Research Article
Histopathological Characteristics of Prostate Carcinoma in Archival Tissues, A Hospital-Based Cross-Sectional Descriptive and Analytical Study
- Ancent Nzioka *
Department of Pathology, Kenyatta University Teaching, Research and Referral Hospital, Kenya.
*Corresponding Author: Ancent Nzioka, Department of Pathology, Kenyatta University Teaching, Research and Referral Hospital, Kenya.
Citation: Nzioka A. (2025). Histopathological Characteristics of Prostate Carcinoma in Archival Tissues, A Hospital-Based Cross-Sectional Descriptive and Analytical Study, International Journal of Clinical and Surgical Pathology, BioRes Scientia Publishers. 1(2):1-11. DOI: 10.59657/3067-0462.brs.25.011
Copyright: © 2025 Ancent Nzioka, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 04, 2024 | Accepted: March 03, 2025 | Published: March 17, 2025
Abstract
Introduction: Prostate carcinoma (PCa) is the leading cancer among middle and elderly men worldwide accounting for 15% of all cancer cases and 6.6% of all cancer associated-deaths in men. PCa is a leading cause of cancer burden among men in Africa with Kenya reporting an incidence of 16.6/100,000. Exceedingly high burden of PCa is due to lack of screening and delayed diagnosis coupled with genetic and environmental predisposing factors. Histopathological features of PCa offer prognostic indicators in the management of PCa.
Methods: Retrospective study determining the PCa histopathology on archival simple prostatectomy specimens at a tertiary faith-based health facility in Kenya. Tissue blocks (n=210) were retrieved, sectioned and stained using standard Hematoxylin and Eosin (H&E) protocols. The slides were examined using light microscopy and histopathological features recorded.
Results: Histopathologically, 97.6% of the PCa were of acinar adenocarcinoma type. Perineural invasion (PNI) was present in 57.6% of the PCa while 25.2% had lymphovascular invasion (LVI). High grade PCa (Gleason score ≥8) constituted 43.4% of the cases. The frequencies of the different ISUP grade groups were as follows; ISUP grade 1 (23.3%), grade 2 (16.2%), grade 3 (17.1%) grade 4 (10.5%) and grade 5 (32.9%). Binary logistic regression analyses revealed that Gleason score ≥8 was associated with higher odds for PNI (OR, 40.407; 95% CI, 11.036-147.949; P<0.0001) and gland involvement of ≥50% (OR, 30.447; 95% CI, 7.220-128.397; P<0.0001). In addition, PCa pathological grade III was associated with PNI (OR, 37.710; 95% CI, 8.762-162.288; P<0.0001), LVI (OR, 33.531; 95% CI, 13.757-81.729; P<0.0001), and gland involvement of ≥50% (OR, 2.291; 95% CI, 1.040-50.500; P=0.040).
Discussions: The findings showed higher percentage of PNI, Gleason score ≥8, ≥ ISUP grade 3and other histopathological features indicative of poorer prognosis as compared to studies by Schreiber and Samaratunga.
Conclusion: This is the first PCa study outlining the histopathological characteristics of PCa in Kenya and the first African study that has used ISUP grade groups for PCa. These findings will influence the screening, diagnosis, treatment and prognosis of PCa.
Keywords: prostate carcinoma; kenya; histopathological characteristics; ISUP grades; archival tissue
Introduction
Globally, prostatic cancer (PCa) is the second most often diagnosed malignancy amongst males and the sixth ranked cause of cancer mortality in men (Jemal et al., 2011). PCa incidence portrays notable and quite baffling geographical and racial differences worldwide (Ekman, 1999). White Americans show a PCa incidence of 50 - 60 males per 100,000 people. The incidence of PCa amongst African Americans is higher than for the White Americans pushing the United States of America national age - adjusted PCa incidence to 69 males per 100,000 people. Among the Japanese, PCa age - adjusted incidence is 3-4 males per 100,000 people while in Hong Kong the age - adjusted PCa incidence is just 1 male per 100,000 people. African population-based data on PCa are scanty. The International Agency for Research on Cancer (IARChttp://www-dep.iarc.fr/) reveals that rates amongst Africans are higher in the Eastern countries (10.7–38.1 per 100,000 man-years, age-adjusted world standard) than in the Western countries (4.7–19.8) (Lisa et al.,2011).
Among Kenyan men, prostate cancer is the commonest malignancy with an age specific rate of 17.3percentage according to the Nairobi Cancer Registry (Mutuma & Anne, 2006). Recently released data indicates that 2,864 new PCa cases were diagnosed in Kenya in 2018 constituting 14.9% of all new cancers in Kenyan males in 2018 (Globocan, 2018).
PCa is not always lethal but rather it is a diverse disease spanning a full spectrum of being asymptomatic to a swiftly lethal systemic cancer (Shah et al., 2004; Hughes et al., 2005). There is minimal published data on the pathological characteristics of PCa in Kenyan setting. PCa characteristics including histological type, Gleason score/ISUP/WHO grade group, angiolymphatic /lymphovascular invasion (LVI), tumor bulk, perineural invasion (PNI), and tumor stage are crucial in determining the management and prognosis of PCa.
