Research Article
Epidural Labor Analgesia: A Comparison of 0.25% Bupivacaine Versus 0.25% Bupivacaine Plus Tramadol
1Department of Respiratory Therapy, University of Lahore, Pakistan
2Department of Biochemistry & Molecular Biology, Abdul Wail Khan University Pakistan.
*Corresponding Author: Sadiq Ullah, Department of Biochemistry & Molecular Biology, Abdul Wail Khan University Pakistan.
Citation: Dar SH, Ullah S. (2024). Epidural Labor Analgesia: A Comparison of 0.25% Bupivacaine Versus 0.25% Bupivacaine Plus Tramadol, Journal of Clinical Research and Clinical Trials, BioRes Scientia Publishers. 3(3):1-11. DOI: 10.59657/2837-7184.brs.24.037
Copyright: © 2024 Sadiq Ullah, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 06, 2024 | Accepted: September 23, 2024 | Published: September 30, 2024
Abstract
Introduction: The objective of this study was to compare the hemodynamic changes, analgesic efficacy and side effects of Tramadol in combination with Bupivacaine used in patients receiving epidural analgesia for labor pain.
Study Design: A double blind, randomized, comparative trial.
Setting: Department of Anesthesiology, Critical Care and Pain Management, Divisional Headquarters Teaching Hospital Mirpur.
Duration: Feb 2020 to October 2020.
Materials and Methods: Fifty patients were recruited who were scheduled to receive epidural analgesia for labor pain. The patients were randomized into two groups (A and B). In patients of group A (control group) 0.25% Bupivacaine was used to relieve labor pain and when indicated, while in group B (study group), patients received 0.25% Bupivacaine plus 1 mg/kg Tramadol at cervical dilatation of 4 cm. Top up doses were given on as needed basis in both the groups. Analgesic efficacy was assessed by Visual Analogue Scale (VAS) and other vital parameters (Blood Pressure, Heart Rate and Respiratory Rate) before the administration of the drug and at different time intervals of 0, 5 min, 10 min, 15 min, 30 min, 45 min, 60 min, 120 min and 240 min. Neonatal out comes were assessed by the use of APGAR scores and the side effects of the drugs in two groups were also evaluated.
Results: Pain intensity as measured using pain scores was lower in women receiving 0.25% Bupivacaine plus tramadol when compared to women who received 0.25% Bupivacaine in Epidural. In hemodynamic parameters, there were no significant differences in both groups. In both groups there was no significant effect on duration of second stage of labor and it wasn’t prolonged in any of the patients. Epidural anesthesia with bupivacaine and tramadol provided better pain relief and reduced the total dose of bupivacaine in majority of the patients with no major adverse effects on mother and fetus. However, incidence of nausea and vomiting was significantly high in tramadol group.
Conclusions: Epidural Labor analgesia can be achieved safely with tramadol in combination bupivacaine without any adverse effects on mother and baby.
Keywords: epidural; analgesia; bupivacaine; tramadol; painless labor
Introduction
Obstetric analgesia studies have often focused on the immediate effects on labor, mother pain, and pain management comfort; maternal satisfaction is generally tested during labor or within 48 hours following birth. We found no studies that investigated the long-term effects of obstetric pain treatment on women's history of labor discomfort and overall satisfaction with delivery [1]. Pain can lead to weariness and emotional disruptions, drastically affecting the mother-baby bond during the early days. 100 pregnant women who achieved the inclusion criteria were enrolled in this study. The majority of the ladies (84%) were between the ages of 20 and 30 years. The average age of the patients was 26.21 years. All patients remained at term (37-41 weeks of gestation), with a mean age at delivery of 38.49 weeks. The study groups consisted of 51% primigravida and 49% multigravida [2]. Pain during childbirth varies greatly in both intensity and location. A similar pro-portion of women experience relatively little pain, compared to 15% who experience quite severe pain. While some women complain mostly of back discomfort, others experience pain primarily in the abdomen. Continuous low-back pain is the most prevalent and intense form of labor pain in many women [3] In our initial study, we were surprised by the significant level of pain experienced by most women during birth. Figure 2 depicts the average pain scores for trained and untrained first-time mothers and multiparas. The median score for labor pain is only surpassed by those for causalgia in long-term pain patients and excision of a digit in acute pain patients [4]. Pain is seen as a significant aspect of the labor situation the coefficients for the sensory and emotional subscales ranged from 0.79 to 0.84 and 0.64 to 0.70, respectively. Alpha coefficients for the overall scale were high and steady, ranging from 0.84 to 0.86 across the stages of labor [5]. The pudendal nerve, which originates from the sacral roots S2, S3, and S4, transmits somatic pain during the second stage of labor Compared to the visceral pain of the first stage of labor. Lumbar epidural analgesia is widely considered the most effective means of giving pain relief during delivery. The use of combination spinal-epidural analgesia in obstetrics is common and growing internationally. Analgesia is quickly and consistently delivered, and the satisfaction