Review Article
Efficacy, Safety, Quality Control, Marketing and Regulatory Guidelines for Sceletium Tortuosum
- Prathibha Guttal Subhas 1*
- Debayan Bhattacharjee 1
- Lalitha Kudlur 2
- Monopati Suchitra 3
- Sabarinath Harshakumar 4
1Assistant Professor, Department of Pharmacognosy, Bapuji Pharmacy College, Davanagere, Karnataka, India.
2UG Student, Department of Pharmacognosy, Bapuji Pharmacy College, Davanagere, Karnataka, India.
3Associate Professor, Department of Pharmaceutical Chemistry, Narayana Pharmacy College, Nellore, Andhrapradesh, India.
4Assistant Professor, Department of Pharmacy Practice, Bapuji Pharmacy College, Davanagere, Karnataka, India.
*Corresponding Author: Prathibha Guttal Subhas,Assistant Professor, Department of Pharmacognosy, Bapuji Pharmacy College, Davanagere, Karnataka, India.
Citation: Subhas. P.G, Bhattacharjee. D, Kudlur. L, Suchitra. M, HarshaKumar S. (2025). Efficacy, safety, quality control, marketing and regulatory guidelines for Sceletium tortuosum: review article. International Journal of Nutrition Research and Health, BioRes Scientia Publishers. 4(2):1-13. DOI: 10.59657/2871-6021.brs.25.042
Copyright: © 2025 Prathibha Guttal Subhas, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 01, 2025 | Accepted: March 15, 2025 | Published: March 26, 2025
Abstract
Sceletium tortuosum (ST), also known as kougoed or kanna, is a succulent plant from South Africa traditionally used by indigenous peoples for stress relief, pain management and mood enhancement. Its primary bioactive compounds, mesembrine and related alkaloids show potential benefits for anxiety, depression, and inflammation. ST is not classified as a narcotic. While it contains psychoactive compounds, it is not considered a narcotic substance like opium, morphine, or heroin. These narcotics have a high potential for addiction and abuse. ST is generally considered a relatively mild and non-addictive substance when used responsibly. It has been used traditionally for centuries, and recent research suggests that it may have potential benefits for anxiety, depression and inflammation. However, more research is needed to fully understand its long-term effects and interactions with other medications. Although research is still in its early stages, Sceletium-based products like Zembrin® have gained popularity for their antidepressant and cognitive-enhancing effects. Early toxicology studies, including animal tests suggest it is generally safe at standard doses with few reported side effects, though long-term effects and drug interactions particularly with serotonin-affecting medications need further study. The plant’s alkaloids are absorbed through various tissues and its unique pharmacological properties including serotonin modulation, neuroprotection and anti-inflammatory activity are under investigation. Recent advances in analytical techniques, such as mass spectrometry and X-ray crystallography, have enabled more precise identification and standardization of Sceletium's alkaloids, which is critical for ensuring consistent quality in commercial products. Though promising, gaps remain in clinical trials, bioavailability and understanding minor alkaloids, underscoring the need for further research to assess the plant’s therapeutic potential fully.
Keywords: kougoed; zembrin®; sceletium alkaloids; sceletium tortuosum; cognitive function
Introduction
Sceletium tortuosum (L.) N.E. Br, also known as kougoed or kanna or channa or Mesembryanthemum tortuosum is a succulent, flowering plant native to South Africa, boasts an ancient history of use by indigenous San and Khoi people (Gericke et al., 2008). It belongs to the Aizoaceae family, which also includes other well-known succulents like Lithops ("living stones") and Faucaria. Today, ST tea bags often combined with other South African teas, are readily available in supermarkets (Gericke et al., 2018). In recent years, Sceletium-based products have become popular online for stress management and overall well-being. Traditionally, ST has also been used for pain relief. While more research is needed, some evidence suggests it may be helpful for anxiety and depression.
ST has anti-inflammatory properties that may be beneficial in chronic diseases. Specifically, mesembrine, a key compound in ST exhibits both cytoprotective and mild anti-inflammatory effects. Notably, it targets enzymes in the adrenal glands, potentially reducing glucocorticoid production, which is linked to chronic inflammation associated with diabetes and obesity. Dried out leaves and bright yellow flowers of ST are shown in figure 1 and 2
Figure 1 &2: Dried out leaves of Sceletium tortuosum (1) and bright yellow flowering plant (2).
South Africa, a global hotspot for biodiversity with 10% of the world's flowering plants, has a long-standing tradition of using medicinal plants for various ailments. This echoes a worldwide trend towards utilizing plant-based medicine for its social and economic benefits. The rich flora of South Africa has yielded bioactive compounds like alkaloids and flavonoids, potentially offering new avenues for future medical advancements (Manganyi et al., 2021).