The findings from this study will provide much needed local baseline data on the pathological characteristic of PCa. This will shed more light on the biology of PCa seen locally.
Methodology
The study aimed to;
- To establish the demographics of PCa subjects and prior gross prostate weight for PCa 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded tissue blocks.
- To determine the histopathological characteristics of PCa on retrieved 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded tissue blocks.
- To determine the associations between different histopathological characteristics on retrieved prostate cancer 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded tissue blocks.
The Study Designs
A hospital-based retrospective descriptive and analytical study design on archival 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded histopathology tissue blocks on previously diagnosed Prostate carcinoma was utilized to examine the histopathological characteristics of PCa.
The study was conducted at the AIC Kijabe hospital pathology department. The AIC Kijabe hospital is located in Kiambu County, Kenya. AIC Kijabe Hospital is a faith-based hospital run by the AIC Church. The Hospital caters for surgical patients from across Kenya and neighboring East African and Horn of Africa countries. The pathology department receives biopsy specimen from the hospital theatres and also from more than 10 other faith-based hospitals in the East African and Horn of Africa region (Muchendu, 2017).
The Study Population
The study targeted archival 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded prostate tissue blocks previously collected from routine simple prostatectomy specimen. The study population included all archived blocks previously diagnosed to have prostatic adenocarcinoma.
Inclusion Criteria
Only well-preserved archived 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded tissue blocks from PCa patients who had undergone simple prostatectomy and whose biodata was available were included into the study.
Sampling Techniques
Purposive sampling technique was used.
Sample Size Determination
Sample size was calculated based on the number of prostate cancer specimens received at AIC Kijabe Hospital, annually. In 2012-2014, the pathology department received 200-450 prostate cancer prostatectomy specimens annually. Therefore, a population size of 450 archival tissue blocks was used to determine the sample size.
Cochran's formula for categorical data for an alpha rank a priori at 0.05 was used to get minimum sample size (Cochran, 1977).
N0 = [(t2) (p) (q)]/ (d2)]
Were,
N0 = minimum sample size required
t = 1.96
p = maximum possible proportion. A value of 0.5 was used as the frequency of PCa at AIC Kijabe was unknown in 2012-2014.
q = 1- p = 0.5
d = acceptable margin of error for the proportion being estimated = 0.05.
Substituting these values in the formula above, gives:
No = [(1.962) (0.5) (0.5)] / (0.052)
No = [(3.842) (0.5) (0.5)] / (0.003)
No = 384
Therefore, the minimum sample size = 384
N1 = N0/ (1 + N0/population)
Were,
N1 = final sample size
N0 = 384
Therefore, final sample size = 384/ (1+384/450) = 207
Adding 1.5% for possible spoilage of tissue blocks = 101.5/100 x 207 = 210.1
Therefore, the final sample size for the study was 210 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded tissue blocks.
Recording of Demographic and Clinical Information
This information was gotten from the pathology laboratory information system at the pathology department (File maker pro-software, Apple Inc, USA). Information on the patient’s age, ethnic origin (Bantu, Nilote, Cushite) and gland weight in grams was retrieved and directly entered into excel spreadsheets (Microsoft® Excel 2016). Unique accession numbers were recorded to aid in retrieval of the paraffin tissue blocks.
Laboratory Procedures and Research Instruments
Retrieval of 10% Neutral Buffered Formaldehyde-Fixed, Paraffin Wax-Embedded Archival Tissue Blocks
The acquired demographic and clinical data was used to help in retrieving 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded archival tissue blocks as per the inclusion criteria. Pathologist assistants retrieved the tissue blocks from the archives using the unique accession numbers. The tissue blocks were assessed grossly and those in good condition included. The best tissue block per case was selected.
Histopathology Procedures
One histopathology section per case was cut out of the retrieved 10% neutral buffered formaldehyde-fixed, paraffin wax-embedded tissue blocks using a microtome as per microtomy protocol (Appendix II). Each tissue section was then stained using routine histological Hematoxylin and Eosin stains as per protocol (Appendix III). These stained slides were then mounted in mounting media and cover slipped. They were then microscopically evaluated by two independent histopathologists. Histopathological features including tumor type, Gleason score / ISUP grade group, perineural invasion, lymphovascular invasion, tumor bulk; tumor margins and stage were determined. Cases with discordant results underwent a consensus review at a multithreaded microscope with a third histopathologist. The PCa histopathological characteristic findings were entered into a specially designed data collection form (Appendix I).
Data Analysis and Presentation
Histopathological Characteristics
The data collected was inputted and sanitized in excel spreadsheets (MS® Office) and exported into IBM® SPSS Statistics 21.0 (SPSS Inc. Chicago, USA) for coding and statistical analyses. Data obtained from the histopathological characteristics of each retrieved PCa tumor block was captured in a consolidated raw registration form and entries done into Excel spreadsheet package workbook (Microsoft office version 2010) by assigning characteristics as either categorical or continuous variables. Data entry was also duplicated by importing Excel captured data onto SPSS statistical application software Programme (IBM SPSS Inc. application software version 21.0.1, 2010). Cleaned computed and coded relevant data was assessed to eliminate non-specific information. Continuous data (age and gland weight) summarized as means and range, and dichotomous measures (ethnicity, histopathological profiles) summarized as frequencies (n,%) were tabulated. PNI, LVI, and Gleason score / ISUP grade groups were presented as plates.
