Case report
Chemical Characterization and Assessment of Public Health Risk Due to Inhalation of PM2.5 in the City of Salamanca, Gunaju
- Israel Castro Ramírez 1
- Diana Olivia Rocha Amador 2
- Juan Manuel López Gutiérrez 1
- Elizabeth Ramírez Mosqueda 1
- Glenda Edith Cea Barcia 1
- Francisco Daniel Ramos Patlán 3
- Rogelio Costilla Salazar 1*
1University of Guanajuato, Environmental Science Department, DICIVA, Irapuato, Mexico.
2University of Guanajuato, Pharmacy Department, DCNE, Guanajuato, Mexico.
3University of Guanajuato, Department of Agronomy, DICIVA, Irapuato, Mexico.
*Corresponding author: Rogelio Costilla Salazar.
*Corresponding Author: Rogelio Costilla Salazar, University of Guanajuato, Environmental Science Department, DICIVA, Irapuato, Mexico.
Citation: Israel C. Ramírez, D.O.R. Amador, J.M.L. Gutiérrez, Elizabeth R. Mosqueda, Rogelio C. Salazar, et al. (2024). Chemical Characterization and Assessment of Public Health Risk Due to Inhalation of PM2.5 in the City of Salamanca, Gunaju, Pollution and Community Health Effects, BioRes Scientia Publishers. 2(1):1-10. DOI: 10.59657/2993-5776.brs.24.016
Copyright: © 2024 Rogelio Costilla Salazar, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 07, 2024 | Accepted: February 21, 2024 | Published: March 16, 2024
Abstract
In this study, we conducted an analysis of health risks faced by residents of Salamanca, Mexico, who were exposed to fine particulate matter with a diameter of 2.5 µm (PM2.5) through inhalation. The characterization and analysis of these particulate matter samples were undertaken. A total of 131 samples were collected from two different sites: 65 from the Red Cross site (RC) and 66 from the Integral Family Development site (DIF). These samples were analyzed for a set of chemical components, including metals and ions. Non-cancerous health risk levels associated with PM2.5 exposure through the human respiratory system, as per the WHO benchmark (assigned a value of 1), revealed notable risk values for two elements: Manganese (Mn) with a range of 1.19-2.12 in the adult population and 1.59-2.84 in the child population, and Nickel (Ni) with a uniform risk value of 1.39 for both evaluated population groups. However, concerns arose regarding potential non-cancerous effects as the cumulative risk levels for various assessed elements showed elevated indices. These ranged from 3.81-4.4 in adults and 4.48-5.24 in children. This study provided comprehensive data on composition and its potential impact on human health, offering valuable insights for the implementation of mitigation measures aimed at reducing inhalation-related exposure.
Keywords: characterization; mitigation; cumulative risk; non-cancer health risk
Introduction
Certain human activities associated with industrial processes contribute to increased atmospheric pollution. These activities include construction, mining, disposal of industrial waste, handling and transportation of raw materials involving combustion processes or earthmoving and building renovation (Azarmi & Kumar, 2016; Fan & Wang, 2016; Ramírez et al., 2019; Takaoka et al., 2016). Such activities are prevalent in most urban environments (Motesaddi Zarandi et al., 2019; J. Sun & Zhou, 2017; Y. Sun et al., 2010; Viana et al., 2008). Atmospheric pollution results from a combination of various anthropogenic activities and natural conditions (Lippmann, 2012; M. Tian et al., 2021). Particulate Matter (hereafter referred to as PM), commonly found in the environment, consists of particles to which heavy metals such as chromium, cadmium, lead, zinc, manganese, and some metalloids like arsenic may adhere. These components can be quantified as part of particles (Kumar et al., 2014; Moreno-Ríos et al., 2022; Santibáñez-Andrade et al., 2017; H. Z. Tian et al., 2015). The concentration of these particles varies regionally and can be characterized by their origin or size. PM2.5 and PM10 refer to particles with an aerodynamic diameter equal to or less than 2.5 and 10 µm, respectively (Perrino, 2010; USEPA, 1996). Significant PM sources include vehicular traffic, which generates precursor gases such as NOx, CO, and volatile organic compounds (Viana et al., 2007; Watson et al., 2001).