of mothers is excellent [6] The Swedish National Birth Register, which uses personal identifying rules, linked data on women's delivery events acquired in a randomized controlled trial of birth center care in Stockholm from 1989 to 1992 [7] Nearly two decades ago, there had been some uncertainty about rising cesarean rates in the United States and the United Kingdom, which were up to 20% in the most sophisticated centers throughout the world. Myers and Gleicher were among the first to provide information on the outcomes of an endeavor to minimize the number of cesarean sections. Although similar successes have been reported in other parts of the world, the percentage of cesarean sections remains high, reaching up to 10% in sophisticated institutions. All cesarean section patients receive some type of anesthetic. Obstetric anesthetists must have specialized training and expertise to offer safe anesthesia in emergency settings for patients who are frequently unprepared for anesthesia and in suboptimal conditions. The anesthetic techniques and substances used should offer safe and effective anesthesia and analgesia with little impact on mother and fetal health.[8] Data on paracervical block, pudendal block, transcutaneous nerve stimulation, and sterilized water papules will not be included due to their limited use during labor or small sample sizes. At two months following birth, the majority of the women remembered labor as well Painful, and 29% (721 women) characterized it as ''worst possible. The more serious pain women reported experiencing during birth, the more pain relief they received, and this held true for both pharmaceutical and non-pharmacological approaches, with the exception of psychoprophylaxis, where no association was discovered. The usage of pharmacological treatments increased with pain intensity, while non-pharmacological approaches climbed from low pain scores (1+2+3) to score 4, but then declined [9]. The sense of pain during the initial stage of labor begins with nociceptive stimulation in the uterus and cervix's mechanical and chemoreceptors. During uterine contractions, high threshold mechanoreceptors are triggered by the severe pressure. In later phases of myocellular damage, repetitive contractions trigger the release of bradykinin, histamine, serotonin, acetyl and potassium ions, activating chemical-based nociceptors [10].
Aim and Objectives
Increasing intervention in birth continues to be a cause for concern and epidural analgesiais an increasingly common intervention in childbirth. The objective of this study was to compare the analgesic efficacy (pain relief), side effects and haemodynamic changes after the use of 0.25% Bupivacaine with 0.25% Bupivacaine plus 1 mg/kg Tramadol used in patients receiving epidural analgesia for labor pain.
Material and Methods
Geographical Area
The state of Azad Jammu and Kashmir lies between longitude 730 – 750 and latitude 330 – 360 and comprises an area of 5134 square miles (13,297 km2). The state of Azad Jammu & Kashmir falls within the Himalayan organic belt. As such, its topography is mainly hilly and mountainous characterized by deep ravines, rugged, and undulating terrain. The northern districts (Neelum, Kotli, Muzaffarabad, Hattian, Bagh, Haveli, Poonch, and Sudhnoti) are generally mountainous while southern districts (Mirpur and Bhimber) are relatively plain. The state of Azad Jammu & Kashmir is full of natural beauty with thick forests, fast flowing rivers and winding streams. Main rivers are Jhelum, Neelum and Poonch. The South has dry sub-tropical climate while the North most moist temperate. District Mirpur is located at the extreme South of the State of Azad Jammu & Kashmir and is linked with Pakistan and other districts of Azad Jammu & Kashmir through several routes. District Mirpur has two tehsils named Dadyal and Mirpur. Topography of District Mirpur comprises partly plane and partly hilly areas. It’s hot climate and other geographical conditions are ideally suited for agriculture.
Study Design
A double blind, randomized, comparative trial.
Study Area
This comparative study of epidural analgesia for painless labor was conducted in Department of Anaesthesiology, Critical Care and Pain Management, Divisional Headquarters Teaching Hospital Mirpur over a study period of 9 months from Feb 2020 to October 2020. A double blind, randomized, comparative trial was conducted on fifty obstetric patients scheduled for painless delivery with American Society of Anesthesiologist's physical status (ASA-PS) I or II. The study was started after receiving institutional ethical committee approval and informed written consent from the patients and was randomly divided into two groups namely A and B with 25 patients in each group. Group A (Control Group): 25 patients received 10 – 12 ml of 0.25% Bupivacaine. Group B (Study Group): 25 patients received 10 – 12 ml of 0.25% Bupivacaine + 1mg/kg Tramadol.
Study Population
Study population included patients visiting for painless labor and childbirth at Div. HQs Teaching Hospital, Mirpur AJ&K. All patients fulfilling inclusion criteria were agreed for epidural as labor analgesia and signed the informed written consent form for the study purpose.
Inclusion Criteria
The patients requesting for painless labor possessing American Society of Anesthesiologist's physical status (ASA-PS) I or II with age between 20 and 35 years were included in the study.