Associations of Histopathological Characteristics
Data analysis was done using several analytical model platforms to test for significance in associations and relationships between the dependent and the independent variables. Results are portrayed as odds ratios (OR) and 95% confidence intervals (CI), and β coefficient for each of the models. Summarized results were tabulated and P values of lessthan0.05 were considered statistically significant. Binary logistic regress models were performed to identify the associations of different histopathological indicators.
Results
Patient Demographics and Prostatectomy Specimen Gross Characteristics
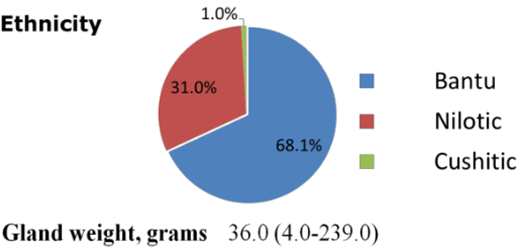
A total of two hundred and ten (210) prostatectomy archival paraffin tissue blocks were studied. The mean (interquartile) age for the patients was 74.0 (45.0-99.0) years. The patients’ ethnic extraction was as follows; Bantu (68%), Nilotic (31.0%) and Cushitic (1.0%) groups. The mean (interquartile) prostate gland weight (g) was 36.0 (4.0-239.0).
Figure 1: Demographics and gross specimen weight. Data presented as means and range for gland mass and as proportion (%) of subjects for ethnicity.
The Histopathological Characteristics of The Prostate Carcinoma
The histopathological features of the archival prostate tissue blocks analyzed in this study are presented in Table 1. The microscopic examination of the prostate specimens revealed that 97.6% were of acinar prostatic adenocarcinoma type while 2.4% were of ductal prostatic adenocarcinoma type. Tumor involving more than 50% of the gland was identified in 19.5% of the specimens while 80.5% of the specimens had less than 50% of the gland involved by tumor. The rate of high-grade Gleason prognostic groups (≥8) was 56.6% and low-grade Gleason prognostic group (lessthan 8) was 43.4% (Plate 1.1). In addition, ISUP grade groups were as follows: ISUP 1, 23.3%, ISUP 2, 16.2%, ISUP 3, 17.1%, ISUP 4, 10.5% and ISUP 5, 32.9%. PNI (Plate 1.2) was present in 56.7% of the specimens and LVI (Plate 1.3) was present in 25.2% of the specimens. Positive surgical margins (pathological stage III) were present in 27.1% of the specimens and negative surgical margins (pathological stage II) were 72.9%.
Table 1: Histopathological characteristics of PCa in archival tissue blocks. Data Presented as number and proportion (%) of subjects after microscopic evaluation. PNI, perineural invasion. LVI, lymphovascular invasion. ISUP, international society of urological pathologists. Pathological stage II, Prostate adenocarcinoma with negative surgical margins. Pathological stage III, Prostate adenocarcinoma with positive surgical margins.
| Histopathological Characteristics | Number of Subject Specimens, n (%) |
| Type of Cancer | |
| Acinar | 205/210 (97.6) |
| Ductal | 5/210 (2.4) |
| Tumor size, (% of Gland Involvement) | |
| ≥50 | 41/210 (19.5) |
| <50> | 169/210 (80.5) |
| PNI | |
| Yes | 119/210 (56.7) |
| No | 91/210 (43.3) |
| LVI | |
| Yes | 53/210 (25.2) |
| No | 157/210 (74.8) |
| Gleason Prognostic Grade | |
| <8> | 119/210 (56.6) |
| ≥8 (High Grade) | 91/210 (43.4) |
| ISUP Grade Groups | |
| Grade 1 | 49/210 (23.3) |
| Grade 2 | 34/210 (16.2) |
| Grade 3 | 36/210 (17.1) |
| Grade 4 | 22/210 (10.5) |
| Grade 5 | 69/210 (32.9) |
| Surgical Margins Positivity | |
| Yes | 57/210 (27.1) |
| No | 153/210 (72.9) |
| Pathological Stage | |
| II | 153/210 (72.9) |
| III | 57/210 (27.1) |
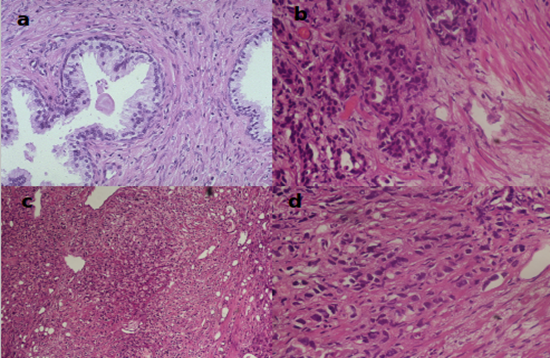
Figure 2: Plate showing prostate cancer grades (x40, H&E). (a) Benign prostatic tissue, (b) PCa Gleason scores 3 + 4 = 7/10, ISUP grade group 2, (c) PCa Gleason scores 4 + 4 = 8/10, ISUP grade group 4, (d) PCa Gleason scores 5 + 5 = 10/10, ISUP grade group 5.