Additionally, meteorological factors, such as climate, temperature, wind speed, precipitation, humidity, radiation, geographical location, topography, land cover, and proximity to arid areas, influence the concentration and PM composition (Bayraktar et al., 2010; Minguillón et al., 2014; Schneider et al., 2015; Teixeira et al., 2009). Previous studies have focused on characterizing and determining variations in the behavior of PM2.5 particles in major urban areas worldwide (Amil et al., 2016; Das et al., 2015; Duarte et al., 2022; Kretzschmar, 1994; Pan et al., 2015; Tan et al., 2017). Elevated concentrations of PM in the environment have been linked to premature deaths (Buxton et al., 2020; Hannam et al., 2014; Hansen et al., 2006) and respiratory diseases, including asthma, chronic obstructive pulmonary diseases, and earth conditions (Goudarzi et al., 2018; Marabini et al., 2017; Sierra-Vargas et al., 2009; Smith, 2007). In the decade from 2010 to 2020, it has been documented those certain cities in the Mexican Republic exhibit elevated levels of air pollution (IQAir, 2018, 2020), such as the case of Salamanca, Guanajuato. This municipality has been classified as one of the most polluted sites in Mexico, where primary sources of pollution include fixed sources like chemical, petrochemical, and thermoelectric industries (Cortina–Januchs et al., 2015; Linares et al., 2010; Pham et al., 2008). Other contributing sources include fertilizer companies, the textile industry, metal processing, and food production (Herrera Murillo et al., 2012).
In 2003, the Mexican Petroleum Institute (IMP) published a study characterizing PM2.5 in the city of Salamanca. The results revealed that particulate matter is composed of 40% organic carbon, 10% elemental carbon, 17% sulfates, 6% nitrates, 5% geological material (silica, oxides, aluminum, calcium, and iron silicates), 2% trace elements (heavy metals), and 20% unspecified compounds. The study describes the sources, indicating that PM10 is prevalent in unpaved streets and agricultural lands, while anthropogenic contributions to PM2.5 primarily originate from combustion processes (Sosa Iglesias & Ortega López, 2004). Given the environmental challenges in this city, it is essential to conduct studies characterizing the chemical composition of PM2.5 and establishing the relationship between potentially toxic chemical elements and the likelihood of adverse health effects through inhalation exposure. This study aims to characterize and assess the public health risk associated with exposure to atmospheric particles smaller than PM2.5 in the city of Salamanca, Guanajuato.
Materials and Methods
Study Site and Location of Monitoring Stations
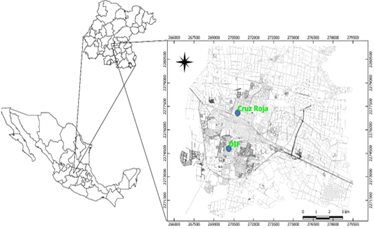
A quantitative study of particulate matter collected in 2014-2015 in the urban area of Salamanca, Mexico, was conducted based on the municipality's industrial activity history. Salamanca is in the central part of the state of Guanajuato, covering an area of 755.6 km2 with a population of 160,682 people in the municipal seat (National Institute for Statistics and Geography, 2021). Situated at 1720 meters above sea level, the city experiences a semi-warm and slightly humid climate, with predominant rainfall from June to August. The annual average temperature is 19.3 °C, with high temperatures from April to June and low temperatures from December to February (Mexican National Meteorological System, 2010). For the location of monitoring stations, two points were selected: RC with UTM coordinates N 270788.95, E 2277078.44, and the offices of the DIF with UTM coordinates N 270142.61, E 2274808.19 (Figure 1). These were considered strategic and representative due to their proximity to main roads and significant pollutant sources in the urban area.