Exclusion Criteria
Patient refusal, local infections at the site of puncture for block / sepsis, known allergy to study drugs, coagulation abnormalities, very obese was excluded. Patients on antihypertensives, diabetics, and pregnancy induced hypertensive patients were excluded. Patients with pre-eclampsia and eclampsia were also excluded from the study. Patients with uncontrolled cardiac/neurological disease and severe systemic disease were excluded from the study. Women with coagulopathy (platelet count less than 80,000), abruptio placentae, placenta previa, acute fetal distress and cord prolapse were also excluded from the study.
Materials
- Sterile tray for epidural blocks
- Drugs for the block
- Inj. Bupivacaine 0.5%
- Inj. tramadol
- Normal saline
- Distilled water
- Inj. Lignocaine 2%
Equipment and drugs for resuscitation and conversion to general anaesthesia in the case of any complication
Preparation for Epidural Anaesthesia
Patients were pre-operatively assessed and the procedure was explained to the patient. Pre-anaesthetic evaluation included general examination, systemic examination of cardiovascular, respiratory, CNS systems and examination of the spine for any disease or deformity. Written informed consent was obtained. They were assessed with particular attention to any contraindications.
The assessment of pain was done using Visual Analogue Pain Scale (VAPS). Patient was explained pre operatively about the visual analogue scale as 0 - No pain and 100 the worst possible pain and was asked the score in visual analogue scale.
Patients were explained the procedure of Epidural anaesthesia at the time of preanesthetic evaluation. After Pre-anaesthesia assessment, counselling and preparation, patients were transferred from the ward to the obstetric operating room. On arrival of the patient in the operating room, monitors like pulse oximeter, non-invasive blood pressure and ECG were connected and baseline values were recorded. An intravenous line with 18G IV cannula was established. Lactated Ringer's solution 15ml/kg was infused to all patients 30 minutes before the Epidural as a standard protocol.
Procedure
Under strict aseptic precautions Epidural Anesthesia was instituted in sitting position. Standard Touhy Needle was used to get access to epidural space after dermal infiltration of 1 ml of 1% lignocaine at L3–L4 with the loss of resistance to saline technique. At first, a test dose was applied through the catheter (with 2-3 mL 2% lidocaine with 5 mcg/mL adrenaline), and if no negative reaction to the test dosage were observed in 5-10 minutes, epidural catheter was then secured and the parturient placed in the supine position with left uterine displacement with the head of the bed elevated 20–30 degrees. At 4 cm cervical dilatation and upon request for labor analgesia 12 mL of bupivacaine 0.25% with tramadol 5 mg/mL and 12 mL of bupivacaine 0.2% with tramadol 5 mg/mL in group a and group b respectively was injected as three fractionated boluses (4 mL each) within 3 min to achieve a bilateral block at ≥T10 sensory level.
Further boluses of 8 mL of the analgesic solution without tramadol were given for breaking through pain. Hypotension (systolic blood pressure below 100 mmHg or a 30% reduction from baseline) was treated with additional left uterine displacement, maternal oxygen administration, IV fluid bolus, or IV ephedrine as indicated.
The visual analogue pain scale (VAPS) [0–100 mm scale: 0 = no pain, 100 = worst pain ever] was measured at the peak of contractions before and 5, 15, and 30 min after the administration of the epidural analgesia and then at hourly intervals.
Sensory level to cold, a Modified Bromage Score (1 = Complete block; unable to move feet or knee, 2 = Almost complete block; able to move feet only, 3 = Partial block; just able to move the knee, 4 =Detectable weakness of hip flexion while supine with full flexion of knees, 5 = No detectable weakness of hip flexion while supine with full flexion of knees, 6 = Able to perform partial knee bend) were obtained 30 min after epidural injection and again at hourly intervals.
Non-invasive blood pressure (NIBP), five leads electrocardiogram (ECG), pulse oximetry, heart rate, oxygen saturation and respiratory rate were recorded. Side effects like sedation, vomiting, drowsiness, tachycardia, and fetal distress were recorded following the administration of the drug.
Data Analysis Process
The data collected includes pain scores, systolic, diastolic, respiratory rates and heart rates. Statistical analysis performed through Statistical Package for Social Sciences (SPSS) version 20. Descriptive statistics were presented as tables. Chi-square test was applied to compare the mean values of systolic blood pressure. P value ≤ 0.05 was considered statistically significant.
Ethical Considerations
Ethical approval was taken from the ethical committee of University of Azad Jammu & Kashmir and Hospital ethical committee of DHQ Mirpur Azad Jammu & Kashmir. Several Ethical considerations were highlighted during the observation.
Consent
The discussion of narrative indicating the plan, alternative plans, purpose of plan, and their advantages and disadvantages (including their relative risks) were presented, understood, and accepted by the patient. A written informed consent was also taken from each study participant.
Privacy and Confidentiality
- The record of the patient was kept confidential and it will be kept in close custody of researcher and supervisor in future.
- It was not unveiled and was maintained at all levels of study.
- Restricted access to the database/ password protection.
- Informed permission will be taken if the data needed to use for some other purpose.