Research on ST faces several gaps. Existing studies utilize diverse extraction methods, hindering comparisons. Furthermore, crucial areas like toxicology, clinical trials and bioavailability studies remain underexplored. Additionally, research has primarily focused on the major alkaloids (mesembrine, mesembranol, etc.) neglecting the potential of minor alkaloid groups. Future advancements in analytical techniques like leaf spray mass spectrometry hold promise for rapid alkaloid identification and quality control. Investigating other ST species could aid in species differentiation and conservation efforts for the entire genus. Finally, a critical gap exists in understanding the molecular and physiological effects of mesembrine alkaloids in a clinical setting. With ST growing popularity as a supplement, further research in these areas is likely to accelerate. This review article comprehensively analyzes the safety profile, efficacy data, standardization approaches, and marketing and regulatory considerations for ST.
History, Taxonomy, Description and Distribution of ST
Prehistoric Origins and Early Encounters
ST history stretches back millennia, with evidence suggesting its use by South African hunter-gatherers and pastoralists as a mood-altering substance long before written records. While the exact date of its first use remains unknown, archaeological evidence and oral traditions point to a deep-rooted connection between ST and these cultures. This connection is further solidified by the various purposes for which ST was traditionally employed, including stress relief, pain management, and inducing trance-like states during rituals. The oldest documented encounter with the plant by Europeans comes from a 1685 painting by Simon van der Stel. This encounter marked the beginning of a written history for Sceletium, which would be further documented in trade records from 1662, where Dutch colonial administrator Jan van Riebeeck noted the exchange of sheep for "Kanna" with native tribes. Interestingly, Europeans initially compared ST to ginseng, highlighting the potential for misunderstanding due to the vast differences between the two plants. More than just a short-lived succulent herb with creeping roots and colorful flowers, the flower petals come in white, pale yellow pale salmon, or pale pink. ST boasts a rich history intertwined with the cultures of South Africa. Evidence suggests its use by indigenous Khoisan people stretches back millennia, employed for various purposes in traditional medicine and rituals. Among its reported uses were alleviating stress and pain, suppressing hunger and thirst during hunting expeditions and inducing hallucinations in spiritual ceremonies (Reddy et al., 2024). The taxonomy of ST is depicted in table 1.
Table 1: Taxonomy of Sceletium tortosum
| Kingdom | Plantae |
| Domain | Eukaryota |
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Subclass | Caryophyllidae |
| Order | Caryophyllales |
| Suborder | Caryophyllineae |
| Family | Aizoaceae |
| Genus | Sceletium /Mesembryanthemum |
| Species | tortuosum |
ST (formerly a genus, now classified within Mesembryanthemum) belongs to the Aizoaceae family and the subfamily Mesembryanthemoideae. Established in 1925 by N.E. Brown, this group of plants is known for their unique leaves. As ST dries out, its leaves take on a skeletal appearance, earning the name "Sceletium" from the Latin word "sceletus" meaning skeleton. ST thrives in dry environments with partial shade, typically found under shrubs growing in sandy loam soil. Geographically, their range stretches from the arid Namaqualand region of Namibia to Montagu in Western Cape, South Africa and eastward to Aberdeen in the Eastern Cape Province.
The World Flora Online (WFO) lists 24 names associated with Sceletium, only 20 are considered valid species (WFO, 2021). This highlights a surprising level of taxonomic confusion within the genus, despite ST being the most well-known and utilized member. This suggests a need for further research to clarify the relationships between the various Sceletium species (Olatunji et al., 2022).
Phytochemistry
The presence of alkaloids in ST was first suggested by Meiring in 1896, later confirmed by Zwicky in 1914. Zwicky's extensive study on Mesembryanthemum species revealed that over half contained alkaloids. This high prevalence led to the abandonment of the genus Mesembryanthemum, with some species (including S. expansum and S. tortuosum) being reclassified as Sceletium within the Aizoaceae family (Patnala 2007).
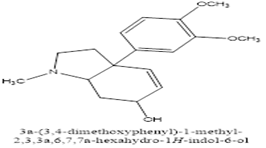
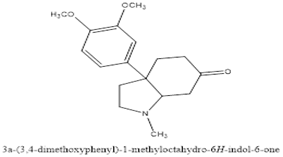
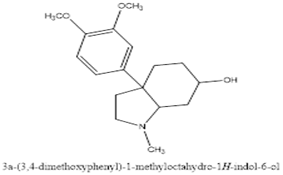
Zwicky isolated a non-crystalline alkaloid from these Sceletium species, naming it "mesembrin" and proposing the formula C16H19NO4. However, Rimington and Roets (1937) re-investigated the compound and found these properties inaccurate. Through their work, they established the current formula (C17H23NO3) for mesembrine and suggested it didn't belong to the tropane ester alkaloid group as previously thought (Olatunji et al., 2022). Sceletium species contain various alkaloids, classified into four main groups based on their chemical structure as shown in figure 3-6.
Figure 4: Mesembrenone
Figure 5: Mesembrine
Figure 6: Mesembranol
The mesembrine group is the most prominent, containing roughly 15 alkaloids. This group takes its name from the first identified alkaloid within it: mesembrine (Patnala et al., 2007). Summary of currently available data on specific pharmacological effects of the most abundant alkaloids found in ST is presented in Table 2.
