Case Report
Hemolytic Uremic Syndrome as A Cause of Postpartum Acute Renal Failure
- Hadhami Aidi 1*
- Sabra Hammami 2
- Mariem Romdhani 1
- Sana Ghades 1
- Hammadi Jawaher
- Ben Ali Yasmine
- Ben Farhat Imen 1
- Knaz Samar 1
- Ridha Fatnassi 1
1Department of Obstetrics and Gynecology, IBN El Jazzar University Hospital, Kairouan, Tunisia.
2Basic Health Care Center Sidi Abdelkader, Kairouan, Tunisia.
*Corresponding Author: Hadhami Aidi, Department of Obstetrics and Gynecology, IBN El Jazzar University Hospital, Kairouan, Tunisia.
Citation: Aidi H., Hammami S., Romdhani M., Ghades S., Jawaher H., et al. (2025). Hemolytic Uremic Syndrome as A Cause of Postpartum Acute Renal Failure, Clinical Obstetrics and Gynecology Research, BioRes Scientia Publishers. 4(1):1-6. DOI: 10.59657/2992-9725.brs.25.021
Copyright: © 2025 Hadhami Aidi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 02, 2024 | Accepted: December 26, 2024 | Published: January 02, 2025
Abstract
HUS is an extremely rare complication of the postpartum period with days to months lag after an otherwise uneventful pregnancy and spontaneous delivery. Classic HUS is most often related to entero-invasive infections caused by Shiga toxin-producing Escherichia coli. Etiological diagnosis is performed by identification of the pathogen, of Shiga toxin, and of the related virulence genes in stool samples. HUS has a strong association with abnormalities in the alternative complement pathway, mutations in factors H, I, or B. Other causes include von Willebrand factor anomalies and vitamin B12 metabolism disorders. We report the case of a patient developing HUS after probable bacterial infection in the postpartum period that did not require dialysis, in whom the course was uncomplicated and rapidly resulted in recovery of normal renal function.
Keywords: hemolytic uremic syndrome; acute renal failure; adult; postpartum
Introduction
Acute renal failure in the puerperium is a serious and common complication in developing countries, bringing a high burden of maternal and fetal morbidity and mortality. Its causes include hemorrhagic delivery, mechanical hemolysis in HELLP syndrome, postpartum sepsis, or thrombotic microangiopathy, which often aggravates preexisting anemia common in these regions [1]. HUS represents a rare cause but is very serious of acute postpartum renal failure, classically arising after entero-invasive E. coli infections. Anemia, thrombocytopenia, and renal failure essentially compose HUS, with or without digestive and neurological manifestations. Adult HUS is seldom encountered as compared to its pediatric counterpart, with the majority of cases predisposed by familial tendencies, recurrence, or concurrent malignancies. HUS is very rarely encountered during pregnancy and in the postpartum period, less than 1/25,000 pregnancies [2]. It may present like other conditions complicating pregnancy, such as preeclampsia and HELLP syndrome, which may delay diagnosis and treatment. Here, we present a case study in an attempt to review the literature for clarification of the diagnostic and therapeutic approaches to this condition.
Observation
Patient W.Z. is a 30-year-old female with no relevant past medical history, was referred to our maternity for an eclamptic seizure 10 hours after delivery. Pregnancy was uneventful, with normal prenatal check-ups throughout and no recordings of high blood pressure. Delivery was at term in a peripheral maternity facility and was both spontaneous and uncomplicated. The patient developed a generalized tonic-clonic seizure at 10 hours postpartum, with tongue biting and ocular globe deviation, lasting for two minutes and resolving with intravenous diazepam administration. She was transferred to our service by EMS (emergency medical service) after receiving magnesium sulfate and Loxen (calcium channel blockers) by electric syringe pump (speed 2). Conscious, oriented, with a blood pressure of 140/90 mmHg, and urine dipstick showing +++ proteinuria on arrival; She described symptoms including bilateral amaurosis, headache, and epigastric pain. Deep tendon reflexes were brisk. Obstetrically, there were no ominous physical signs; specifically, the uterus was well-contracted, there was minimal dark bleeding, and the episiotomy scar was not bleeding.