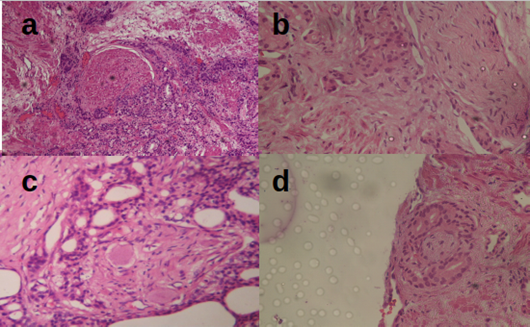
Figure 3: Plate showing different forms of PCa PNI (x40, H&E). (a) Semi circumferential PNI, (b) Longitudinal PNI, (c) Complete circumferential PNI, (d) PNI around a small nerve.
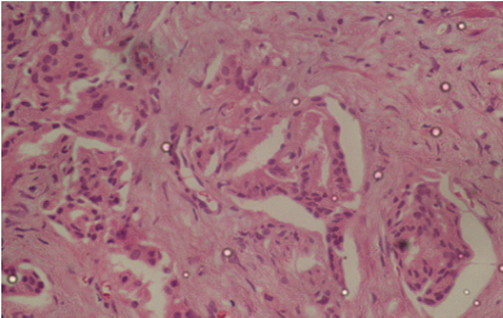
Figure 4: Plate showing LVI (x40, H&E). Shows tumor emboli within several small sized endothelial lined lymphovascular channels.
The Association Between Different Histopathological Characteristics on Archival PCa Tissue at The AIC Kijabe Hospital
Several histopathological characteristics were selected evaluated for associations. This was performed using binary logistic regression models.
Association of High-Grade Gleason Score (≥8) with PNI, Level of Gland Involvement
Evaluation of the link between the high-grade Gleason score (≥8) and the histopathological characteristics of the PCa (Table 2) showed a likelihood of PNI (β=-3.699; OR, 40.407; 95% CI, 11.036-147.949; P lessthan 0.0001) and tumor size greaterthan 50% (β=3.416; OR, 30.447; 95% CI, 7.220-128.397; P lessthan 0.0001).
Table 2: Association of high-grade Gleason score (≥8) with PNI and % gland involvement. Data shown as odds ratios (OR) and 95% confidence interval (CI). β coefficient for the model. P value less than 0.05, significance level. Ref, reference group. PNI, perineural invasion.
| Gleason Score ≥8 | |||
| Indicator | β | OR (95% CI) | P |
| PNI | |||
| Absent | Ref | ||
| Present | 3.699 | 40.407 (11.036-147.949) | Lessthan 0.0001 |
| % Gland Involved | |||
| Lessthan 50% | Ref | ||
| ≥50% | 3.416 | 30.447 (7.220-128.397) | Lessthan 0.0001 |
Association of pathological stage III with PNI, LVI and level of gland involvement. PCa samples exhibiting pathological stage III (Table 3) were more likely to have PNI (β=3.630; OR, 37.710; 95% CI, 8.762-162.288; P lessthan 0.0001); LVI (β=3.512; OR, 33.531; 95% CI, 13.757-81.729; P lessthan 0.0001); and tumor size greaterthan 50% (β=0.829; OR, 2.291; 95% CI, 1.040-50.50; P=0.040).
Table 3: Association of Pathological stage III with PNI, LVI and % gland involvement. Data shown as odds ratios (OR) and 95% confidence interval (CI). β coefficient for the model. P value less than 0.05, significance level. Ref, reference group. PNI, perineural invasion. LVI, lymphovascular invasion.
| PCa Pathological Stage III | |||
| Indicator | β | OR (95% CI) | P |
| Perineural Invasion | |||
| Lacking | Ref | ||
| Evident | 3.630 | 37.710 (8.762-162.288) | Lessthan 0.0001 |
| Lymphovascular Invasion | |||
| Lacking | Ref | ||
| Evident | 3.512 | 33.531 (13.757-81.729) | Lessthan 0.0001 |
| %Gland Involvement | |||
| Lessthan 50% | Ref | ||
| ≥50% | 0.829 | 2.291 (1.040-50.500) | 0.040 |
| Evident | -0.980 | 0.375 (0.091-1.551) | 0.176 |
Discussion
Patient Demographics
The mean age of the subjects with prostate cancer was 74 years. This is 5 years older compared to a mean age of PCa diagnosis of 69 years in the United States and 69.5 years in a Ugandan study (Okuku et al., 2016). These differences might be due to the high age-standardized incidence of prostate cancer (per 100,000) in USA (124.8) and in Uganda (38) compared to Kenya’s (16.6) (Haas, Delongchamps, Brawley, Wang & Roza, 2008). The difference with the USA means age can also be attributed to the intensity of screening and diagnostic efforts that leads to detection of occult prostate cancer disease among Americans.
Regarding ethnicity of the subjects, 68% were Bantu, 31% Nilotes and 1% of Cushitic extraction. These proportions roughly correspond to the Kenyan national population of these different ethnic groups (Kenyan National Bureau of Statistics, 2010).
