Case Report
Fever and Skin Lesions: Does It Have to Do with the Heart?
- González-Estriégana Soraya *
- Del Río-Lechuga Ana, Oneto-Otero Jesús
Department of Cardiology, Jerez de la Frontera University Hospital, Cádiz, Spain.
*Corresponding Author: González-Estriégana Soraya, Department of Cardiology, Jerez de la Frontera University Hospital, Cádiz, Spain.
Citation: Soraya G, Del R. Ana, Jesús O. (2024). Fever and Skin Lesions: Does It Have to Do with the Heart?, Journal of Clinical Medicine and Practice, BioRes Scientia Publishers. 1(2);1-3. DOI: 10.59657/3065-5668.24.002
Copyright: © 2024 González-Estriégana Soraya, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 27, 2024 | Accepted: March 13, 2024 | Published: July 30, 2024
Abstract
We present the clinical case of a 73-year-old patient who after performing cardiac catheterization, begins with erythematous maculopapular skin lesions associated with high fever. The key to the final diagnosis, like most cases involving several possible differential diagnoses, lies in a complete history and careful physical examination.
Keywords: fever; rash; clopidogrel; erythema multiforme
Introduction
When presented with fever and skin lesions, the differential diagnoses can vary widely. While we possess an extensive array of complementary tests to explore potential underlying causes, it's imperative not to overlook the significance of patient history and physical examination, which are fundamental pillars of daily clinical practice.
Clinical history
73-year-old woman with personal history of no known drug allergies or toxic habits. Diagnosed with diabetes mellitus in 2001 and dyslipidemia, stroke in 2013 with no sequelae and effort angina, also cholecystectomized.
Regular treatment: Simvastatin 20 mg every 24 hours orally, Citalopram 20 mg every 24 hours orally, ferrous sulfate 80 mg every 24 hours orally, sublingual nitroglycerin as needed, Omeprazole 20 mg every 24 hours orally, isosorbide mononitrate 60 mg every 24 hours orally, Bisoprolol 5 mg every 24 hours orally, Bromazepam 1.5 mg every 24 hours orally, aspirin 100 mg every 24 hours orally, Metformin/sitagliptin 850/50 mg every 24 hours orally, Insulin glargine 20 international units every 24 hours subcutaneously, clopidogrel 75 mg every 24 hours orally.
Current illness: admitted to Puerta del Mar University Hospital (Cádiz) for scheduled cardiac catheterization, revealing two-vessel disease with the placement of two drug-eluting stents in the right coronary artery and another in the circumflex artery. Four days later, erythematous submammary skin lesions began to appear. After three days she developed fever of up to 39.5°C. She presented to the emergency department of the University Hospital of Jerez de la Frontera due to worsening symptoms, with more skin lesions appearing, some affecting the facial region.
Physical examination
Blood pressure: 109/46 mmHg; Heart rate: 72 beats per minute; Temperature: 36.5°C.
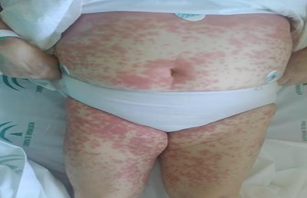
Acceptable general condition, conscious, oriented, and cooperative. Well-hydrated and perfused. Eupneic at rest, no signs of neurological focalization, normal coloration, with skin lesions mainly on the trunk and limbs, consisting of pruritic, erythematous, and coalescent maculopapules, forming plaques submammarily. The lesions have a more purplish center and a clearer annular border (target-like). Blanching under pressure. Scaling in the surrounding skin. Intraoral lesions but none on palms and soles. No palpable lymphadenopathy.
Cardiopulmonary auscultation: rhythmic without murmurs. Preserved vesicular murmur without added noises.
Abdomen: soft, depressible, non-tender to palpation. No masses or hepatomegaly palpable. No signs of peritonism. Peristalsis preserved.
Lower limbs: no edema or signs of deep venous thrombosis. Preserved and symmetrical pedal pulses.
Figure 1: Dermatological lesions on the trunk and limbs: maculopapules, erythematous, and coalescent, forming plaques at the submammary level.