Participants were informed verbally that no payment or special benefit would be directed to them and no withholding of services in case of non-participation.
Results
The information collected in our study Group A (Control Group) and Group B (Study Group) were recorded in a Master Chart. Data analysis was done with the help of computer using SPSS. For statistical analysis students t test was used for comparison between the groups. Using this range, frequencies, percentages, means, standard deviations, chi square and ‘p’ values were calculated. A ‘p’ value less than 0.05 was considered statistically significant.
Pain was assessed by VAS. Assessment was done before and after the administration of the drug and till full dilatation at (0) then after 5, 10, 15, 30, 45 minutes then at 1 h, 2 h, 3h, 4h and 5 h. All the participants were haemodynamically observed prior to the conduct of analgesia and every 5 minutes following injection. Non-invasive blood pressure (NIBP), five leads electrocardiogram (ECG), pulse oximetry, heart rate, oxygen saturation and respiratory rate were recorded. Side effects like sedation, vomiting, drowsiness, tachycardia, and fetal distress were noted following the administration of the drug. Intrapartum monitoring was done according to the standard labor ward protocol using the partogram. The time interval between drug administration and delivery was recorded. Labor progress, mode of delivery and side effects of analgesia either maternal or fetal were recorded. Neonatal evaluation was done by the neonatologist using APGAR score. The data were collected from each patient, compiled in a chart and analysed statistically at the end of the study. Results were expressed as mean ± standard deviation (SD). Parametric data was compared using Student’s t-test. Nonparametric data were compared with Mann-Whitney U test. Proportionate data was compared with Chi-square test. A P value of <0>
Both groups were compared for effectiveness of labor analgesia, hemodynamic changes and maternal and fetal outcomes. For time to first painless contraction, data from all women were used. Patient characteristics (age, height, weight, and parity) were similar in each group.
Demographic Profile of The Patients
Shows the demographic profile of Group A and Group B.
| Parameters | Group A (n = 25) | Group B (n = 25) | P Value |
| (Mean ± SD) | (Mean ± SD) | ||
| Age in years | 24.54 ± 3.74 | 24.22 ± 3.65 | 0.7608 |
| Weight in kg | 60.86 ± 6.24 | 61.90 ± 6.14 | 0.5619 |
| Height in cm | 164.05 ± 4.58 | 163.04 ± 5.40 | 0.4792 |
| BMI | 23.60 ± 1.39 | 23.47 ± 1.09 | 0.7145 |
The above table shows that the average age was 23.54 ± 3.74 years in Group A and 24.22 ± 3.65 years in Group B. Youngest patient in our groups was 20 years and oldest was 34 years. The average weights of the patients were 60.86 ± 6.24 kgs in group A and 61.90 ± 6.14 kgs in group B. The average height of the patients was 164.05 ± 4.58 cm in group A and 163.04 ± 5.40 cm in group B. The average BMI was 23.60 ± 1.39 in Group A and 23.47 ± 1.09 in Group B. There was no significant difference in age, weight BMI and height distribution (p>0.05).
Age Distribution
Shows the age distribution in Group A and Group B.
| Age in Years | Group A (n = 25) | Group B (n = 25) | ||
| No | % | No | % | |
| 20-24 | 9 | 36 | 8 | 32 |
| 25-29 | 12 | 48 | 13 | 52 |
| 30-32 | 2 | 8 | 3 | 12 |
| 33-35 | 1 | 4 | 1 | 4 |
| Total | 25 | 100 | 25 | 100 |
| Mean ± SD | 24.54 ± 3.74 | 24.22 ± 3.65 | ||
The mean age in group A was 24.54 with standard deviation of 3.74 years, while the mean age in group B was 24.22 with standard deviation of 3.65 years. Maximum patients were in age group of 25-29 years (48% in group A and 52% in group B). The age distribution showed no significant difference with means of age between the two groups (p=0.7608).
Parity Distribution
Shows parity distribution in both the groups
| Parity | Group A (n = 25) | Group B (n = 25) | ||
| No | % | No | % | |
| Nulliparous | 16 | 64 | 18 | 72 |
| Multiparous | 9 | 36 | 7 | 28 |
| Total | 25 | 100 | 25 | 100 |
Most of the patients were nulliparous (64% in Group A and 72% in Group B). The two groups were compared for parity. The difference was not significant (p>0.05).
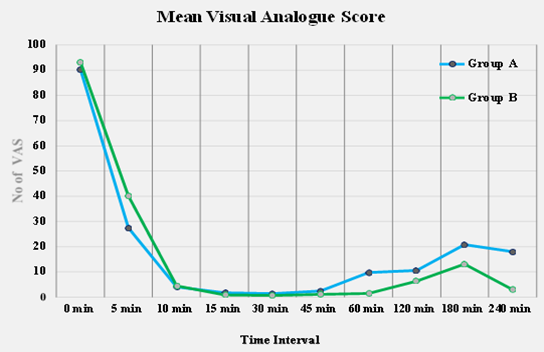
Visual Analogue Score for Pain
Graph Shows Visual Analogue Score.