Histopathological Characteristics of the PCa
Upon histopathological evaluation, 97.6% of the PCa were of acinar adenocarcinoma type while the rest were of ductal adenocarcinoma type. This is comparable to published data showing that 95 to 99.6 % of PCa are of acinar type while 0.4 to 5% is of ductal type (Bock & Bostwick, 1999).
Evaluation for PNI showed that 56.7% of the cases had PNI. Prostatic perineural invasion is thought to be mediated by tumor expression of nerve cell adhesion molecule (Li, Wheeler, Ai& Ayala, 2003). PNI in PCa is a poor prognostic factor since it has been shown that these tumors acquire a growth advantage compared with PCa without perineural invasion (Fromont et al., 2012). It is a less appreciated metastatic pathway for a number of malignancies including prostatic carcinoma, pancreatic carcinoma, colorectal carcinoma, head and neck malignancies, biliary tract malignancies and gastric carcinomas. In most of these malignancies, PNI has been demonstrated to be an indicator of worse prognosis and signifies shorter survival (Liebig et al., 2009). D'Amico et al. (2001) demonstrated PNI to be an autonomous projecting factor for PCa reappearance following radical prostatectomy. However, Reeves et al. (2015) in their study claimed that PNI on radical prostatectomy specimen is not an autonomous forecaster of biochemical cancer reappearance and hence they don’t advocate for routine reporting of PNI.
A study by DeLancey et al (2013) established that PNI is autonomously connected to unfavorable pathologic characteristics and poor survival outcomes following radical prostatectomy. Therefore, PNI in prostatic biopsy specimens is recommended to be factored in prostate cancer management assessment planning and clinical care plan. Work based on the action by the histomorphology-based extrapolative characteristic working group of the 2004 WHO-facilitated International Consortium on handling and reporting of prostate cancer protocol (Epstein et al., 2005) recommends that PNI status be included in all prostate cancer reports. Further studies are recommended targeting the biology of PNI to increase the understanding of its function in prostate cancer disease progression.
LVI is defined as the spread of a cancer cells into the blood vessels and lymphatic vessels. It is identified morphologically in the peritumoral area. It is a crucial step in the infiltration-metastasis progression of malignancies and is considered a marker of metastatic capability strongly linked with a worse outcome in many solid neoplasms. Malignant cells have the potential to interrelate with the endothelium, permeate the lymphovascular wall and tolerate the intravascular forces through different molecular mechanisms. LVI is the commonest route of metastases for malignancies. The other routes are direct spread and trans coelomic spread. When the tumor cells invade the lymphatics or blood vessels, the chance of this malignancy to spread to regional and distant parts of the body is very high (Cheng et al., 2005). In this study, LVI was demonstrated in 25.2% of the prostate cancer cases. This is comparable to findings by Yong et al. (2016) that showed LVI incidence of 20% and 21.5% respectively. However, other studies by Mitsuzuka et al., (2015) and Matthias et al., (2006) showed significantly lower incidences of LVI of 10.4% and (10.2%). This can be attributable to the fact that these studies were done in populations where prostate cancer screening is intensive and hence the study population has generally low stage prostate cancers. LVI has been demonstrated to be a very important predictive feature in prostate cancer since it is significantly linked to higher presurgical serum PSA, high pathological stage, higher Gleason grade score, positive surgical excision margins, extra prostatic spread, seminal vesicle infiltration, nodal spread and perineural infiltration (Yong et al., 2016; Mitsuzuka et al., 2015; Matthias et al., 2006). This is also backed by the histomorphology-based predictive characteristics committee of the 2004 World Health Organization-facilitated International Consortium on handling and reporting of prostate cancer protocol (Epstein et al., 2005).
In this study, low grade prostatic adenocarcinoma (Gleason score ≤7) constituted 56.6 % while high grade prostatic adenocarcinoma (Gleason score ≥8) constituted 43.4%. This is dissimilar to findings by Schreiber et al., 2016 that indicated that low grade carcinoma constituted 90.2% while high grade carcinoma constituted only 9.8%. This indicates that compared with other study populations, prostate cancer patients at AIC Kijabe Hospital tend to present with higher grade disease. This might be due to late diagnosis.
In this study, the proportions of the different ISUP grades groups were as follows; ISUP Grade group 1 (23.3%), Grade group 2 (16.2%), Grade group 3 (17.1%), Grade group 4 (10.5%) and Grade group 5 (32.9%). This contrasts with findings by Samaratunga & Delahunt (2015). In their study, they found ISUP Grade group1 (13.5%), Grade group 2 (49.6%), Grade group 3 (17.6%) Grade group 4 (3.7%) and Grade group 5 (15.6%), respectively. Their study showed that only 36.9% of the cases were ≥ ISUP grade 3 while this study showed that 60.5% of the cases were ≥ ISUP grade 3. These findings indicate that PCa patients in this study had higher ISUP grade prostate carcinoma compared with the western population.