Table 2: Pharmacological effects of alkaloids found in ST
| Alkaloids | Pharmacological | Plant part used | Model used | References | |
| Mesembrine | SERT inhibition | Aerial part of the plant | Mammalian in vivo models using Zebra fish larvae. | Gericke et al 2024 | |
| PDE-4 inhibition | Dried Plant Extract | The chick anxiety-depression model | Carpenter et al., 2016; Gericke and Viljoen, 2008 | ||
| Anti-inflammatory; cytoprotective | Lyophilised extract (Trimesamine®) | In vitro: human monocytes Immunomodulatory effects – cytokine release and mitochondrial viability | Bennett and Smith (2018) | ||
| Up regulates VMAT-2 | Trimesamine High in Mesembrine | In vitro studies Human astrocytes and mouse hippocampal cells | Bennett and Smith (2018) | ||
| Mild inhibition of AChE | Alkalod enrichd fraction | In vivo animal study Chick anxiety-depression model | Coetzee et al. (2016) | ||
| limited reuptake of NE and DA at high concentrations | Not specifically stated. | Clinical case reports: 1) Depressed woman | Coetzee et al. (2016) | ||
| 2) Dysthymic woman with personality disorder | |||||
| 3) Woman with MDD. Failed treatment with St. John's Wort | |||||
| Acute dose-dependent antidepressant effects and Potential chronic procognitive effects. | Zembrn® | In vivo rat model of depression Flinders Sensitive Line rat study: acute dose response and chronic treatment study. Tested antidepressant properties using the forced swim test (FST) and open field test (OFT) | Gericke (2020) | ||
| Protection against experimentally induced neurotoxicity. | Petroleum ether or ethyl acetate extracts | In vitro: Neuroprotective effect of extract-fractions using UPLC-qTOF-MS with collision energy and MassFragment Software in mouse MPP+-injured N2a cells and glutamate-injured PC12 cells. | Luo et al. (2021) | ||
| Mesembrenone and Mesembrenol | SERT inhibition and PDE-4 inhibition | Petroleum ether or ethyl acetate extracts | In vitro studies on mammalian cells Receptors, enzymes and other targets | Carpenter et al.,2016; Harvey et al., 2011 | |
| Mesembranol | No data found | ||||
| Alkaloid enriched fraction (in vivo behavioural) | Anxiolytic | Alkaloid enriched fraction | In vivo animal study Chick anxiety-depression model | Carpenter et al. (2016) | |
| Antidepressant in FST | Alkaloid extract | In vivo animal study Healthy Sprague Dawley rats | Loria et al. (2014) | ||
| Behavioral tests: | |||||
| Conditioned Place | |||||
| Preference (CPP)* | |||||
| Hotplate test | |||||
| Forced Swim Test (FST) | |||||
| Elevated Plus Maze (EPM) | |||||
| Rotarod | |||||
Safety
Even though herbal medicines have been in use according to long-standing indications, their safety and efficacy are a major concern. While a few toxicological studies using a standardized extract of ST (Zembrin®) in rats and humans haven't reported severe adverse effects, ST research is still in its early stages. More extensive clinical trials are needed to definitively assess its safety and efficacy for various therapeutic applications. Additionally, the long-term effects of ST use remain unknown. Extensive research has been conducted on the unique alkaloid profile of ST. However, studies have shown significant variations in alkaloid composition among wild ST plants. This highlights the importance of using naturally occurring and traditionally used plant varieties (chemotypes) for commercial production. Additionally, standardization of ST extracts is crucial to ensure consistent quality and predictable effects.
Standardization should consider both the total alkaloid content and the specific alkaloid composition within the extract. Notably, research suggests that the four main ST alkaloids (mesembrine, mesembrenone, mesembrenol, and mesembranol) can be absorbed through the intestine, sublingual tissue, and buccal mucosa.
Animal Studies on ST
Several studies have investigated the safety of ST in animals. Hirabayashi et al. and Hirai et al. reported no adverse effects in dogs treated with ST powder (assumed to contain 2% alkaloids) at doses ranging from 20 mg/kg to 100 mg/kg per day, even in cats (Brendler et al., 2021). Further research has explored the specific effects of ST alkaloids. Mesembrine, a major alkaloid, showed analgesic properties without signs of dependence or impaired coordination (ataxia). However, a crude extract containing mesembrine also exhibited antidepressant effects, but unfortunately caused ataxia.
Safety Studies of ST Extract (Zembrin®)
Studies on the standardized ST extract Zembrin® provide more robust data. In a 14-day study, rats received daily doses of Zembrin® ranging from 0 to 5000 mg/kg body weight (equivalent to 0-20 mg/kg of total mesembrine alkaloids). Similarly, a 90-day study used Zembrin® doses from 0 to 600 mg/kg (equivalent to 0 - 2.4 mg/kg of total mesembrine alkaloids). Both studies included detailed behavioral observations alongside standard toxicity assessments. Importantly, neither study found any deaths or adverse effects related to Zembrin® treatment. These studies established No Observed Adverse Effect Levels (NOAEL) for Zembrin® at 5000 mg/kg and 600 mg/kg in the 14- and 90-day studies, respectively. Translated to total mesembrine alkaloids, these NOAELs translate to 20 mg/kg and 2.4 mg/kg per day (Olatunji et al., 2022).