Ophthalmological assessment showed grade II hypertensive retinopathy with serous detachment of the bilateral peripapillary area that explains amaurosis. Brain CT and abdominal-renal ultrasound proved to be normal. Initial lab work showed significant hepatic and renal dysfunction represented by AST/ALT 427/69U/L, platelets 62,000, hemoglobin 12.3 g/dL in the first day of hospitalization. The 24-hour proteinuria was 5352.4mg/24h. The diagnosis made was severe preeclampsia complicated by dissociated HELLP syndrome, and eclampsia. After a multidisciplinary management made by a team of intensive care, obstetricians, neurologists and ophthalmologists- the patient presented a remarkable clinical and biological improvement, regaining her sight, and with blood parameters that returned to normal values. (ASAT/ALAT :50/31.2 UI/ml, hemoglobin 11 g/dl and Platelets count136000). She developed high fever (41°C), confusion, dysarthria, right ptosis, on the sixth day.
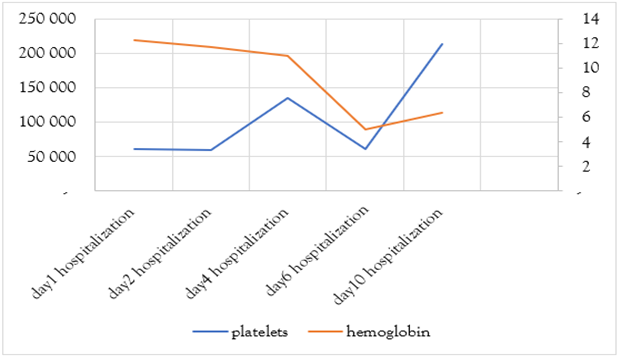
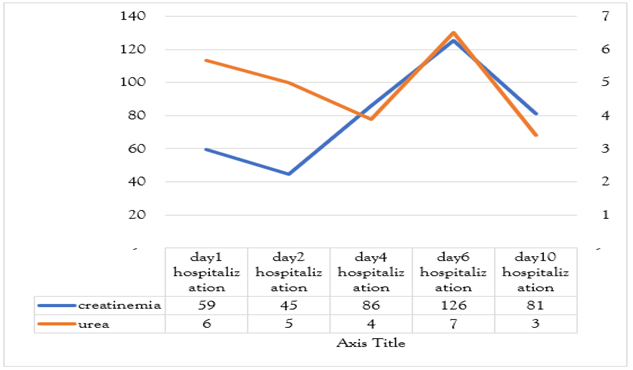
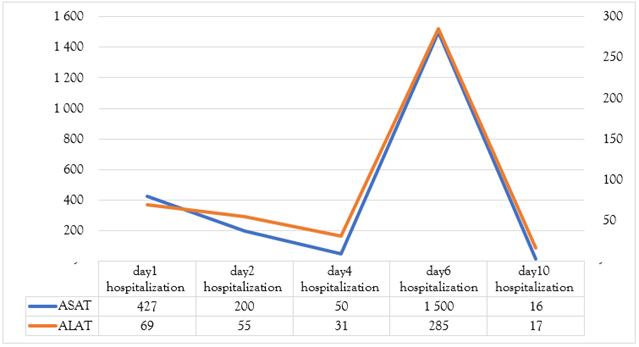
Etiological investigations did not evidence any specific infectious source, except three episodes of diarrhea in the days preceding the event. Brain imaging and lumbar puncture were normal. Further lab results showed regenerative normochromic normocytic anemia (Hb 5 g/dL), thrombocytopenia (61,400), marked hepatic cytolysis (AST 1500 U/L), LDH 8400 U/L, and CPK 196 U/L. Creatinemia was 125.6 umol/l with a clearance of 39 ml/min with preserved diuresis. Blood smear revealed schistocytes. A diagnosis of typical hemolytic uremic syndrome was made based on anemia, thrombocytopenia, and acute renal failure, likely secondary to a bacterial infection. The patient refused complement system and stool tests. Ptosis is thought to be secondary to circulating microthrombi (the substrate of this pathology). The fever subsided within 48 hours of third generation cephalosporin and metronidazole therapy, with the resolution of ptosis in a week’s time. Liver function tests came back to normal on the tenth day with AST/ALT of 16/16.9, the platelet count was 214,000 (Figure 1,2,3) and hemoglobin following the transfusion of two leukodepleted phenotype matched RBC units and four units of fresh frozen plasma was 6.4 g/dL. The patient was referred for nephrology follow-up.