Figure 1: The location of the study area (monitoring stations) was georeferenced in UTM coordinates, map was created using QGIS 3.16.6 (Alkmaar, Netherlands).
Sample Collection
High-volume TISCH ENVIRONMENT samplers with an average flow rate of 68 m3h-1 and a PM2.5 head, based on the principle of inertial impaction to classify particles by the desired size, were used for sample collection. Sampling was conducted over 24 hours every 6 days, following the schedule of the National Air Quality Information System (SINAICA), with an air capacity of 1.3 m3min-1. A total of 65 filters were collected at the DIF station, and 66 filters at the station. Filters were stabilized before and after sampling at 22 ± 3°C and 40 ± 5% relative humidity for 24 hours (NOM-035-SEMARNAT-1993, 1993). Particles were collected on 8x10" quartz (SiO2) filters from Whatman, known for high collection efficiency, inert character, and high purity.
Chemical Analysis of PM
The procedure for chemical characterization, considering the handling of particulate matter on the filter, followed the approach described by Campos and colleagues in their 2010 publication. Approximately 10% of the space impacted by particles on the quartz filter (approximately 150 cm2) was cut and placed in test tubes. To this, 20 mL of HNO3-HCl (2.5M-0.5M) was added. Ultrasonic extraction was performed using a Branson Model 5800 ultrasonic bath for 3 hours at a temperature of 60-70°C (Herrera Murillo et al., 2012), followed by centrifugation of the mineralized sample at 6000 revolutions per minute for 10 minutes using a Solbat J40 centrifuge.
Chemical identification was carried out using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent Technologies 7500CE). For quality control of the analytical procedure, a small amount (1 mg) of Standard Reference Material® 2706 New Jersey Soil was loaded to obtain recovery percentages. Table 1 shows the recovery data for each analyzed element. External calibration was used for quantification, with six levels of concentration of the multi-elemental standard for environmental samples (V, Cr, Mn, Co, Ni, Cu, Zn, As, Se, Mo, Cd, Ba, Tl, and Pb) in a calibration range of 0-100 mgL-1 for all elements.
For the soluble fraction, deionized water (Milli-Q) filtered in 50 mL of water in a tube was used. A 150 cm2 filter fragment was introduced, and ultrasonic extraction was carried out for one hour. The obtained solution was analyzed by ion chromatography for the determination of Cl-1, SO4-2, and NO3-1, and by Flow Injection Analysis (FIA) colorimetry for NH4+ on an ICnet METROOHM chromatograph. For quality control in the soluble fraction, blanks and tubes with fragments were used, employing the standard addition method to evaluate the recovery percentage. Table 1 presents the results obtained for the different evaluated anions and cations. External calibration was used for quantification, with concentrations of 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, and 50 µgmL-1 for Cl-1, NO3-1, SO4-2, Na+1, NH4+1, and K+1.
Table 1: Recovery percentages and detection limits for different elements, anions, and cations in the chemical characterization of particulate matter.
