21]. This trend has been reported to be common to all food materials and it is an indication that Ighu samples would adsorb more water at higher relative humidity/water activity. Consequently, in an environment of constant relative humidity, it can absorb more moisture at lower than at higher temperatures. At constant moisture content, an increase in temperature caused lowering of isotherm curves which increased aw thereby making Ighu shreds more susceptible to microbial spoilage. It could also be observed from Figures 2 and 3 that at water activity above 0.5, higher amount of water was adsorbed for a small rise in aW. This is because at low water activities physical sorption occurs on strongly active binding sites of substrate, such as –OH groups present on the surface film. In the intermediate aw range, adsorption took place at less active sites and showed that the region could be a zone highly unstable in terms of spoilage [10]. also reported such behavior for different hygroscopic foods. The displayed moisture adsorption isotherm curves (Figures 2 and 3) are typically a sigmoid shape corresponding to type II isotherms [20,22]. The type II isotherm is known for the formation of multiple layers of adsorbate molecules at the internal surface of food solid and is a characteristic specific to most organic tissues and commonly observed in hygroscopic food products [21,23]. However, reported a reverse in this trend for moisture adsorption studies of Awara which was attributed to high protein content of the product.
Figure 2: Effect of water activity on equlibrum moisture content of ighu (isuochi method)
Figure 3: Effect of water activity equlibrum moisture content of ighu (udi mehod)
Evaluation of sorption isotherm models of Ighu shreds
The sorption isotherms model derivatives (GAB and BET) used are presented in Table 3.
The GAB Isotherm parameters and derivatives
The GAB constants (C and K) varied with temperature for adsorption isotherms of Ighu from different processing methods. The variation of G-values with temperature was greater than those of K-value. The G- values decreased with increasing temperature while K values for adsorption isotherm did not show a definite pattern across the temperature, and higher values were obtained for Ighu processed using Isuochi method. Contrastively, higher monolayer moisture content was recorded for Ighu processed using UDI method (16.03 to 27.03 gH 20/100g solid) compared to ISUOCHI method (9.59 to 10.13 gH20/100g solid). The variation in monolayer moisture content at different temperature could be summarized as stated by that arise from the kinetic model [20]. Increasing temperature or decreasing the energy of activation (Ea) of particles of reactants will lead to increase in the rate of moisture adsorption and vice versa. At lower storage temperature, the level of excitation of the molecules of the reactants could be similar and implied that the number of collisions per second that might lead to moisture adsorption or not, behaved similarly. The G-values for adsorption isotherm ranged from 14.26 to 55.99 for Isuochi method while 7.35 to 10.6 for Udi method of processing Ighu. K-values ranged from 0.95 to 0.96 Isuochi method and 0.58 to 0.73 Udi method. The variation in G and K values with respective to processing method could be attributed to different shape of nko used in the shredding which resulted to high energy. This decrease in GAB’s constants (G and K values) was also reported from several authors [10, 20, 24,25].
The BET Isotherm parameters and derivatives
The BET model was used to calculate the surface area (S0) of monolayer moisture according to equation 5 (Table 3). Higher surface area of momolayer moisture content was recorded for Ighu processed using UDI processing method (470.80 to 642.96 gH2O/100 g solid compared to 290.13 to 333.80 recorded for Ighu from ISUOCHI method. A significantly lower BET monolayer moisture content compared to GAB’s were recorded for the samples. The range of 8.35 to 9.46 gH2O/100 g and 13.32 to 19.31 gH2O/100 g were recorded for Ighu from ISUOCHI and UDI methods respectively. A decrease in both monolayer moisture content and surface area of sorption were observed (Table 3) with increasing temperature was observed, indicating the effect of thicckness of the Ighu strands on the parameters evaluated. The BET model is known to fit experimental sorption data in the range of water activities below 0.5[26]. However, the BET model provides values of monolayer moisture content, which is an important parameter in food deterioration studies. According to the main limitation of BET theory appears to be the assumption that all absorbed molecules in layers other than the 1st have liquid like evaporation-condensation properties [26].