Three-month study
A randomized, double-blind, placebo-controlled study assessed the safety of daily Zembrin® doses (8mg and 25mg) in 37 healthy adults for three months. No significant differences in vital signs, physical exams, heart function (ECG- Electrocardiogram), blood tests, or urine tests were observed between the Zembrin® and placebo groups. Notably, the most common side effect, headaches, occurred more frequently in the placebo group. Interestingly, some participants taking Zembrin® reported unsolicited improvements in well-being, including better stress management and sleep.
Cross-over study
A separate study evaluated both safety and cognitive effects of Zembrin®. Here, 21 healthy adults received either a daily dose of 25mg Zembrin® or a placebo for three weeks, followed by a switch to the opposite treatment. This design allowed researchers to compare each participant's response to both Zembrin® and placebo. Similar to the first study, Zembrin® was well-tolerated with no major side effects reported. Importantly, this study found that the 25mg dose improved cognitive flexibility and executive function compared to placebo. Additionally, participants reported positive changes in mood and sleep while taking Zembrin® [source needed for this specific study].
Metabolism of ST Alkaloids
Studies have investigated how the body breaks down (metabolizes) the main alkaloids in ST- mesembrine and mesembrenone. This involved analyzing rat urine and human liver samples. The research identified various metabolites, which are modified versions of the original compounds. These modifications included the removal of methyl groups (demethylation), addition of water molecules (hydration) and introduction of hydroxyl groups. Interestingly, most metabolites found in rat urine were also detected in human liver samples. The specific enzymes responsible for metabolizing mesembrine and mesembrenone were also identified. This information might be crucial for future research on potential drug interactions. However, the exact impact of this metabolism on the body's overall response to ST remains unclear (Brendler et al., 2021).
Side effects and Potential Interactions with Medications
While ST is often well-tolerated, some users experience headaches, high blood pressure, nausea and trouble sleeping. There's also a potential concern about interactions with medications that affect serotonin, such as antidepressants. Combining ST with these medications could theoretically raise serotonin levels too high, although no such cases have been reported. Because of this potential interaction, it's important to be cautious, especially if you take antidepressants (SSRIs- selective serotonin reuptake inhibitors or SNRIs- serotonin and norepinephrine reuptake inhibitors). Similarly, ST might interact with medications known as PDE-4 (Phosphodiesterase-4) inhibitors. It is better to talk to doctor before using ST, especially if already taking medications.
2.7 Dosage Summary and Safety:
Traditional use involves a wide range of unstandardized ST intake, making it difficult to determine a safe and consistent dose. Clinical studies using the standardized Zembrin® extract suggest a potential safe range of 100-200 μg of total alkaloids daily. Case reports mention successful treatment with 75-100 mg of Zembrin® daily (roughly 300-400 μg of alkaloids).
Efficacy
In vitro studies
Scientists have conducted various lab tests (in vitro studies) to understand the potential effects of ST are as follows
Antidepressant Effects
ST might work differently from some antidepressants. Instead of blocking the reuptake of mood-regulating chemicals (monoamines), it might increase their release. A study using a high-mesembrine extract (Trimesemine™) at a dose of 0.01 mg/mL showed it mimicked the effects of the antidepressant citalopram, reducing serotonin reuptake and boosting its release.
Neuroprotective Potential
Studies are investigating ST's ability to protect nerve cells. One study identified specific compounds in ST extracts, like delta7-mesembrenone, that exhibited neuroprotective effects in mouse cells. However, the specific dosage used wasn't reported.
Anti-inflammatory Effects: ST may also help manage inflammation. A study using Trimesemine™ at 0.01 mg/mL demonstrated its ability to increase the viability of immune cells exposed to an inflammatory stimulus (Bennett et al., 2017).
Other Potential Benefits: Some studies suggest ST extracts might possess antimalarial and anti-retroviral properties, but further research is needed.
In vivo studies
Researchers have conducted in vivo (live animal) studies to investigate the potential effects of ST extracts and isolated compounds. These studies provide clues about ST potential benefits for various conditions.
Stress and Anxiety
Studies in rats suggest that ST extracts may help manage stress and anxiety. One study found that a low dose of unfermented ST extract (5 mg/kg) in rats daily for 17 days helped manage stress-induced behaviors in rats. Another study showed that a specific ST alkaloid (mesembrine) exhibited antidepressant-like effects in mice (Smith et al., 2010).
Depression: Several studies explored the potential antidepressant effects of ST extracts in rats and chicks. Some studies showed promising results, with ST extracts reducing signs of depression-like behavior in these animals. However, more research is needed to confirm these findings in humans. A lower dose of mesembrine extract (10 mg/kg) in mice showed antidepressant effects compared to higher doses. Zembrin® (at 25 mg/kg and 50 mg/kg) showed promise in reversing depressive-like behavior in rats, with 50 mg/kg being the most effective dose (Carpenter et al.,2016).
Cognitive Function: Studies suggest that ST extracts might enhance cognitive function in healthy adults. One study observed improvements in tasks related to focus and mental flexibility after ST extract administration. Another study indicated increased brain wave activity associated with improved attention and memory.