Shows comparison of mean pain score at different time intervals (VAS Min: Maximum pain relief achieved during the study period) between two groups of patients. The 0 minutes visual analogue pain score of both groups was comparatively insignificant. Pain score of patients in Group B was significantly higher at 5th minute but it was significantly less at 60th minute than Group A (p lessthan 0.05). Overall pain relief was seen higher in Group B.
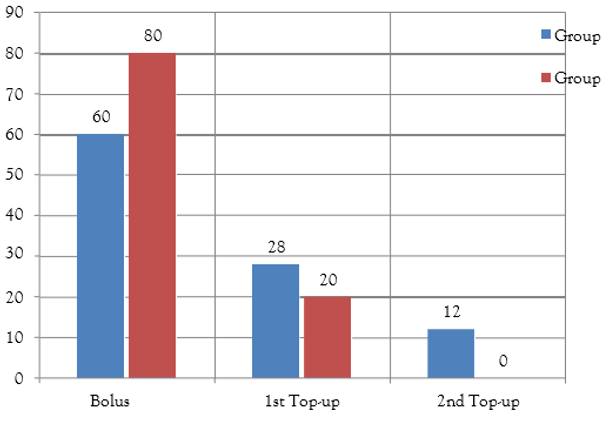
Doses Require
Graph shows the distribution of number of patients according to number of doses required in Group A & Group B.
Duration of Analgesia
Table Shows the duration of analgesia of different doses in Group A & Group B.
| Parameters | Group A | Group B | P Value |
| (Mean± SD) mins | (Mean± SD) mins | ||
| Bolus Dose | 105.97 ± 60.23 | 160.37 ± 55.23 | 0.0017 |
| 1st Top-up | 90.25 ± 25.65 | 106.75 ± 63.15 | 0.457 |
| 2nd Top-up | 86.64 ± 38.24 | NA | NA |
Duration of analgesia of initial bolus dose, defined as the time until parturient requests for additional analgesia (first top-up), was calculated for all 25 patients in each group and was found to be significantly more in Group B (160.37 ± 55.23 min) than in group A (105.97 ± 60.23 min) (P lessthan 0.005). In Group B only 5 (20%) patients required a single top-up dose and 20 (80%) patients had adequate analgesia until the delivery after initial bolus dose, whereas in Group A only 15 (60%) parturient could achieve effective analgesia until the delivery after initial bolus dose, 7 (28%) required a single top-up dose and 3 (12%) required two top-ups (P lessthan 0.001). Overall, significantly higher number of parturients in Group A (n = 10, 40%) required one or more top-up doses. First top-up dose was required significantly earlier in Group A (105.97 ± 60.23 min) than in Group B (160.37 ± 55.23 min), P <0>
Total dose of Bupivacaine
| Parameters | Group A | Group B | P Value |
| (Mean± SD) | (Mean± SD) | ||
| Total dose of Bupivacaine (mg) | 53.15 ± 21.83 | 36.05 ± 13.97 | 0.0018 |
| Duration of 2nd stage in mins | 30.88 ± 28.42 | 24.70 ± 18.03 | 0.3632 |
The total dose of Bupivacaine (mg) required by Group A (53.15 ± 21.83) was significantly high than Group B (36.05 ± 13.97) (p lessthan 0.05). While the duration of 2nd stage of Labor was 30.88 ± 28.42 & 24.70 ± 18.03 minutes in Group A and group B, respectively. The result was statistically insignificant (p=0.3632). The second stage was not prolonged in any of the groups.
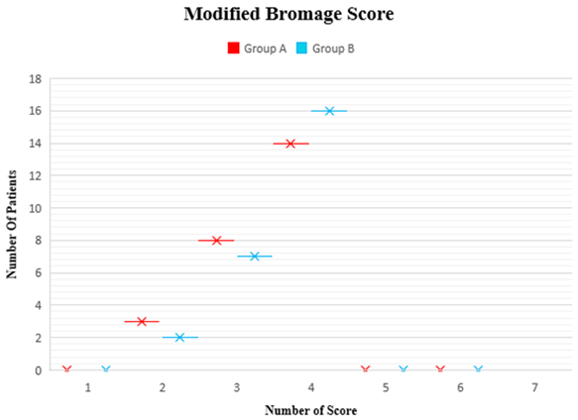
Modified Bromage Score
Most of the patients 14 (56%) in Group A and 16 (64%) in Group B had 4 Modified Bromage Score. They were detectable weakness of hip flexion while supine with full flexion of knee in those patients.