Cancer staging scheme is a universal way for the malignancy care teams to illustrate the extend of malignancy spread. The commonly used cancer staging system for PCa is the American Joint Committee on Cancer (AJCC) that utilizes tumor characteristics, nodal spread, and distant Metastases (TNM) system. Prostate cancer patients undergo two types of staging; the clinical cancer stage is the clinician’s best approximation of the degree of prostate cancer disease, using the results of the digital rectal examination, laboratory tests, prostatic biopsy and any imaging studies performed. After surgery, the pathologists establish the pathologic stage based on the clinical stage and the histomorphology features of the submitted specimen. Pathological stage is superior to the clinical staging (Buyyounouski et al., 2017).
Deciding the cancer stage gives a methodical way of describing the quantity and the scope of a neoplasm at a specific point in time. In this study, 27.1% of the cases had positive surgical margins and hence were stage III prostate cancers. This compares with findings by Liang et al. (1999) who found that margin positivity rate for prostate cancer was 29%. They also found that surgical margin status was a sovereign forecaster of evolution post radical prostatectomy.
Relationship Between Different Histopathological Characteristics
The Gleason grading and scoring system is one of the best predictive parameters for prostate cancer. This study showed that there is a positive association of high-grade Gleason score (≥8) with PNI (β=-3.699; OR, 40.407; 95% CI, 11.036-147.949; P lessthan 0.0001) and tumor involving greaterthan 50% of the prostate gland (β=3.416; OR, 30.447; 95% CI, 7.220-128.397; P lessthan 0.0001). These findings are consistent with studies showing that high Gleason score, PNI and high tumor bulk are poor prognostic factors. DeLancey et al. (2013) demonstrated that PNI is independently linked with unfavorable pathologic characteristics among them, a high Gleason score. In addition, previous studies showed that PNI and Gleason score to important presurgical determinants of evolution of PCa after surgery (Sebo et al., 2002), while tumour volume correlates with the Gleason grade (Schmid & McNeal, 1992).
The higher the PCa tumor volume, the higher the Gleason score. However, it is questionable whether measurement of tumor volume provides any additional prognostic value more than that provided by the Gleason score. Pathologic stage is the ultimate indicator of tumour extend and thus it is the most accurate prognostic factor in PCa (Buyyounouski et al., 2017). This study showed a strong positive association between PCa pathological stage III and PNI (β=3.630; OR, 37.710; 95% CI, 8.762-162.288; P lessthan 0.0001), LVI (β=3.512; OR, 33.531; 95% CI, 13.757-81.729; P lessthan 0.0001); and tumor size greaterthan 50% (β=0.829; OR, 2.291; 95% CI, 1.040-50.50; P=0.040). These results are similar to previous study indicating a strong relationship between the level of tumor infiltration (PNI, LVI) and the cancer volume and the rate of cancer recurrence (Wheeler et al., 1998).
Limitations of The Study
The ethnicity of the study subjects was based purely on what the patient or care giver had indicated as the patient’s ethnicity. Cases of mixed ethnicity or mistaken ethnicity could have existed. Risk factors including sexual behavior, genital infections and family history of prostate cancer could not be elucidated since this was a retrospective study using archival prostatectomy specimens. Other diagnostic parameters including PSA level, digital rectal examination findings and prior prostate needle core biopsy findings were not factored in this cross-sectional study.
Conclusion
The mean age of the prostate cancer patients seen at AIC Kijabe was 74 years. This is 5 years older than the mean age in Ugandan and United States studies. Ethnicity of the subjects, were as follows; 68% were Bantu, 31% Nilotic and 1% of Cushitic extraction. These proportions roughly correspond to the national population of these different ethnic groups. Histopathologically, 97.6% of the prostate cancers were of acinar adenocarcinoma type while the rest were of ductal adenocarcinoma type. The tumor bulk in most of the subjects was voluminous with 80.5% of the cases have more than 5
List of Abbreviations and Acronyms
AIC - Africa Inland Church; AJCC - American Joint Committee on Cancer; DRE - Digital rectal examination; H & E - Hematoxylin and Eosin stain; IARC - International Agency for Research on Cancer; ISUP - International society of urological pathology; LVI - Lymphovascular invasion; NACOSTI - National commission for science, technology and innovation; NPH - Nodular prostatic hyperplasia; PCa - Prostate carcinoma; PNI - Perineural invasion; PSA - Prostate specific antigen; TNM - Tumor, Node, and Metastases; WHO - World Health Organization
Declarations
Ethical Approval and Consent
Ethical approval and consent for this study was sought and granted by the AIC Kijabe hospital IRB, Medical education and research division. Ethical approval was also sought and granted by NACOSTI (National commission for science technology and innovation, permit number - NACOSTI /P/18/4201/21390. (Approval available on request).
Consent for Publication
Not Applicable.
Availability of Data and Material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
This study was part of PhD thesis for the corresponding author and there were no competing interests.
Funding
The study was fully funded by the corresponding author as part of PhD thesis.
Authors' Contributions
AN analyzed and interpreted the data regarding the histopathological characteristics of prostate cancer and performed histopathological examination of prostate cancer. TM performed the statistical analysis and regression models and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
References
- Ashok Kumar, A. P., Dilip Kumar, G., David, S., Eyas, M. H., Ulysses, J. B., et al. (2007). Availability and Quality of Paraffin Blocks Identified in Pathology Archives: A Multi-Institutional Study by The Shared Pathology Informatics Network (SPIN). BMC Cancer. 7:37.