Complementary tests
Emergency laboratory results
General biochemistry: Glucose 282 mg/dL (80-110 mg/dL), Creatinine 0.95mg/dL (0.57-1.11 mg/dL), CK 291 U/L (29-168 U/L), Na 133 mEq/L (136-145mEq/L), K 4.5 mEq/L (3.5-5.1 mEq/L), Troponin I (high sensitivity) 51.30 ng/L (2.00 - 15.60 ng/L), CRP 87.1 mg/L (0.0-5.0 mg/L).
Venous blood gas analysis: pH 7.42 (7.32 - 7.43), PCO2 43.0 mmHg (41.0 - 54.0mmHg), PO2 23.0 mmHg (35.0 - 44.0 mmHg), Bicarbonate 27.9 mmol/L (24.0 -mmol/L).
Urinalysis: normal except leukocytes 500/µL (0-25/µL) and erythrocytes 60/µL (0- 15/µL).
Red blood cell count: Hb 11.1 g/dL (11.8 - 15.8 g/dL), MCV 74.3 fL (80.0 - 101.0fL), MCH 22.8pg (27.0-34.0 pg).
White blood cell count: Leukocytes 23.47x10^3/µL (3.60-10.50x10^3/µL), Neutrophils 95.70% (42.00-77.00%), Lymphocytes 2.50% (20.00-44.00%).
Platelets 273 x 10^3/µl (130 – 400 x 10^3/µl).
Urinalysis: normal except leukocytes 500/µL (0-25/µL) and erythrocytes 60/µL (0- 15/µL).
Standard laboratory results (noteworthy): LDH 256U/L (125-220U/L), TG 207mg/dL (4-150mg/dL), LPa 54 mg/dL (0-30mg/dL), Vit D 18.4ng/mL (30-80ng/mL), normal proteinogram, B12 161 pg/mL (187-883pg/mL), Fe 26ng/mL (50-170ng/mL), IST 7.4% (20-50%), TSH 0.22µUI/mL (0.35-4.94µUI/mL), T3 1.54pg/mL (1.71-3.71pg/mL).
Autoimmunity: Negative antineutrophil cytoplasmic antibodies (ANCA), Negative antinuclear antibodies (ANA), Negative anti-mitochondrial antibodies (IgG), Negative anti-cardiolipin antibodies 5.8GPL/mL (0-20), Positive anti- cardiolipin antibodies 1.2MPL/mL (0.0 - 20.0), Negative anti-beta-2-glycoprotein I antibodies <6>Urine culture: negative
Blood culture x 2: negative
Stool occult blood test: negative
Serologies: Negative HIV, Negative HBV, Negative HCV, Negative EBV, Negative CMV, Negative Rickettsia, Negative Borrelia, Negative syphilis.
Chest X-ray: Normal cardiac silhouette. No parenchymal consolidations. No costophrenic angle blunting.
Electrocardiogram: Sinus rhythm at 75 bpm, normal axis. Narrow QRS. PR interval 200 ms. No acute repolarization abnormalities.
Follow-up
Given the clinical and laboratory findings, a protocol including serologies, proteinogram, autoimmunity, ESR, chest X-ray, blood cultures x2, anemia study, and fecal occult blood test is requested.
Intravenous corticosteroid therapy, oral antihistamine treatment, and empirical intravenous antibiotic therapy are initiated. Additionally, clopidogrel 75 mg once daily is replaced with Ticagrelor 90 mg every 12 hours.
With these measures, there is a remission of the skin lesions and fever, along with a clear clinical improvement in the patient, allowing discharge to home in a hemodynamically stable condition, with follow-up for hypochromic microcytic anemia in the medical day hospital.
Diagnosis
Toxicoderma: Clopidogrel-induced erythema multiforme (EM).
Discussion and conclusions
One of the differential diagnoses considered was a secondary cutaneous rash due to infection (elevated acute-phase reactants in the bloodwork: PCR, leukocytosis with neutrophilia, and abnormal urine sediment, consistent with urinary tract infection, in addition to the history of an interventional procedure: cardiac catheterization).