Mode of Delivery
Table shows distribution of patient’s Mode of delivery
| Mode of delivery | Group A | Group B | ||
| No | % | No | % | |
| Spontaneous Vertex Delivery | 20 | 80 | 22 | 88 |
| Instrumental Delivery | 2 | 8 | 1 | 4 |
| Cesarean Section | 3 | 12 | 2 | 8 |
| Total | 25 | 100 | 25 | 100 |
There was no significant difference between the patients who needed various mode of delivery between two groups (p>0.05). Spontaneous Vertex Delivery occurred in 20 (80%) parturient in Group A and 22 (88%) in Group B. 2 (8%) parturient in Group A and 1 (4%) parturient in Group B required forceps and 3 (12%) and 2 (8%) had cesarean delivery in Group A and Group B respectively.
Maternal Respiratory Rate
Table shows Maternal Respiratory Rate variability at different time intervals in Group A and Group B.
| Parameters RR | Group A (n = 25) | Group B (n = 25) | P Value |
| 0 Min | 16.65 ± 2.07 | 16.56 ± 2.16 | 0.8811 |
| 15 Mins | 16.89 ± 2.01 | 16.78 ± 2.51 | 0.8649 |
| 30 Mins | 16.45 ± 2.71 | 17.09 ± 1.82 | 0.3319 |
| 60 Mins | 16.72 ± 2.09 | 17.09 ± 1.70 | 0.4956 |
| 75 Mins | 17.23 ± 1.82 | 17.31 ± 1.90 | 0.8798 |
| 90 Mins | 17.43 ± 1.59 | 17.30 ± 2.05 | 0.8032 |
| 120 Mins | 17.10 ± 1.89 | 17.30 ± 2.08 | 0.7235 |
| 240 Mins | 16.50 ± 1.81 | 17.01 ± 2.09 | 0.3610 |
Maternal Systolic BP Variations
Table shows Maternal Systolic BP variability at different time intervals in Group A and Group B.
| Time Interval | Group A (n = 25) | Group B (n = 25) | P Value |
| 0 Min | 123.70 ± 6.98 | 125.39 ± 7.20 | 0.4036 |
| 15 Mins | 122.78 ± 7.63 | 124.21 ± 6.65 | 0.4833 |
| 30 Mins | 111.90 ± 5.19 | 112.75 ± 5.95 | 0.5929 |
| 60 Mins | 109.41 ± 5.66 | 109.41 ± 5.70 | 1.000 |
| 75 Mins | 111.40 ± 5.27 | 112.63 ± 5.39 | 0.5103 |
| 90 Mins | 115.60 ± 4.45 | 117.19 ± 4.72 | 0.2264 |
| 120 Mins | 119.95 ± 5.95 | 122.39 ± 5.65 | 0.1436 |
| 240 Mins | 125.99 ± 3.64 | 124.71 ± 3.42 | 0.2062 |
Maternal Diastolic BP Variations
Table shows Maternal Diastolic BP variability at different time intervals in Group A and Group B.
| Time Interval | Group A (n = 25) | Group B (n = 25) | P Value |
| 0 Min | 76.40 ± 5.85 | 76.99 ± 5.65 | 0.7184 |
| 15 Mins | 75.23± 6.58 | 75.19 ± 6.80 | 0.9832 |
| 30 Mins | 67.78 ± 5.52 | 66.27 ± 5.11 | 0.3206 |
| 60 Mins | 66.59 ± 3.51 | 64.69 ± 4.39 | 0.2322 |
| 75 Mins | 68.43 ± 2.85 | 70.87 ± 3.59 | 0.1228 |
| 90 Mins | 73.07 ± 2.91 | 73.13 ± 3.74 | 0.1228 |
| 120 Mins | 76.37± 4.10 | 74.49 ± 4.56 | 0.1319 |
| 240 Mins | 78.80 ± 3.97 | 77.57 ± 5.09 | 0.3455 |
Maternal Heart Rate Variations
Table shows Maternal Heart Rate variability at different time intervals in Group A and Group B.
| Time Interval | Group A (n = 25) | Group B (n = 25) | P Value |
| 0 min | 87.71 ± 12.91 | 91.05 ± 13.59 | 0.3774 |
| 5 min | 90.78 ± 13.59 | 87.89 ± 11.14 | 0.4150 |
| 10 min | 84.40 ± 9.90 | 83.58 ± 10.90 | 0.7819 |
| 15 min | 83.40 ± 14.90 | 82.99 ± 8.54 | 0.9055 |
| 30 min | 85.75 ± 10.97 | 83.80 ± 7.40 | 0.4648 |
| 45 min | 88.01 ± 8.89 | 84.60 ± 8.08 | 0.1623 |
| 60 min | 88.22 ± 8.87 | 84.18 ± 8.98 | 0.1371 |
| 120 min | 88.02 ± 9.89 | 84.56 ± 9.83 | 0.2208 |
| 180 min | 85.49 ± 24.68 | 88.10 ± 12.23 | 0. .6378 |
| 240 min | 88.25 ± 9.98 | 85.09 ± 12.05 | 0.3176 |
The hemodynamic parameters such as HR, SBP, DBP and RR were comparable between the groups at different points of time from administration of epidural anaesthesia. There was no significant difference (p>0.05). There was decreasing trend in heart rate, systolic and diastolic blood pressure in both the groups initially. But these falls were within normal range on clinical ground. None of the patients in any group required either ephedrine or atropine in the above three tables.