Figure 1: A graph showing the evolution of hemoglobin and platelet values during hospitalization.
Figure 2: A graph showing the evolution of urea and creatinemia values during hospitalization.
Figure 3: A graph showing the evolution of liver transaminase values during hospitalization.
Discussion
Characterized by abrupt onset of acute renal failure, hemolytic anemia, and thrombocytopenia, HUS has variable clinical manifestations depending on the age of the patient and the circumstances surrounding the onset: enterohemorrhagic colitis, coma, cognitive slowing, seizures. Pregnancy-associated thrombotic microangiopathy is uncommon, occurring in approximately 1 in 25,000 pregnancies [2].
Histologically, three types of lesions most commonly associated with HUS include:
Cortical Necrosis: It presents with diffusely dense cortical necrosis. The obstruction involves interlobular arteries with sparing of the arcuate and interlobar arteries. This lesion is seen in 30% of typical and 14% of atypical HUS cases [3].
Glomerular Thrombotic Microangiopathy: It is characterized by swelling and detachment of endothelial cells, with the creation of a clear subendothelial space; thus, glomerular capillary walls often show a double-contour appearance. Mesangial cells become hypertrophic, with fibrillar matrix deposits obstructing capillaries. Capillaries frequently contain erythrocytes, platelets, or fibrin thrombi. This pattern is typical for classic HUS in 57% of cases and in 28% of atypical forms [3].
Arterial Predominant: It is characterized by the involvement of mainly medium-caliber arteries, with particular predilection for the interlobular arteries, showing in the initial phase’s intimal proliferation, fibrin thrombi, and necrosis of media. These changes progress towards a fibrous endarteritis with an “onion-skin” appearance. This lesion type represents 57% of atypical pediatric HUS cases and results from ischemic processes. Tubulointerstitial lesions are nonspecific and reflect only the severity and duration of disease.
In immunofluorescence, anti-fibrin serum binds to certain glomeruli, in the mesangium and on the walls of glomerular capillaries. It can also bind to thrombi in capillary lumens and arterioles. The long-term prognosis for atypical HUS (aHUS) in pregnancy remains particularly challenging, especially in women with complement gene variants. Atypical HUS during pregnancy accounts for 16-20% of all aHUS cases in women of reproductive age [4].
Among these, 50-71% of cases exhibit an identifiable complement pathway mutation, leading to poor renal outcomes; 41-66% of them require acute dialysis [5]. Long-term outcomes are worse among the patients with complement specific genetic mutations or polymorphisms that predispose to chronic kidney disease and risk of relapse. In one study, 64% of women with complement gene variants progressed to end-stage renal disease, compared to 36% in those without variants. Additionally, the risk of relapse was significantly higher, with 38% of women with complement gene variants experiencing relapse, compared to only 16% of those without [4]. In the postpartum period, inflammation due to childbirth, hemorrhage, and passage fetal cells into maternal circulation may activate the alternative pathway of complement. In the absence of effective regulatory mechanisms, this activation may trigger Hemolytic Uremic Syndrome (HUS). Pregnancy and postpartum periods can also unmask a latent dysfunction within the complement system, predisposing certain individuals to HUS [5].
Effects of HUS on Pregnancy
Thrombotic microangiopathy can present as acute TTP, HUS, or HELLP syndrome during pregnancy. The differentiation of all these conditions enables distinct regimens of treatment. Measuring antithrombin III level may help differentiate preeclampsia from HUS or TTP before 28 or after 34 weeks of gestation. HUS most frequently arises in the third trimester or during the postpartum period and is often associated with intrauterine growth restriction (IUGR), symptoms similar to those of preeclampsia, or HELLP syndrome. In Such cases, delivery by cesarean section is required, though it can increase the risk for preterm birth [6]. HUS is associated with an increased risk of early or late spontaneous miscarriage, fetal death in utero and IUGR. Maternal prognosis is also seriously compromised without treatment. Upon HUS suspicion especially in the absence of fetal distress, plasma infusions or plasma exchange therapy is the mainstay of treatment. Treatment is generally continued daily until remission. However, in cases where plasma therapy is only partially effective, dialysis may become necessary. Management is multidisciplinary, involving hematology, nephrology, and obstetrics. Familial cases warrant genetic counseling, although no prenatal marker is currently available [6]. Postpartum HUS appears between 48 hours and 10 weeks after delivery. The clinical course is usually fulminant. Treatment is symptomatic but crucial. It includes dialysis, transfusions and careful fluid intake. Antiplatelet agents, heparin and antithrombotic are contraindicated because of the risk of bleeding. Renal failure may become chronic [6].