Serological testing helped rule out multiple infectious etiologies (such as brucellosis, rickettsiosis...) and vasculitis associated with these infections (cryoglobulinemic vasculitis associated with hepatitis C virus, vasculitis associated with hepatitis B virus).
Additionally, the chest X-ray was normal, and blood and urine cultures taken prior to antibiotic treatment initiation were negative.
The negativity of ANAs reduced the likelihood of autoimmune conditions such as rheumatoid arthritis and lupus erythematosus [1].
It is noteworthy that the skin can be affected in the majority of systemic vasculitides, especially those involving medium and small vessels.
Regarding medium vessel vasculitides, polyarteritis nodosa and Kawasaki disease seemed unlikely given the exclusive cutaneous involvement.
Similarly, the clinically incongruent presentation and negative ANCA reduced the possibility of this subset of small vessel vasculitides according to the Chapel Hill classification (microscopic polyangiitis, granulomatosis with polyangiitis, and eosinophilic granulomatosis with polyangiitis).
While it is true that for the group of immune complex vasculitides, the study of complement proteins and cryoglobulins would have been useful, the rest of the analytical parameters and clinical presentation made these entities less likely [2]. The last of the differential diagnoses considered was toxicoderma (due to the recent initiation of clopidogrel), for which it was replaced with another antiplatelet drug. In this case, a skin biopsy was not performed (which would have allowed for samples to be sent for microbiology and histopathology, as well as molecular biology techniques) given the invasive nature of the test and the fact that we were dealing with a situation of clinical stability, improvement with empirical treatment, and the patient's immunocompetence. Based on the results of the tests performed, clinical evolution, and the morphology of the skin lesions, the most likely diagnosis was clopidogrel-induced erythema multiforme (EM). Erythema multiforme (EM) is an acute eruptive syndrome defined by the typical morphology of the lesions: target-like, with three concentric zones including a central necrotic disk, an edematous intermediate ring, and an erythematous outer ring, which can affect mucous membranes in major forms. Erythema multiforme is caused by an immune-mediated reaction. The most common cause is infectious, especially HSV-1... The second most frequent cause is drugs (allopurinol, antiepileptics, antibiotics, aspirin, statins…) [3]. In the clopidogrel technical data sheet, erythema multiforme is described as a very rare adverse effect (frequency less than 1/10,000) [4]. Fever is present in 30% of minor forms, and skin lesions heal within a period of 1-3 weeks with only symptomatic treatment. If drug-induced EM is suspected, the suspected drug should be discontinued [5].
Highlights
Fever associated with skin lesions is a common clinical presentation of multiple pathologies, which can range from trivial to potentially serious. In order to initiate appropriate treatment as early as possible, it is essential to establish a correct differential diagnosis.
References
- Jeniffer S, Jonathan NG. (2008). Pregnancy and low back pain. Curr Rev Musculoskeletal Med, 1:137-141.
Publisher | Google Scholor - Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell-Andrews AA, Kain ZN. (2004). Low back pain during pregnancy: prevalence, risk factors, and outcomes. Obstet Gynecol, 104:65-70.
Publisher | Google Scholor - Mens JM, Vleeming A, Stoeckart R, Stam HJ, Snijders CJ. (1996). Understanding peripartum pelvic pain. Implications of a patient survey, 21:1363-1369.
Publisher | Google Scholor - Van De Pol G, Van Brummen HJ, Bruinse HW, Heintz AP, Van Der Vaart CH. (2007). Pregnancy-related pelvic girdle pain in the Netherlands. Acta Obstet Gynecol Scand, 86:416-422.
Publisher | Google Scholor - Pierce H, et al. (2012). Pregnancy-related lumbopelvic pain: listening to Australian women. Nursing Research and Practice, 387428:1-10.
Publisher | Google Scholor - Bergström C, Persson M, Mogren I. (2014). Pregnancyrelated low back pain and pelvic girdle pain approximately 14 months after pregnancy–pain status, self-rated health and family situation. BMC Pregnancy Childbirth, 14(1):48.
Publisher | Google Scholor - Persson M, Winkvist A, Dahlgren L, Mogren I. (2013).