Moisture adsorption models and their adequacies
The adequacy of the chosen sorption models was evaluated using correlation coefficient (R2) and percentage root mean square error (RMSE). The GAB’s monolayer moisture content was significantly higher (16.03 to 27.03 gH20/100g solid UDI) and (9.59 to 10.13 gH20/100g solid for ISUOCHI method) compared to 8.35 to 9.46 gH2O/100 g and 13.32 to 19.31 gH2O/100 g recorded for Ighu from ISUOCHI and UDI methods respectively for BET monolaayer. This is in agreement to statement of and [25,26]. That GAB equation is an improved version of BET model and is based on theoretically sound principles. The GAB isotherm was used to fit sorption isotherms up to water activities of 0.9 in many cases [27]. The use of GAB parameters has physical significance and it is considered to be the best equation available for representing monolayer isotherms for many food materials [25,26,28]. The GAB model provided monolayer moisture content values and other useful information to the heat of sorption and monolayer and multilayer of Ighu. However, correlation coefficient was higher in BET’s model than the GABs’ which is an indication of better model fitting esspecailly for Ighu processed using UDI indigenous method which recorded R2 below 0.9 for GAB model. The correlation coefficient was however improved by using the BET model (0.95 for 30oC, 0.96 20oC and to 0.98 for 40oC). The percentage root means square error (RMSE) which is a measure of magnitude of varying quantity. Low RMSE values below 1 (as recorded for BET model) show that model makes more accurate prediction and fits the data well.
Table 2: Water activity of sulphuric acid at selected temperature
| Treatment | Quantity of H2SO4 to water | 20 oC | 30 oC | 40 oC |
| 1 | 15% | 0.8237 | 0.9245 | 0.9253 |
| 2 | 25% | 0.8218 | 0.8252 | 0.8285 |
| 3 | 30% | 0.7491 | 0.7549 | 0.7604 |
| 4 | 35% | 0.6607 | 0.6693 | 0.6773 |
| 5 | 40% | 0.5599 | 0.5711 | 0.5816 |
| 6 | 45% | 0.4524 | 0.4653 | 0.4775 |
| 7 | 50% | 0.3482 | 0.3674 | 0.3702 |
| 8 | 55% | 0.2440 | 0.2563 | 0.2658 |
| 9 | 65% | 0.0895 | 0.0972 | 0.1052 |
Table 3: GAB and BET models for adsorption parameters for Ighu processed from local variety (Sandpaper)
| Ighu from Isuochi method | Ighu from Udi method | |||||
| Models/temp | 20 oC | 30 oC | 40 oC | 20 oC | 30 oC | 40 oC |
| GAB Model | ||||||
| Mo (gH20/100 g solid) | 10.13 | 9.59 | 9.65 | 26.39 | 27.03 | 16.03 |
| G | 55.99 | 37.11 | 14.26 | 10.6 | 7.35 | 10.0 |
| K | 0.96 | 0.95 | 0.95 | 0.68 | 0.58 | 0.73 |
| R2 | 0.9654 | 0.9833 | 0.7787 | 0.8822 | 0.8704 | 0.8182 |
| RMSE | 1.548 | 1.569 | 1.598 | 1.548 | 1.568 | 1.597 |
| Predicted aw | 0.08 | 0.085 | 0.09 | 0.1 | 0.098 | 0.081 |
| BET Model | ||||||
| Mo (gH20/100 g solid) | 9.43 | 9.46 | 8.35 | 19.31 | 17.77 | 13.32 |
| So (gH20/100 g solid) | 330.26 | 333.80 | 290.13 | 642.96 | 621.88 | 470.80 |
| C | 208.182 | 38.403 | 12.725 | 13.618 | 7.884 | 8.471 |
| R2 | 0.9972 | 0.9941 | 0.9172 | 0.9608 | 0.9576 | 0.9823 |
| RMSE | 0.644 | 0.673 | 0.701 | 0.641 | 0.671 | 0.698 |
Conclusion
The moisture sorption isotherm of Ighu processed from two indigenous methods showed type II isotherm curve. The BET sorption model gave better prediction and fits the data well compared to GAB model with higher monolayer moisture content, correlation coefficient and root mean square error. Higher monolayer moisture content and surface area of sorption recorded for UDI processing method is indication that more energy was expended during its processing. Calculated water activity value (0.08-0.1) from quadratic integration of GAB model is an indication that the samples will have high shelf stability and will also be useful in shelf-life prediction studies.
Declarations
Acknowledgment
Authors would like to thank the Directorate for University Research Administration (DURA) and TETFUND for supporting this research.
Declaration Of Conflict of Interest
The authors declare no conflict of interest
References
- Nwagbara, I. I and Iwe, M. O. (2008). Critical control point in processing of cassava for ighu production. Nigerian Food Journal, 26(2):114-124.