Other Effects: Some studies investigated the potential pain-relieving effects (analgesic properties) of ST and its lack of addictive properties. Other research explored the effects on brain electrical activity and potential benefits for epilepsy treatment. It's important to note that these are animal studies, and the results may not directly translate to humans. More research is needed to confirm the effectiveness and safety of ST for specific conditions in humans.
Three studies in cats and dogs using powdered ST material (20-100 mg/kg body weight daily for 7-180 days) showed no adverse effects and suggested positive impacts on mood and behavior.
Brain Activity: Studies using Zembrin®, a branded ST extract, examined its effects on brain electrical activity in rats. Doses of 2.5, 5.0, and 10.0 mg/kg (equivalent to 10, 20, and 40 μg/kg of mesembrine alkaloids) showed similar effects to a known antidepressant medication (Dimpfel et al., 2016). Clinical Studies of ST (Zembrin®)
While no clinical studies have been conducted on ST in people with diagnosed conditions, several studies explored its effects in healthy volunteers (Gericke et al., 2017). Here's a summary of the findings with highlighted doses of Zembrin®, a branded ST extract:
Reduced Anxiety Response: A single 25 mg dose (equivalent to 100 μg total mesembrine alkaloids) showed reduced activity in the brain's anxiety center (amygdala) compared to placebo in healthy adults (Terburg et al., 2013).
Improved Cognitive Function: Daily intake of 25 mg Zembrin® for three weeks significantly improved executive function and cognitive flexibility in healthy older adults compared to placebo (Chiu et al., 2014).
Enhanced Brain Activity: Single doses of 25 mg or 50 mg Zembrin® increased specific brain wave activity associated with focus, memory and calmness during cognitive tasks in healthy adults (Dimpfel et al., 2016).
Potential Benefits for Anxiety and Cognitive Function in Older Adults: Daily intake of 25 mg or 50 mg Zembrin® for six weeks showed modest improvements in some cognitive functions and a decrease in anxiety scores in healthy older adults. However, the 50 mg dose appeared more effective (Nell et al., 2012).
Limited Effects on Stress and Mood: Some studies using a single 25 mg dose of Zembrin® did not find
Table 3: Tabular column summarizing the case studies mentioned for ST and its standardized extract Zembrin®
| Study Type | Participants/Subjects | Duration | Outcome | Reference |
| Three-Month Study on Zembrin® | 37 healthy adults | 3 months | No significant adverse effects; unsolicited improvements in stress management and sleep. | Nell et al., 2013 |
| Cross-Over Study on Zembrin® | 21 healthy adults | 3 weeks | Improved cognitive flexibility and executive function; positive effects on mood and sleep. | Dimpfel et al., 2016 |
| Single-Dose Study on Anxiety | Healthy adults | Single dose within two hours of the dose. | Reduced activity in the brain's anxiety center (amygdala) compared to placebo. | Terburg et al., 2013 |
| Cognitive Function Study | Healthy older adults | 3 weeks | Significant improvement in executive function and cognitive flexibility compared to placebo. | Chiu et al., 2014 |
| Brain Activity Study | 60 Healthy adults | Single dose within two hours of the dose. | Increased brain wave activity associated with focus, memory, and calmness during tasks. | Dimpfel et al., 2016 |
| Six-Week Study on Cognitive Function and Anxiety | Healthy older adults | 6 weeks | Modest cognitive improvement; decreased anxiety scores, with 50 mg dose more effective | Nell et al., 2012 |
| Animal Safety Study | Cats and dogs | 7-180 days | No adverse effects observed; positive impacts on mood and behavior. | Hirabayashi et al. |
| Zembrin® Safety Study in Rats | Rats | 14 days, 90 days | No observed adverse effects; NOAEL established at 5000 mg/kg (14-day) and 600 mg/kg (90-day). | Olatunji et al., 2022 |
Standardization
Standardizing ST extracts using improved TLC (Thin layer chromatography)
The study developed a new and improved TLC system specifically designed for ST extract analysis. This novel system offers enhanced resolution compared to previous methods, allowing for more detailed alkaloid profile. It effectively separates more alkaloid components, providing a clearer picture of the various alkaloids present in the extract. This improved separation capability makes the new TLC system a valuable tool for researchers studying ST extracts.
A Look at Advanced Characterization Techniques
The sophisticated methods employed to unlock the mysteries surrounding isolated and semi-synthesized ST alkaloids. These techniques provide crucial insights into the exact structure and purity of these fascinating chemical compounds. Researchers utilized a powerful arsenal of analytical tools to unlock the mysteries of ST alkaloids
Liquid Chromatography-Mass Spectrometry (LCMS)
This technique acts like a detective duo. LC separates the alkaloids, and MS meticulously identifies them based on unique mass fingerprints.
Electrospray Ionization (ESI)
This gentle method within LCMS transforms a liquid sample into a cloud of charged particles for analysis by MS.
LCMS/MS: Taking analysis further, LCMS/MS fragments the molecules (parent ions) identified by LCMS, revealing even deeper structural details (daughter ions)
Nuclear Magnetic Resonance (NMR)
This technique acts like a mapmaker, revealing the intricate atomic architecture of the molecules by analyzing interactions with a magnetic field.