The Effects of Pregnancy on the Disease
Generally, pregnancy increases the risk of HUS recurrence, whose likelihood varies by type. Typical HUS due to Shiga toxin seldom recurs, while medication-induced HUS is precipitated by exposure to the offending drug rather than pregnancy. Recurrence in congenital HUS in pregnancy is almost a given and requires prophylactic plasma exchange. Diopathic HUS is more likely to recur during a future pregnancy, provided the first episode is not a contraindication to pregnancy [6].
Clinical and Biological Features
The incidence of post-infectious HUS is particularly high in children but also affects adults, including the elderly, prompting the need for routine testing for Shiga toxin in stool samples from all patients with HUS [7]. In France, approximately 100 cases of post-infectious HUS are reported each year, with an incidence of two to three cases per 100,000 children under five [8]. Cases tend to cluster in rural areas and be more prevalent during summer months, and though often sporadic, outbreaks can occur. Transmission is primarily foodborne, with common sources including water, unpasteurized milk or contaminated meat, but also sometimes via fruit or vegetables. The interval between ingestion of contaminated food and onset of diarrhea varies from 2 to 12 days. Typically, E. coli O157:H7 infections induce non-bloody diarrhea for one to three days, followed by bloody diarrhea. Colonic involvement can be relatively severe. However, minor infections also occur, and E. coli O157:H7 can be found in patients without diarrhea. Most patients are not febrile during diarrhea. In the state phase of the disease, the main clinical signs are related to ARF and TAM syndrome.
they include asthenia, fever, abdominal pain, purpura and neurological disorders. More rarely, icterus, myalgias and arthralgias are noted. Central neurological disorders range from simple headache with confusion to motor deficits, aphasia, visual disturbances, seizures and coma. Myocardial damage is also possible, and should be systematically detected. In the lungs, acute respiratory distress syndrome (ARDS) has also been described. These serious visceral disorders are more frequent in adults, as reported in an American registry [9]. Renal involvement is prominent, characterized by microscopic hematuria, more rarely macroscopic, and proteinuria. AKI, often oligo-anuric, is usually associated with severe arterial hypertension (AH), and complicates more than 50% of cases of HUS [10]. Fundoscopic examination may reveal papilledema, retinal and vitreous hemorrhages.
Microangiopathy-type hemolytic anemia is characteristic: regenerative anemia, with the presence of schizocytes. Reticulocytosis is increased, as are serum lactic dehydrogenase (LDH), hemoglobinemia, hemoglobinuria and unconjugated bilirubin. Serum haptoglobin is decreased. The Coombs test is negative. Thrombocytopenia is constant, rarely very deep, unlike thrombotic thrombocytopenic purpura (TTP), with no evidence of disseminated intravascular coagulation. Quick and activated partial thromboplastin times are normal. Levels of fibrinogen and other coagulation factors are also normal in over 90% of cases. However, there is a discrete increase in fibrin degradation products in blood and urine, indicating minimal activation of fibrinolysis. Bacteriological testing is required for E. coli O157:H7, which can be detected by culturing feces on sorbitol-containing agar (Mac Conquey agar). In the absence of diarrhea, rectal swabbing is useful. The stool should also be tested for Shiga toxins by polymerase chain reaction (PCR) (11).
A reduction in serum complements proteins (CH50, C3, C4) has been documented in some cases, and complement pathway components such as factors H and I, along with MCP protein, should be assessed before any plasma transfusion. ADAMTS-13 protease activity, responsible for von Willebrand factor cleavage, should be measured to differentiate HUS from TTP, as ADAMTS-13 activity is typically normal or moderately reduced in HUS but severely reduced in acquired autoimmune TTP [12].