Adverse Side Effects
Table shows incidence of Nausea and Vomiting in Group A and Group B.
| Parameters | Group A (n = 25) | Group B (n = 25) | P Value |
| Nausea | 1 | 5 | 0.0135 |
| Vomiting | 3 | 8 | 0.0344 |
A total of 1 and 5 patients of Group A and B respectively had developed nausea which was found to be statistically significant (p=0.0135). 3 patients in Group A had vomiting whereas in Group B, 8 patients developed vomiting which was also statistically significant (p=0.03).
Neonatal Apgar Score
Table shows Apgar score at different time intervals in Group A and Group B.
| Time Interval | Group A (n = 25) | Group B (n = 25) | P Value |
| Mean ± SD | Mean ± SD | ||
| APGAR score at 1 min | 6.92 ± 0.60 | 7.11 ± 0.56 | 0.2528 |
| APGAR score at 5 min | 8.11 ± 0.54 | 8.31 ± 0.54 | 0.1966 |
| APGAR score at 10min | 9.19 ± 0.51 | 9.20 ± 0.52 | 0.9461 |
Neonatal outcome was favorable in both the groups. The mean APGAR score of babies at one minute in Group A was 6.92 with standard deviation of 0.60 and at 5 minutes it was 8.11 with standard deviation of 0.54. While mean APGAR score at one minute in group (B) was 7.11 with standard deviation of 0.56 and at 5 minutes it was 8.31 with standard deviation of 0.54. The difference was insignificant (p>0.05).
Discussion
Tramadol is a cost-effective opioid that can be used safely alongside local anesthetics for epidural analgesia. When compared to bupivacaine plus tramadol for epidural postoperative analgesia, ropivacaine with tramadol provided equal and effective analgesia for a longer period of time with minimal cardiovascular depression. There was no difference in motor blockage or adverse reactions among the two subgroups. The duration of analgesia differed significantly between the two groups. The duration of analgesia in group RT was substantially longer than in group BT (p lessthan Tramadol is a cost-effective opioid that can be used safely alongside local anesthetics for epidural analgesia. When compared to bupivacaine plus tramadol for epidural postoperative analgesia, ropivacaine with tramadol provided equal and effective analgesia for a longer period of time with minimal cardiovascular depression. There was no difference in motor blockage or adverse reactions among the two subgroups. The duration of analgesia differed significantly between the two groups. The duration of analgesia in group RT was substantially longer than in group BT (p lessthan 0.001). The period of analgesia was somewhat greater with the Ropivacaine fentanyl (6.1hrs) than with the bupivacaine opioid group (5.6hrs), but the difference was not statistically inconsequential. The baseline heart rate, diastolic and systolic blood pressure, and respiratory rate of both groups were similar. The pulse rate decreased in group BT, but not much in group RT. Group BT had considerably lower systolic and diastolic blood pressures compared to group RT, especially from 10 minutes to 4 hours (p lessthan 0.001) [11]. The best procedure should be safe, effective, simple to apply, and have no negative effects on the mother or fetus. Neuraxial blockage in the form of lumbar epidural is excellent [1]. This procedure enables the patient to participate in the childbirth process while being conscious [9]. This study examined the effectiveness and side effects of Tramadol for labor pain when combined with bupivacaine. There were two groups with fifty individuals overall. There was no significant difference in the duration of the second stage of labor between the two groups. Babies in group A had a mean APGAR score of 6.98± 0.55 at one minute and 8.02 ± 0.47 at 5 minutes, while group B had a mean APGAR score of 7.18± 0.60 at one minute and 8.22 ± 0.58 at 5 minutes. Patients in Group B had significantly greater pain scores at the 5th minute, but considerably lower scores at the 60th minute compared to Group A [12]. This research will benefit nursing and the public by addressing the problem of pain management during labor. It is quite challenging for labor and delivery nurses to take care of the laboring patient in today developed world, with the notion that labor can be absolutely painless [13]. Our investigation found no clinically significant changes in hemodynamic or respiratory indicators. The incidence of PONV has been fairly high, particularly when opioids are delivered in the epidural or intrathecal region. An incidence of 20-80% has been recorded. Our study found a 40% incidence of PONV in Group III. Tramadol hydrochloride is a safe and effective analgesic for post- operative pain treatment when injected epidurally, with no significant side effects such as hemodynamic imbalance or breathing difficulties. Raising the tramadol dose enhanced the length of post-operative analgesia, but also raised the risk of PONV. When administered epidurally, tramadol hydrochloride at a dose of 2mg/kg body weight provides the best post- operative analgesia without significantly increasing side effects. However, a 3mg/kg dose can be administered safely with anti-emetics to reduce the high-risk Postoperative nausea and vomiting [14]. Despite equal pain levels and painkiller use, the 100 mg tramadol group showed significantly higher postoperative thermal pain tolerance after 48 hours compared to the placebo and baseline groups. The challenges of using clinical pain parameters to measure dose-response interactions in analgesia have recently been addressed. This study examined pain thresholds because psychophysical testing gives a measure of alterations in perioperative painful-processing [15].