Publisher | Google Scholor - Schröder G, Kundt G, Otte M, Wendig D, Schober HC. (2016). Impact of pregnancy on back pain and body posture in women. J Phys Ther Sci, 28 (4):1199-1207.
Publisher | Google Scholor - Branco MR. Santos-Rocha, Vieira F. (2014). Biomechanics of gait during pregnancy. The Scientific World Journal, 2014:1-5.
Publisher | Google Scholor - Fitzgerald CM. (2013). Pregnancy-related lumbopelvic pain: what have we learned? Am J Obstet Gynecol , 208(4):242.
Publisher | Google Scholor - Elden H, Lundgren i, Robertson e. (2013). Life’s pregnant pause of pain: pregnant women’s experiences of pelvic girdle pain related to daily life: a Swedish interview study. Sexual Reproductive Healthcare, 4(1):29-34.
Publisher | Google Scholor - Close C, Sinclair M, Liddle D, Mc Cullough J, Hughes C. (2016) Women's experience of low back and/ or pelvic pain (LBPP) during pregnancy. Midwifery, 37:1-8.
Publisher | Google Scholor - Verstraete EH, Vanderstraeten G, Parewijck W. (2013). Pelvic Girdle Pain during or after Pregnancy: a review of recent evidence and a clinical care path proposal. Facts Views Vis ObGyn, 5(1):33.
Publisher | Google Scholor - Mahishale AV, Borkar SSS. (2015). Prevalence of Patterns of Pregnancy induced Pelvic Girdle Pain and Low Back Pain in a Tertiary Care Centre-a Cross Sectional Study. IJTRR, 4(4):122-124.
Publisher | Google Scholor - Ansari NN, Hasson S, Naghdi S, Keyhani S, Jalaie S. (2010). Low back pain during pregnancy in Iranian women: Prevalence and risk factors. Spine Journal, 26(1):40-48.
Publisher | Google Scholor - Meyer LC, Peacock JL, Bland JM, Anderson HR. (1994). Symptoms and health problems in pregnancy: their association with social factors, smoking, alcohol, caffeine and attitude to pregnancy. Pediatric and Prenatal Epidemiology, 8:145-155.
Publisher | Google Scholor - Ostgaard HC, Andersson GB, Karlsson K. (1991). Prevalence of back pain in pregnancy. Pubmed, 16(5):549-555.
Publisher | Google Scholor - Sabino J, Grauer JN. (2008). Pregnancy and low back pain. Current Review of Musculoskeletal Medicine, 1:137-140.
Publisher | Google Scholor - Mogren IM, Pohjanen AI. (2002). Low back pain and pelvic pain during pregnancy: prevalence and risk factors. Obstetrics and Gynecology.
Publisher | Google Scholor - Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell AA, Kain ZN. (2004). Low back pain during pregnancy: prevalence, risk factors, and outcomes. Obsterical Gynecology, 104(1):65-68.
Publisher | Google Scholor - Mantle MJ, Greenwood R, Currey HLF. (1997). Prevelence of backache in pregnancy. Rheumatologic Rehabilitation, 16:95-99.
Publisher | Google Scholor - Stapleton DB, MacLennan AH, Kristiansson P. (2002). The Prevalence of Recalled Low Back Pain During and After Pregnancy: A South Australian Population Survey. Obsterical Gynaecology, 42(5):482-487
Publisher | Google Scholor - Joseph JF, Cragin L. (1998). Biomedical and Feminist perspectives on low back pain in pregnancy. Nursing clinics of North America, 33(4):713-4.
Publisher | Google Scholor - Ostgaard HC, Anderson GB, Wennergren M. (1991). The impact of low back and pelvic pain in Pregnancy on the pregnancy outcome. Acta Obstetricia et Gynecologica Scandinavica, 70(1):21-4.
Publisher | Google Scholor - Ingrid M, Anna M, Pohjanen I. (2007). Low back pain and pelvic pain during pregnancy: prevalence and risk factors. Pain, 30(8):983-991.
Publisher | Google Scholor - Hills EC. (2010). Mechanical Low Back Pain. Medicine, 2(4):56-58.
Publisher | Google Scholor