Treatment
The development of ARF in patients with HUS calls for very precise, regular monitoring of hydration status to avoid hydro sodium overload and episodes of hypertension and pulmonary edema. Dialysis is required in over 60% of cases. In cases of fluid overload, high-dose diuretics such as intravenous furosemide (125-500 mg) may be used to increase urine output; Subtractive dialysis or isolated ultrafiltration are generally more effective. Hypertension should be treated with vasodilators, in particular ACE inhibitors and/or angiotensin II receptor antagonists, with or without calcium channel blockers or beta-blockers [13]. Similarly, it is not recommended to administer digestive motility inhibitors or opiate derivatives, as these also appear to increase the risk of HUS or neurological complications. Isolation can be lifted as soon as the stool culture is negative, which is often within a few days. Patients who develop HUS can quickly become severely anemic, and transfusions may be necessary. Transfusions must be cautious, however, as they can promote hypertension. It is preferable to avoid platelet transfusions, unless there is clinically significant bleeding or an invasive procedure is planned. Indeed, platelet infusions seem to aggravate micro thrombosis. ARF often regresses completely after HUS, but depending on the severity of the initial episode and the need for dialysis, sequelae remain possible in 10-20% of cases [14].
In pediatric population, plasma exchange is reserved for severe HUS forms. In adults, distinguishing between TTP and HUS mechanisms can be challenging making it impossible to predict whether ADAMTS13 deficiency or another mechanism is involved. It is therefore recommended to initiate plasma infusions or plasma exchange as soon as possible, and to monitor the clinical course [15]. The optimal duration of treatment is not yet known. Daily plasma exchange is usually continued until stable platelet normalization is achieved. LDH levels, which reflect both tissue ischemia and hemolysis, are also a good marker of response to treatment [16].
Treatment Advances
Corticosteroid therapy and immunosuppressants are not justified in post-infectious HUS. In contrast, Eculizumab, a humanized anti-C5 antibody that blocks C5 and prevents the formation of the C5b-9 membrane attack complex, appears to be effective in post-infectious HUS. Eculizumab has been shown to be remarkably effective in atypical HUS, allowing correction of thrombocytopenia within days or weeks, cessation of hemolysis and, in a large number of cases, recovery of renal function [17]. During the E. coli O104:H4 HUS epidemic in Germany in spring 2011, eculizumab was used successfully, with rapid improvement in hematological and renal signs and a considerable reduction in mortality [10]. Although typical HUS is not yet an official indication for eculizumab, the use of this new treatment should be discussed rapidly in severe forms, or in the absence of rapid symptomatic improvement among children or adults.
Conclusion
Typical HUS following Shiga toxin-producing E. coli is an acknowledged public health concern, diagnosed by the identification of either the pathogen or its Shiga toxin genes. Abnormalities of the complement pathway should be looked for in the atypical forms. The progress in understanding the mechanism of aHUS has paved the way for totally innovative therapies, which we can now expect to prevent progression to end-stage renal failure and enable aHUS patients to be successfully transplanted.
Declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors are sincerely grateful to the patients for giving the permission to share this informative report.
Data Availability
No data is associated with this article.
References
- Abdelkader, F., Saleh, A. M. (2020). Acute Postpartum Renal Failure: About 102 Cases at the National Hospital Center of Nouakchott, Mauritania. PAMJ-Clinical Medicine, 4(48).
Publisher | Google Scholor - Machado S, Figueiredo N, Borges A, São José Pais M, Freitas L, et al. (2012). Acute Kidney Injury in Pregnancy: A Clinical Challenge. J Nephrol. 25(1):19-30.
Publisher | Google Scholor - Loirat, C., Baudouin, V., Sonsino, E., Mariani-Kurdjian, P., Elion, J. (1992). Hemolytic Uremic Syndrome in Children: Clinical and Etiological Aspects, Prognostic Elements and Therapeutic Results. Nephrology News Jean Hamburger, 133-158.
Publisher | Google Scholor - Bruel, A., Kavanagh, D., Noris, M., Delmas, Y., Wong, E. K., et al. (2017). Hemolytic Uremic Syndrome in Pregnancy and Postpartum. Clinical Journal of the American Society of Nephrology, 12(8):1237-1247.