| Analyte | SRM Recovery Percentage 2706 | Limit of Detection (µg /m3) |
| V | 96.48 ± 6.14 | 0.106 |
| Cr | 95.93 ± 4.34 | 0.133 |
| Mn | 95.77 ± 3.69 | 0.013 |
| Co | 80.54 ± 0.11 | N. F |
| Ni | 52.02 ± 3.01 | 0.063 |
| Cu | 39.60 ± 2.86 | 0.084 |
| Zn | 105.61 ± 2.62 | 0.156 |
| As | 126.65 ± 1.15 | 0.096 |
| Se | 56.96 ± 0.61 | 0.126 |
| Mo | 91.16 ± 0.51 | 0.013 |
| Cd | 76.65 ± 0.40 | 0.001 |
| Ba | 61.44 ± 3.27 | 0.006 |
| Tl | 103.44 ± 0.01 | 0.002 |
| Pb | 60.82 ± 3.050 | 0.034 |
| Na+1 | 112.00 ± 15.71 | 0.002 |
| NH4+1 | 92.98 ± 5.72 | 0.003 |
| K+1 | 148.59 ± 2.45 | 0.001 |
| Ca+2 | 96.48 ± 5.30 | 0.002 |
| Cl-1 | 112.40 ± 9.65 | 0.0005 |
| N03-1 | 87.35 ± 5.677 | 0.006 |
| SO4-2 | 93.00 ± 1.038 | 0.011 |
Exposure Assessment and Inhalation Risk Characterization
Risk analysis is employed to assess issues arising in the environment and human health due to the presence of potentially toxic substances, hazardous activities, and the management of toxic substances. The risk assessment method was conducted based on the procedures described by Peña et al. (2001) and the United States Environmental Protection Agency US EPA (2009). The methodology involves the following steps: risk identification, dose-response assessment, exposure assessment, risk characterization, and the development of regulatory options.
For the calculation of the exposure dose, the following formula was utilized:
Exposition dose(mg/Kg/día) = (C x I x r x A x F x D)/ (t x W) (1)
Where: C = concentration of the pollutant in the atmosphere (mg m-3), I = rate of inhaled air (m3day-1), r = retention rate for inhaled air, A = absorption rate of inhaled air, F = exposure frequency (days), D = exposure duration (years), t = average time, W = body weight.
The values used are listed in Table 2.
Regarding the calculation for risk assessment (R), the expression used is:
R=DE/RD (2)
Where: DE = exposure dose (mg/kg/day), RD = reference dose (mg/kg/day) (Table 3), or the concentration of the pollutant and the Minimal Risk Levels (MRL) in the following expression:
R=C/MRL (3)
Table 2: Reference Dose (RD) and Minimal Risk Level (MRL) values for risk calculation.
| Element | RD (mg/kg/día) | MRL (mg/m3) |
| V | 1.00E-04 | |
| Cr | 2.86E-05 | 3.00E-04 |
| Mn | 1.43E-05 | 3.00E-04 |
| Co | 5.00E-06 | 1.00E-04 |
| Ni | 2.00E-02 | 9.00E-05 |
| Cu | 1.00E-01 | 7.50E-02 |
| Zn | 3.00E-01 | 3.00E-01 |
| As | 3.00E-04 | |
| Se | 5.00E-03 | |
| Mo | 5.00E-03 | |
| Cd | 1.00E-05 | |
| Ba | 2.00E-01 | |
| Pb | 5.00E-04 |
Results and Discussion
Chemical Characterization of Particulate Matter
The number of valid samples by Mexican environmental regulations (NOM-025-SSA1-2014) resulted in 57 filters for the RC monitoring station and 62 filters at the DIF site. In Table 4, concentrations of the analyzed chemical elements are presented, indicating the general behavior of average concentrations (µg m-3) of soluble compounds. For both study sites, the order is the same: SO4-2 less then NH4+1 less then NO3-1less then Ca+1less then K+1 less then Na+1 less then Cl-1 less then NO2-1less then Li+1 (µg m-3). The soluble compound with the least occurrence in the sampling sites is NO2-1, present in 20 samples at the RC monitoring station and 31 samples at the DIF site. The ions with the highest presence and concentration are SO4-2, NH4+1, and NO3-1. Some studies analyzing soluble ions describe SO4-2, NH4+1, and NO3-1 as the most representative in concentration (Gao et al., 2015; Liao et al., 2018; Peter et al., 2018).