X-ray Crystallography: Imagine using X-rays to see the atomic arrangement within a crystal. This technique provided a 3D view of key alkaloids like mesembrine hydrochloride.
Differential Scanning Calorimetry (DSC)
This technique focuses on the thermal behavior, measuring heat absorbed or released by the alkaloids as temperature changes. It determined the melting points of key alkaloids.
Benefits of this Analytical Arsenal
Researchers use advanced techniques like LCMS/MS and NMR to confirm the precise structures of isolated ST alkaloids, ensuring accuracy in their identification. These methods also play a crucial role in detecting and analyzing any impurities, maintaining consistency in research. Additionally, X-ray crystallography provides a detailed 3D view of the crystal structure, revealing the exact atomic arrangement, while DSC helps determine the melting points of these crystalline alkaloids. This comprehensive approach enables a deeper understanding of the structure and purity of ST alkaloids, paving the way for exploring their potential applications.
Unveiling ST Alkaloid Structures with NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful tool for deciphering the molecular structures of organic compounds, and it was employed in this study to confirm the structures of isolated and semi-synthesized ST alkaloids. The study focused on five ST alkaloids: Mesembrine, Mesembranol, Δ⁷ Mesembrenone, Mesembrenone, and Epimesembranol. For each alkaloid, researchers acquired Proton (¹H) NMR spectra at 400 MHz to reveal the hydrogen atom environment and Carbon-13 (¹³C) NMR spectra at 100 MHz to elucidate the carbon atom framework. The results are reported as chemical shifts (δ) in ppm for each proton and carbon atom in the molecules, which helps identify the specific chemical environments and the carbon-to-carbon framework of the alkaloids (Patnala et al., 2007).
Marketing
ST Products: A Market of Variety and Ambiguity
ST comes in various forms like powder, capsules, tinctures, and tablets. While convenient, the wild west of ST marketing is concerning. Products often make exaggerated claims without scientific backing and lack key information on alkaloid content or even the exact Sceletium species. This lack of transparency makes it hard for consumers to choose wisely. Online sales further muddy the waters, with platforms hosting products of questionable quality and unsubstantiated claims. Clearer regulations, especially for online vendors, are needed to protect consumers in this confusing marketplace. Various types of ST plant product available in the market are shown in the figures 7-10.
Figure 8: Tablet
Figure 9: Tincture
Figure 10: Power
Regulations
Today's more stringent regulations make it challenging for promising new plant-based remedies to reach consumers. Companies need more resources to navigate complex rules and ensure fair treatment of those sharing traditional knowledge. This can be expensive, discouraging investment in new botanical ingredients. Further complicating things, regulations vary wildly between countries. The same product might face different rules or even be blocked entirely due to high entry costs. Open discussions can help bridge the gap between companies and regulators. Let's explore how ST, a promising botanical ingredient, navigates these challenges in different markets (Patnala et al., 2007).
Accessing ST in South Africa: Legal Complexities and Considerations
Accessing ST in South Africa is a bureaucratic maze. The National Environmental Management Biodiversity Act (NEMBA) strictly regulates access and use of indigenous resources like ST Permits are needed for various activities, from initial research to commercial production. Additionally, companies must share benefits with communities holding traditional knowledge about ST. This can be complex, as ownership of such knowledge isn't always clear-cut. The process is further entangled by lengthy permit applications and limited monitoring, which can incentivize illegal trade. While these regulations ensure fair treatment of local communities and protect biodiversity, navigating them can be time-consuming and a major obstacle for businesses seeking to bring ST products to market. Careful planning, transparency and a commitment to ethical practices are essential for anyone hoping to navigate this complex system (Gericke et al., 2019).
The status of ST product in European Union
Bringing ST a promising South African herb to Europe is a regulatory maze. Unlike familiar foods it's considered "novel" and needs lengthy safety checks, slowing down companies who want to sell it quickly. Another option being classified as a traditional herbal remedy also doesn't work. These remedies need to be safely used in Europe for over 15 years, which isn't the case for ST. The most straightforward path is full drug development with trials but this is super expensive and risky. Even if successful, competitors could jump in and share the profits. With all these hurdles and low potential rewards companies are hesitant to bring ST to Europe. So, for now, this interesting herb remains unavailable in the legal European market (Brendler et al., 2014).
The status of ST product in United State (US)
The US regulates ST through "GRAS"(Generally Recognized as Safe) status, unlike the EU's (European Union) pre-approval system. Traditional drug approval is expensive, so other avenues are explored. For food/drinks, GRAS status is ideal. Supplements are possible but only if classified as an "Old Dietary Ingredient" (ODI) used before 1994. Otherwise, a lengthy pre-market notification is required. A specific extract (Zembrin®) achieved GRAS for supplements offering some hope. Additionally, historical non-US food consumption evidence might help bypass NDI (New Dietary Ingredient) notification. Despite the complexities over 40 ST supplements exist in the US but it's absent from prescription drugs (US Food and Drug Administration 2016).