There are some obvious limitations of our study. First, although epidural administration of tramadol has been extensively used for analgesia by numerous investigators in clinical studies, more studies are needed to assess the safety of its intrathecal administration for labor analgesia. Second, the result of our study could have been more precise if the sample size of study group would have been large, but the patients willing for labor analgesia were limited in our institution.
Conclusion
In this, randomized, double-blinded study, we found that tramadol is a safe adjunct bupivacaine for pain management in labor epidural analgesia. Our opinion is that epidural analgesia is a safe, widely used, effective means of pain relief during labor and cesarean delivery. Nonetheless, many questions remain to be answered, and side effects of pharmacologic pain relief during labor continue to be a matter of concern. Bupivacaine separately and Tramadol in combination with bupivacaine can be used safely for pain relief in labor without any adverse effects on mother and baby. Addition of Tramadol significantly improves the quality of pain relief and also decreases requirement of total dose of drug by delaying top-ups but the problem of nausea and vomiting needs to be addressed. However, these results need to be confirmed with larger prospective trials.
References
- Waldenstrom, U., Hildingsson, I., Rubertsson, C., Radestad, I. (2004). A Negative Birth Experience: Prevalence and Risk Factors in A National Sample. Birth, 31(1):17-27.
Publisher | Google Scholor - Anwar, S., Anwar, M. W., Ahmad, S. (2015). Effect of Epidural Analgesia on Labor and Its Outcomes. J Ayub Med Coll Abbottabad, 27(1):146-150.
Publisher | Google Scholor - Melzack, R., Belanger, E. (1990). Labour Pain: Correlations with Menstrual Pain and Acute Low-Back Pain Before and During Pregnancy. Obstetrical & Gynecological Survey, 45(1):41-42.
Publisher | Google Scholor - Melzack, R. (1984). The Myth of Painless Childbirth (The John J. Bonica Lecture). Pain, 19(4):321-337.
Publisher | Google Scholor - Lowe, N. K. (1991). Critical Predictors of Sensory and Affective Pain During Four Phases of Labor. Journal of Psychosomatic Obstetrics & Gynecology, 12(3):193-208.
Publisher | Google Scholor - Kuczkowski, K. M. (2007). Labor Pain and Its Management with The Combined Spinal–Epidural Analgesia: What Does an Obstetrician Need to Know? Archives of Gynecology and Obstetrics, 275:183-185.
Publisher | Google Scholor - Gottvall, K., Waldenstrom, U. (2002). Does A Traumatic Birth Experience Have an Impact on Future Reproduction? BJOG: An International Journal of Obstetrics and Gynaecology, 109(3):254-260.
Publisher | Google Scholor - Sukhera, S. A., Ahmed, S. (2006). Neonatal Outcome: A Comparison Between Epidural and General Anesthesia for Cesarean Sections. The Professional Medical Journal, 13(1):72-78.
Publisher | Google Scholor - Waldenström, U., Irestedt, L. (2006). Obstetric Pain Relief and Its Association with Remembrance of Labor Pain at Two Months and One Year After Birth. Journal of Psychosomatic Obstetrics & Gynecology, 27(3):147-156.
Publisher | Google Scholor - Gupta, Sunanda; Kumar, GS Anand; Singhal, Hemesh. (2016). Acute Pain - Labour Analgesia. Indian Journal of Anaesthesia, 50(5):363-369.
Publisher | Google Scholor - Shilpashri AM, et al. (2019). Postoperative Epidural Analgesia between 0.25% Ropivacaine Plus Tramadol and 0.25% Bupivacaine Plus Tramadol in Abdominal and Lower Limb Surgeries - A Comparative Study. Anaesth Critic Care Med J., 4(2):000152.
Publisher | Google Scholor - Original Research Article, (2016).
Publisher | Google Scholor - Morris, Kelly Powell, (2010). Expectations of Pain Relief Utilizing Epidural Analgesia. Nursing Theses and Capstone Projects. 187.
Publisher | Google Scholor - Sidiq S, Bacha U Q, Mir A W, Najib R, Shah M A, (2015). Optimum Dose of Epidural Tramadol Hydrochloride for Post Operative Analgesia. Indian J Clin Anaesth, 2(4):262-268.
Publisher | Google Scholor - Wilder-Smith, C.H., Wilder-Smith, O.H.G., Farschtschian, M., Naji, P. (1998), Preoperative Adjuvant Epidural Tramadol: The Effect of Different Doses on Postoperative Analgesia and Pain Processing. Acta Anaesthesiologica Scandinavica, 42:299-305.
Publisher | Google Scholor