Table 3: Elemental Chemical Composition of PM2.5 at DIF and RC Station
| RC | DIF | |||||||
| Element | N | Maximum (µg/m3) | Medium (µg/m3) | SD | N | Maximum (µg/m3) | Medium (µg/m3) | SD |
| Li+1 | 57 | 0.007 | 0.005 | 0.001 | 62 | 0.006 | 0.005 | 0.001 |
| Na+1 | 57 | 0.563 | 0.065 | 0.070 | 62 | 0.505 | 0.080 | 0.074 |
| NH4+ | 57 | 3.765 | 1.826 | 0.870 | 62 | 3.316 | 1.233 | 0.637 |
| k+1 | 57 | 0.715 | 0.244 | 0.153 | 62 | 1.229 | 0.254 | 0.207 |
| Ca +2 | 57 | 0.401 | 0.112 | 0.083 | 62 | 1.228 | 0.257 | 0.211 |
| Cl-1 | 51 | 0.511 | 0.031 | 0.083 | 57 | 0.955 | 0.048 | 0.135 |
| NO2-1 | 20 | 0.049 | 0.011 | 0.013 | 31 | 0.134 | 0.021 | 0.028 |
| NO3-1 | 57 | 3.502 | 0.283 | 0.507 | 62 | 2.918 | 0.384 | 0.511 |
| SO4-2 | 57 | 10.287 | 5.901 | 2.656 | 62 | 9.454 | 4.181 | 1.845 |
| V | 57 | 5.480 | 2.473 | 0.949 | 62 | 8.600 | 3.959 | 1.861 |
| Cr | 57 | 3.690 | 1.368 | 0.433 | 62 | 6.580 | 1.421 | 0.732 |
| Mn | 57 | 7.060 | 1.348 | 1.093 | 62 | 6.880 | 2.409 | 1.355 |
| Co | 57 | 0.060 | 0.031 | 0.013 | 62 | 0.140 | 0.053 | 0.028 |
| Ni | 57 | 8.230 | 2.601 | 1.641 | 62 | 8.030 | 1.651 | 1.222 |
| Cu | 57 | 78.840 | 14.410 | 16.399 | 62 | 39.770 | 5.900 | 7.840 |
| Zn | 57 | 88.320 | 11.025 | 11.615 | 62 | 37.250 | 12.519 | 6.627 |
| As | 57 | 1.840 | 0.442 | 0.262 | 62 | 1.550 | 0.464 | 0.235 |
| Se | 53 | 1.030 | 0.378 | 0.181 | 59 | 0.960 | 0.383 | 0.174 |
| Mo | 57 | 2.010 | 0.774 | 0.598 | 62 | 2.130 | 0.663 | 0.493 |
| Cd | 56 | 0.270 | 0.067 | 0.055 | 62 | 0.250 | 0.075 | 0.054 |
| Ba | 57 | 7.410 | 2.069 | 1.363 | 62 | 7.770 | 2.710 | 1.469 |
| Tl | 34 | 0.020 | 0.011 | 0.002 | 39 | 0.020 | 0.012 | 0.004 |
| Pb | 57 | 16.450 | 1.543 | 2.224 | 62 | 7.280 | 1.758 | 1.298 |
The soluble compound SO4-2 has the highest concentrations in the monitoring sites, with a maximum concentration of 10.287 µgm-3. This value is lower than reported in the city of Xinxiang, where a PM2.5 study was conducted in 2014, describing significant atmospheric pollution issues (Feng et al., 2017). The sulfate ion concentration is like the value (10.2 µgm-3) reported by Matawle and colleagues in 2017 in an area with heavy industries, a characteristic shared by the city of Salamanca. The presence of SO4-2 is described to originate from sulfur in fuel, oxidizing into sulfur oxides (Mukta et al., 2020; Wen et al., 2018).