The status of ST product in Australia
Australia's regulations for ST depend on its intended use. The TGA (Therapeutic Goods Administration) requires approval for therapeutic purposes as ST isn't on the pre-approved "Listed Medicines" list. As a food source, FSANZ (Food Standards Australia New Zealand) might classify it as a "novel food" requiring safety assessments. The best scenario involves demonstrating ST as a traditional food, exempting it from these hurdles. Currently, neither approval nor traditional food status exists, meaning companies must navigate the TGA process or prove traditional use. Additionally, potential psychotropic effects add another layer of regulatory complexity. In short, bringing ST to the Australian market is a challenging task.
The status of ST product in Canada
Canada takes a stricter approach to ST compared to the US's GRAS system. Here approval by Health Canada. This approval hinges on evidence for the product's safety and effectiveness. While Health Canada offers some guidance the review process itself has tiers of scrutiny. Products aligning with existing standards sail through the fastest (Class 1), while those relying on traditional uses or lacking established benchmarks face a more intense evaluation (Class 2 & 3). Currently, only NHP (Natural Health Product) licenses exist for ST in Canada. Three are single-ingredient products approved for cognitive function, while others combine ST with other ingredients. Importantly, Canada's self-care product ST falls under the NHP category, requiring pre-market regulations are evolving, potentially impacting future regulations for ST-based products (Government of Canada 2020).
The status of ST product in Russia
Bringing ST products to Russia is a bureaucratic maze. Initially considered a food additive, health benefit claims are restricted. This eliminates the food route. The medicinal path seems viable due to traditional uses but mesembrine, Sceletium's key compound is classified as a psychotropic substance in Russia. This relegates ST to the tightly controlled realm of state-developed psychotropic medicines. Even navigating the lengthy approval process wouldn't suffice as only state-owned entities can develop such medications. Special import/export licenses add another hurdle. The combined effect an insurmountable obstacle for companies seeking to introduce ST products in Russia (Russian Federation 2000).
The status of ST product in India
ST products might be available in India, but proceed with caution. One online marketplace offers ST powder labeled as an Ayurvedic extract. However, information on the legality and regulation of ST in India is limited. Since ST can have psychoactive effects and its extract might have different properties, it’s advisable to be cautious. Research ST legal status and safety in India from reliable sources.
Discussion and conclusion
In this study we collectively layout the information upon the safety profile, efficacy data, standardization approaches, and marketing and regulatory considerations for ST. ST shows promise as a natural remedy for stress, anxiety, depression and inflammation with its key alkaloid mesembrine playing a central role. While early studies suggest its efficacy and safety especially in products like Zembrin®, there is still a need for further research to confirm long-term safety, drug interactions and optimize its standardization. Advances in analytical techniques and a deeper understanding of its minor alkaloids will be crucial for ensuring quality control and maximizing its therapeutic potential. Rigorous clinical trials are necessary to fully validate its benefits and address current research gaps.
Future directions
The future goals of this review are attributed to
Confirming the Long-Term Safety of ST
Investigating Drug Interactions related to ST
Optimizing Standardization of ST Extracts
Advancement in Analytical Techniques to Support Quality Control Measures of ST
Declarations
Acknowledgements
The authors would like to acknowledge to the Principal of Bapuji Pharmacy College, Davanagere, Karnataka, for his immense support during our work.
Ethical consideration
Not Applicable.
Consent of Publication
Not Applicable.
Availability of data and materials
Not Applicable.
Competing interests
The authors declare that they have no known competing interests.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All the authors are significantly contributed to this review article. Lalitha K and Prathibha G S were responsible for the conceptualization of the study, literature review and drafting the initial manuscript. Debayan Bhattacharjee, M Suchitra contributed to the analysis of the data, Sabarinath H revising the manuscript for the intellectual content and final approval of the version to be published. All authors have read and approved the final manuscript
References
- Bennett, A. C., & Smith, C. (2018). Immunomodulatory effects of Sceletium tortuosum (Trimesemine™) elucidated in vitro: Implications for chronic disease. Journal of Ethnopharmacology, 214:134-140.
Publisher | Google Scholor - Brendler, T., & Denzil Phillips, L. (2014). European Union market access categories and regulatory requirements for novel natural products. In Novel Plant Bioresources: Applications in Food. Medicine and Cosmetics, 107-124.
Publisher | Google Scholor - Brendler, T., Brinckmann, J. A., Feiter, U., Gericke, N., Lang, L., Pozharitskaya, O. N., Shikov, A. N., Smith, M., & Van Wyk, B. E. (2021). Sceletium for managing anxiety, depression, and cognitive impairment: A traditional herbal medicine in modern-day regulatory systems. Current Neuropharmacology, 19(9):1384-1400.
Publisher | Google Scholor - Carpenter, J. M., Jourdan, M. K., Fountain, E. M., Ali, Z., Abe, N., Khan, I. A., & Sufka, K. J. (2016). The effects of Sceletium tortuosum (L.) NE Br. extract fraction in the chick anxiety-depression model. Journal of Ethnopharmacology, 193:329-332.