Publisher | Google Scholor - Boyle, P., Severi, G., Giles, G. G. (2003). The Epidemiology of Prostate Cancer. Urol Clin North Am. 30:209.
Publisher | Google Scholor - Buyyounouski, M. K., Choyke, P. L., Mckenney, J. K., Sartor, O., Sandler, H. M., et al. (2017). Prostate Cancer - Major Changes in The American Joint Committee on Cancer Eighth Edition Cancer Staging
Publisher | Google Scholor - Carter, B., Bova, G., Beaty, T., Steinberg, G. D., Childs, B., et al. (1993). Hereditary Prostate Cancer: Epidemiologic and Clinical Features. J Urol. 150:797-802.
Publisher | Google Scholor - Cheng, L., Jones, T. D., Lin, H., Eble, J. N., Zeng, G., et al. (2005). Lymphovascular Invasion is an Independent Prognostic Factor in Prostatic Adenocarcinoma. J Urol. 174(6):2181-2185.
Publisher | Google Scholor - Cochran, W. G. (1977). Sampling Techniques (3rd Ed.). New York: John Wiley & Sons.
Publisher | Google Scholor - D'Amico, A. V., Wu, Y., Chen, M. H., Nash, M., Renshaw, A. A., et al. (2001). Perineural Invasion as a Predictor of Biochemical Outcome Following Radical Prostatectomy for Select Men with Clinically Localized Prostate Cancer. The Journal of Urology, 165(1):126-129.
Publisher | Google Scholor - Delancey, J. O., Wood, D. P., Jr., He, C., Montgomery, J. S., Weizer, A. Z., et al. (2013). Evidence Of Perineural Invasion on Prostate Biopsy Specimen and Survival After Radical Prostatectomy. Urology. 81(2):354-357.
Publisher | Google Scholor - Dhurba, G. (2015). Hematoxylin And Eosin Staining. Histopathology.
Publisher | Google Scholor - Epstein, J. I. (2011). Prognostic Significance of Tumor Volume in Radical Prostatectomy and Needle Biopsy Specimens. J Urol. 186:790-797.
Publisher | Google Scholor - Epstein, J. I., Amin, M., Boccon-Gibod, L., Egevad, L., Humphrey, P. A., et al. (2005). Prognostic Factors and Reporting of Prostate Carcinoma in Radical Prostatectomy and Pelvic Lymphadenectomy Specimens. Scand J Urol Nephrol. 216:34-63.
Publisher | Google Scholor - Epstein, J. I., Allsbrook, W. C., Jr., Amin, M. B., Egevad, L. L. (2005). ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 29(9):1228-1242.
Publisher | Google Scholor - Epstein, J. I., Egevad, L., Amin, M. B., Delahunt, B., Srigley, J. R., et al. (2016). The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for A New Grading System. Am J Surg Pathol. 40(2):244-252.
Publisher | Google Scholor - Epstein, J. I., Zelefsky, M. J., Sjoberg, D. D, Nelson, J. B., Egevad, L., et al. (2016). A Contemporary Prostate Cancer Grading System: A Validated Alternative to The Gleason Score. Eur Urol. 69(3):428-435.
Publisher | Google Scholor - Fromont, G., Godet, J., Pires, C., Yacoub, M., Dore, B., et al. (2012). Biological Significance of Perineural Invasion (PNI) in Prostate Cancer. Prostate. 72(5):542-548.
Publisher | Google Scholor - Gleason DF. (1992). Histologic Grading of Prostate Cancer: A Perspective. Hum Pathol. 23(3):273-279.
Publisher | Google Scholor - Global Cancer Incidence, Mortality and Prevalence (Globocan) (2018). The Global Cancer Observatory. 404 Kenya Fact Sheet.
Publisher | Google Scholor - Grönberg H. (2003). Prostate Cancer Epidemiology. Lancet. 361(9360):859-864.
Publisher | Google Scholor - Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. (2008). The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 15(1):3866-3871.
Publisher | Google Scholor - Humphrey, P.A. (2014). Cancers of the Male Reproductive Organs: World Cancer Report, Stewart B W, Wild C P (Eds), World Health Organization, Lyon.
Publisher | Google Scholor - Humphrey PA. (2017). Histopathology of Prostate Cancer. Cold Spring Harb Perspect Med. 7(10):a030411.
Publisher | Google Scholor - James, N. (2014). Primer on Prostate Cancer, Springer Healthcare.
Publisher | Google Scholor - Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011). Global cancer statistics. CA Cancer J Clin. 61(2):69-90.
Publisher | Google Scholor - Srigley JR, Humphrey PA, Amin MB, Chang SS, Egevad L, et al. (2009). Members of the Cancer Committee, College of American Pathologists. Protocol For the Examination of Specimens from Patients with Carcinoma of The Prostate Gland. Arch Pathol Lab Med. 133(10):1568-1576.
Publisher | Google Scholor - Kenyan National Bureau of Statistics (2012). Kenya 2009 Population and Housing Census Highlights. Nairobi.
Publisher | Google Scholor - Lepor H, Donin NM. (2014). Gleason 6 Prostate Cancer: Serious Malignancy or Toothless Lion? Oncology (Williston Park). 28(1):16-22.