Publisher | Google Scholor - Linus-Chibuezeh, Adindu. (2016). Effects of High-Quality Cassava Flour (HQCF) from different Cassava Varieties on the Nutritional Quality of Wheat-Cassava Bread - A Response Surface Analysis. A Master of Science Degree Thesis submitted to the Postgraduate School, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria.
Publisher | Google Scholor - [Njoku, B. A and Banigo, E. O. I. (2006). Physic-chemical properties of precooked cassava (Manihot esculenta Crantz) flour prepared by adaptation of a traditional process. Nigerian Food Journal, 24(1):98-91.
Publisher | Google Scholor - Iglesias, H.A. and Chirife, J. (1976). Prediction of effect of temperature on water sorption of food materials. Journal of Food Technology, 11:109-116.
Publisher | Google Scholor - Sengev, I. A., Ariahu, C. C., Abu, J. O. and Gernah, D. I. (2016). Moisture Adsorption and Thermodynamic Properties of Sorghum-Based Complementary Foods. International Journal of Food Engineering and Technology, 2(1):26-33.
Publisher | Google Scholor - Duckworth, R. B. (1983). Future needs in water sorption in food stuffs. In Physical Properties of Foods; Jowitt, R., Escher, F., Hallstrom, B., Mefert, H., Spiess, W. and Vos, G., Eds. Elsevier Applied Science, 93-102.
Publisher | Google Scholor - Rizvi, S.S.H. (1986). Thermodynamics of foods in dehydration. In Engineering Properties of Food; Rao, M.and Rizvi, S., Eds.; Marcel Dekker: New York, NY, USA, 133-214.
Publisher | Google Scholor - Saberi, B., Vuong, Q. V., Chockchaisawasdee, S., Golding, J. B., Scarlett, C. J and Stathopoulos, C. E. (2016). Water Sorption Isotherm of Pea Starch Edible Films and Prediction Models. Foods, 5(1):1-18.
Publisher | Google Scholor - Sebti, I., Delves-Broughton, J. and Coma, V. (2003). Physicochemical properties and bioactivity of nisin-containing cross-linked hydroxypropylmethylcellulose films. Journal of Agriculture and Food Chemistry, 51:6468-6474.
Publisher | Google Scholor - Ariahu, C. C., Kaze, S. A. and Achem, C. D. (2006). Moisture sorption characteristics of tropical fresh water crayfish (Procamarus clakia). Journal of Food Engineering, 75:315-363.
Publisher | Google Scholor - Eke, M., Ariahu, C. C. and Okonkwo, T. M. (2011). Moisture sorption characteristics of Dambu-nama-A Nigerian dried meat Product. Nigerian Food Journal, 29(2):103-110.
Publisher | Google Scholor - Arogba, S.S. (2001). Effect of temperature on the moisture isotherm of a biscuit containing processed mango (Mangifera indica) kernel flour. Journal of Food Engineering, 34:234.
Publisher | Google Scholor - Akubor, P. I. and Egbekun, M. K. (2017). Browning, viscosity and moisture sorption characteristics of fresh and stored African breadfruit kernel flour. Chemistry Research Journal, 2(4):139-145.
Publisher | Google Scholor - Palou, E., Lopez-Malo, A. and Argaiz, A. (1997). Effect of temperature on the moisture sorption isotherms of some cookies and corn snacks. Journal of Food Engineering, 31:85-93.
Publisher | Google Scholor - Shittu, T.A., Awonorin, S.O. and Raji, A.O. (2004). Evaluating some empirical models for breadfruit (Treculia Africana) seeds. International Journal of Food Properties, 7:585-602.
Publisher | Google Scholor - Nutama, B. and Lin, J. (2010). Moisture sorption isotherm characteristics of taro flour. World Journal of Dairy and Food Science, 5(1):1-6.
Publisher | Google Scholor - Gal, S. (1988). The need for and practical application of sorption data. In: Physical Properties of Foods. R. Jowitt, F. Escher, B. Hall Strem, H. F. Meffert, W. E. L. Spiess and G. Vos. (eds). London Applied science publishers, 13-25.
Publisher | Google Scholor - Iguedjtal, T., Louka, N., and Allaf, K. (2008). Sorption isotherms of potato slices dried and texturized by controlled sudden decompression. Journal of Food Engineering 85, 180-190.
Publisher | Google Scholor