Publisher | Google Scholor - Chiu, S., Gericke, N., Farina-Woodbury, M., Badmaev, V., Raheb, H., Terpstra, K., Antongiorgi, J., Bureau, Y., Cernovsky, Z., Hou, J., & Sanchez, V. (2014). Proof-of-concept randomized controlled study of cognition effects of the proprietary extract Sceletium tortuosum (Zembrin) targeting phosphodiesterase-4 in cognitively healthy subjects: Implications for Alzheimer’s dementia. Evidence-Based Complementary and Alternative Medicine, 2014(1):682014.
Publisher | Google Scholor - Dimpfel, W., Gericke, N., Suliman, S., & Dipah, G. N. C. (2016). Psychophysiological effects of Zembrin® using quantitative EEG source density in combination with eye-tracking in 60 healthy subjects: A double-blind, randomized, placebo-controlled, 3-armed study with parallel design. Neuroscience and Medicine, 7(3):114.
Publisher | Google Scholor - Food, U. S. (2016). Drug Administration Food for Human Consumption, Part 182—Substances Generally Recognized as Safe. Federal Register, 81:54960-55055.
Publisher | Google Scholor - Gericke, J. (2020). Evaluating the antidepressant-like properties of Sceletium tortuosum, alone and as adjunctive treatment (Doctoral dissertation, North-West University, South Africa).
Publisher | Google Scholor - Gericke, N. (2018). Kabbo’s! Kwaiń: The past, present, and possible future of kanna. The Ethnopharmacological Search for Psychoactive Drugs, 122-150.
Publisher | Google Scholor - Gericke, N., & Viljoen, A. M. (2008). Sceletium—A review update. Journal of Ethnopharmacology, 119(3):653-663.
Publisher | Google Scholor - Gericke, O., Gericke, N., & Stein, D. J. (2017). Sceletium tortuosum. American Psychiatric Association Publishing.
Publisher | Google Scholor - Jensen, M. G., Goode, M., & Heinrich, M. (2024). Herbal medicines and botanicals for managing insomnia, stress, anxiety, and depression: A critical review of the emerging evidence focusing on the Middle East and Africa. Pharma Nutrition, 100399.
Publisher | Google Scholor - Luo, Y., Shan, L., Xu, L., Patnala, S., Kanfer, I., Li, J., Yu, P., Jun, X. A. (2022). A network pharmacology-based approach to explore the therapeutic potential of Sceletium tortuosum in the treatment of neurodegenerative disorders. PLOS One, 17(8):e0273583.
Publisher | Google Scholor - Manganyi, M. C., Bezuidenhout, C. C., Regnier, T., & Ateba, C. N. (2021). A chewable cure “kanna”: Biological and pharmaceutical properties of Sceletium tortuosum. Molecules, 26(9):2557.
Publisher | Google Scholor - Murbach, T. S., Hirka, G., Szakonyiné, I. P., Gericke, N., & Endres, J. R. (2014). A toxicological safety assessment of a standardized extract of Sceletium tortuosum (Zembrin®) in rats. Food and Chemical Toxicology, 74:190-199.
Publisher | Google Scholor - Nell, H., Siebert, M., Chellan, P., & Gericke, N. (2013). A randomized, double-blind, parallel-group, placebo-controlled trial of extract Sceletium tortuosum (Zembrin) in healthy adults. The Journal of Alternative and Complementary Medicine, 19(11):898-904.
Publisher | Google Scholor - Ng, J. Y., & Luong, M. (2020). Evaluation of the Canadian natural health product regulatory framework in academic research: A scoping review. European Journal of Integrative Medicine, 37:101159.
Publisher | Google Scholor - Olatunji, T. L., Siebert, F., Adetunji, A. E., Harvey, B. H., Gericke, J., Hamman, J. H., & Van der Kooy, F. (2022). Sceletium tortuosum: A review on its phytochemistry, pharmacokinetics, biological, preclinical, and clinical activities. Journal of Ethnopharmacology, 287:114711.
Publisher | Google Scholor - Patnala, S. S. R. R. S. (2007). Pharmaceutical analysis and quality of complementary medicines: Sceletium and associated products (Doctoral dissertation, Rhodes University).
Publisher | Google Scholor - Reddy, K., Stafford, G. I., & Makunga, N. P. (2024). Skeletons in the closet? Using a bibliometric lens to visualize phytochemical and pharmacological activities linked to Sceletium, a mood enhancer. Frontiers in Plant Science, 15:1268101.
Publisher | Google Scholor - Smith, C. (2011). The effects of Sceletium tortuosum in an in vivo model of psychological stress. Journal of Ethnopharmacology, 133(1):31-36.
Publisher | Google Scholor - Stafford, G. I., Pedersen, M. E., van Staden, J., & Jäger, A. K. (2008). Review on plants with CNS effects used in traditional South African medicine against mental diseases. Journal of Ethnopharmacology, 119(3):513-537.
Publisher | Google Scholor - Terburg, D., Syal, S., Rosenberger, L. A., Heany, S., Phillips, N., Gericke, N., Stein, D. J., & Van Honk, J. (2013). Acute effects of Sceletium tortuosum (Zembrin), a dual 5-HT reuptake and PDE4 inhibitor, in the human amygdala and its connection to the hypothalamus. Neuropsychopharmacology, 38(13):2708-2716.
Publisher | Google Scholor