Publisher | Google Scholor - Li R, Wheeler T, Dai H, Ayala G. (2003). Neural Cell Adhesion Molecule is Upregulated in Nerves with Prostate Cancer Invasion. Hum Pathol. 34(5):457-461.
Publisher | Google Scholor - Liang, C., Michael, F. D., Erik J. B., Jeff, S., Robert, P. M. (1999). Correlation of Margin Status and Extra Prostatic Extension with Progression of Prostate Carcinoma, Cancer. 86(9):1775-1782.
Publisher | Google Scholor - Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. (2009). Perineural Invasion in Cancer: A Review of The Literature. Cancer. 115(15):3379-3391.
Publisher | Google Scholor - Chu LW, Ritchey J, Devesa SS, Quraishi SM, Zhang H, et al. (2011). Prostate Cancer Incidence Rates in Africa. Prostate Cancer. 947870.
Publisher | Google Scholor - Lowrance WT, Elkin EB, Yee DS, Feifer A, Ehdaie B, et al. (2012). Locally Advanced Prostate Cancer: A Population-Based Study of Treatment Patterns. BJU Int. 109(9):1309-1314.
Publisher | Google Scholor - May M, Kaufmann O, Hammermann F, Loy V, Siegsmund M. (2007). Prognostic Impact of Lymphovascular Invasion in Radical Prostatectomy Specimens. BJU Int. 99(3):539-544.
Publisher | Google Scholor - McNeal JE. (1981). The Zonal Anatomy of The Prostate. Prostate. 2(1):35-49.
Publisher | Google Scholor - McNeal JE. (1988). Normal Histology of The Prostate. Am J Surg Pathol. 12(8):619-633.
Publisher | Google Scholor - Mitsuzuka K, Narita S, Koie T, Kaiho Y, Tsuchiya N, et al. (2015). Lymphovascular Invasion Is Significantly Associated with Biochemical Relapse After Radical Prostatectomy Even in Patients with Pt2n0 Negative Resection Margin. Prostate Cancer Prostatic Dis. 18(1):25-30.
Publisher | Google Scholor - Muchendu, M. (2017). History of AIC Kijabe Hospital. Mt. Kenya Times.
Publisher | Google Scholor - Mutuma, G. Z., Anne, R. K. (2006). Nairobi Cancer Registry. Kenya Medical Research Institute. Nairobi, Kenya. Cancer Incidence Report 2000-2002.
Publisher | Google Scholor - Okuku F, Orem J, Holoya G, De Boer C, Thompson CL, et al. (2016). Prostate Cancer Burden at the Uganda Cancer Institute. J Glob Oncol. 2(4):181-185.
Publisher | Google Scholor - Pierorazio PM, Walsh PC, Partin AW, Epstein JI. (2013). Prognostic Gleason Grade Grouping: Data Based on The Modified Gleason Scoring System. BJU Int. 111(5):753-760.
Publisher | Google Scholor - Prostate Cancer Treatment Guide. Prostate Cancer Information from The Foundation of The Prostate Gland.
Publisher | Google Scholor - Reeves F, Hovens CM, Harewood L, Battye S, Peters JS, et al. (2015). Does Perineural Invasion in A Radical Prostatectomy Specimen Predict Biochemical Recurrence in Men with Prostate Cancer? Can Urol Assoc J. 9(5-6):e252-e255.
Publisher | Google Scholor - Samaratunga H, Delahunt B, Gianduzzo T, Coughlin G, Duffy D, et al. (2015). The Prognostic Significance of the 2014 International Society of Urological Pathology (ISUP) Grading System for Prostate Cancer. Pathology. 47(6):515-519.
Publisher | Google Scholor - Schmid HP, McNeal JE. (1992). An Abbreviated Standard Procedure for Accurate Tumor Volume Estimation in Prostate Cancer. Am J Surg Pathol. 16(2):184-191.
Publisher | Google Scholor - Sebo TJ, Cheville JC, Riehle DL, Lohse CM, Pankratz VS, et al. (2002). Perineural Invasion And MIB-1 Positivity in Addition to Gleason Score Are Significant Preoperative Predictors of Progression After Radical Retropubic Prostatectomy for Prostate Cancer. Am J Surg Pathol. 26(4):431-439.
Publisher | Google Scholor - Shariat SF, Scardino PT, Lilja H. (2008). Screening for Prostate Cancer: An Update. Can J Urol. 15(6):4363-4374.
Publisher | Google Scholor - Wheeler TM, Dillioglugil O, Kattan MW, Arakawa A, Soh S, et al. (1998). Clinical and Pathological Significance of The Level and Extent of Capsular Invasion in Clinical Stage T1-2 Prostate Cancer. Hum Pathol. 29(8):856-862.
Publisher | Google Scholor - Park YH, Kim Y, Yu H, Choi IY, Byun SS, et al. (2016). Is Lymphovascular Invasion a Powerful Predictor for Biochemical Recurrence in Pt3 N0 Prostate Cancer? Results from the K-CaP database. Sci Rep. 6:25419.
Publisher | Google Scholor - Zhang K, Bangma CH, Roobol MJ. (2017). Prostate Cancer Screening in Europe and Asia. Asian J Urol. 4(2):86-95.
Publisher | Google Scholor