Publisher | Google Scholor - Huerta, A., Arjona, E., Portoles, J., Lopez-Sanchez, P., Rabasco, C., et al. (2018). A Retrospective Study of Pregnancy-Associated Atypical Hemolytic Uremic Syndrome. Kidney International, 93(2):450-459.
Publisher | Google Scholor - Berrebi, A. (2008). Rare Diseases and Pregnancy from A To Z. Flammarion Medicine-Sciences.
Publisher | Google Scholor - Dundas, S., Todd, W. A., Stewart, A. I., Murdoch, P. S., Chaudhuri, A. K. R., et al. (2001). The Central Scotland Escherichia Coli O157: H7 Outbreak: Risk Factors for The Hemolytic Uremic Syndrome and Death Among Hospitalized Patients. Clinical Infectious Diseases, 33(7):923-931.
Publisher | Google Scholor - Decludt, B., Bouvet, P., Mariani-Kurkdjian, P., Grimont, F., Grimont, P. A. D., et al. (2000). Haemolytic Uraemic Syndrome and Shiga Toxin-Producing Escherichia Coli Infection in Children in France. Epidemiology & Infection, 124(2):215-220.
Publisher | Google Scholor - Karpac, C. A., Li, X., Terrell, D. R., Kremer Hovinga, J. A., Lammle, B., et al. (2008). Sporadic Bloody Diarrhoea-Associated Thrombotic Thrombocytopenic Purpura-Haemolytic Uraemic Syndrome: An Adult and Paediatric Comparison. British Journal of Haematology, 141(5):696-707.
Publisher | Google Scholor - Noris, M., Remuzzi, G. (2005). Hemolytic Uremic Syndrome. Journal of the American Society of Nephrology, 16(4):1035-1050.
Publisher | Google Scholor - Servais, A., Devillard, N., Frémeaux-Bacchi, V., Hummel, A., Salomon, L., et al. (2016). Atypical Haemolytic Uraemic Syndrome and Pregnancy: Outcome with Ongoing Eculizumab. Nephrology Dialysis Transplantation, 31(12):2122-2130.
Publisher | Google Scholor - Demir, E., Yazici, H., Ozluk, Y., Kilicaslan, I., Turkmen, A. (2016). Pregnant Woman with Atypical Hemolytic Uremic Syndrome Delivered a Healthy Newborn Under Eculizumab Treatment. Case Reports in Nephrology and Dialysis, 6(3):143-148.
Publisher | Google Scholor - Wong, C. S., Jelacic, S., Habeeb, R. L., Watkins, S. L., Tarr, P. I. (2000). The Risk of The Hemolytic-Uremic Syndrome After Antibiotic Treatment of Escherichia Coli O157: H7 Infections. New England Journal of Medicine, 342(26):1930-1936.
Publisher | Google Scholor - Garg, A. X., Suri, R. S., Barrowman, N., Rehman, F., Matsell, D., et al. (2003). Long-Term Renal Prognosis of Diarrhea-Associated Hemolytic Uremic Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. Jama, 290(10):1360-1370.
Publisher | Google Scholor - Rock, G. A., Shumak, K. H., Buskard, N. A., Blanchette, V. S., Kelton, J. G., et al. (1991). Comparison of Plasma Exchange with Plasma Infusion in The Treatment of Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine, 325(6):393-397.
Publisher | Google Scholor - Colic, E., Dieperink, H., Titlestad, K., Tepel, M. (2011). Management of an Acute Outbreak of Diarrhoea-Associated Haemolytic Uraemic Syndrome with Early Plasma Exchange in Adults from Southern Denmark: An Observational Study. The Lancet, 378(9796):1089-1093.
Publisher | Google Scholor - Greinacher, A., Friesecke, S., Abel, P., Dressel, A., Stracke, S., et al. (2011). Treatment of Severe Neurological Deficits with IgG Depletion Through Immunoadsorption in Patients with Escherichia Coli O104: H4-Associated Haemolytic Uraemic Syndrome: A Prospective Trial. The Lancet, 378(9797):1166-1173.
Publisher | Google Scholor