The concentrations of potentially toxic elements for the RC site, in order of concentration levels, are Cu less then Zn less then Ni less then V less then Ba less then Pb less then Mn less then Cr less then Mo less then As less then Se less then Cd less then Co less then Tl (µg m-3). The DIF sampling site exhibited the following concentration behavior: Zn less then Cu less then V less then Ba less then Mn less then Pb less then Ni less then Cr less then Mo less then As less then Se less then Cd less then Tl less then Co (µg m-3). For the DIF station, the elements V, Mn, Cu, Zn, Ba, and Pb were identified in the 62 analyzed samples, while the RC station had V, Mn, Cu, Zn, and Ba identified in the 57 analyzed samples. The rest of the elements were found in a variable number of samples at both monitoring stations. Lead and vanadium are known to be released into the environment during combustion processes and are considered byproducts of the refining process of certain hydrocarbons (Hassanvand et al., 2015; Hernandez & Rodriguez, 2012; Schauer, 2006). Copper is currently a heavily studied element due to its strong association with anthropogenic activities. Its presence in the urban environment is attributed to high levels of vehicular traffic and the wear of certain automotive parts, contributing to its presence in atmospheric particles (Hulskotte et al., 2007; Izydorczyk et al., 2021; Straffelini et al., 2015).
Table 4: Average concentration (µg/m3) of potentially toxic elements (PTE) in PM2.5 particulate matter from studies conducted in different cities around the world.
| Element | Salamanca, Mexico | Shandong China | Tianjin China | Hong Kong China | Chennai India | Jeddah Saudi Arabia | Tehran Iran | |
| DIF | RC | |||||||
| V (µg/m3) | 2.47±0.94 | 3.95±1.86 | 0.007 | 0.011 | 0.001 | 0.013 | 0.051 | |
| Cr (µg/m3) | 1.37±0.43 | 1.42±0.73 | 0.02 | 0.035 | 0.002 | 0.058 | 0.001 | 2.19 |
| Mn (µg/m3) | 1.35±1.09 | 2.40±1.35 | 0.09 | 0.065 | 0.019 | 0.015 | 0.01 | 1.07 |
| Co (µg/m3) | 0.03±0.01 | 0.05±0.02 | 0.014 | 0.006 | ||||
| Ni (µg/m3) | 2.60±1.64 | 1.65±1.22 | 0.009 | 0.017 | 0.005 | 0.132 | 0.004 | 1.03 |
| Cu (µg/m3) | 14.41±16.4 | 5.90±7.84 | 0.03 | 0.043 | 0.005 | 1.25 | ||
| Zn (µg/m3) | 11.02±11.62 | 12.51±6.62 | 0.07 | 0.573 | 0.177 | 0.219 | 3.09 | |
| As (µg/m3) | 0.44±0.26 | 0.46±0.23 | 0.02 | 0.02 | 0.004 | 0.021 | 0.008 | 2.31 |
| Se (µg/m3) | 0.38±0.18 | 0.38±0.17 | 0.02 | 0.017 | ||||
| Mo (µg/m3) | 0.77±0.60 | 0.66±0.49 | ||||||
| Cd (µg/m3) | 0.07±0.05 | 0.07±0.05 | 0.017 | 0.003 | 0.001 | 0.016 | 0.01 | 0.76 |
| Ba (µg/m3) | 2.07±1.36 | 2.71±1.46 | 0.07 | 0.019 | 0.039 | |||
| Tl (µg/m3) | 0.01±0.002 | 0.01±0.004 | ||||||
| Pb (µg/m3) | 1.54±2.22 | 1.75±1.29 | 0.31 | 0.144 | 0.054 | 0.008 | 0.25 | 1.58 |
| Reference | Present study | (J. Zhang et al., 2018) | (Zhang et al., 2015) | (Liao et al., 2018) | (Peter et al., 2018) | (Harrison et al., 2017) | (Kermani et al., 2018) | |
Table 5 presents data from studies conducted on PM2.5 in different cities worldwide. The concentrations found in this study exceed those reported in various studies. Only the data from the study conducted in Tehran, Iran, show concentrations of Cr, As, Cd, and Pb that are higher than those found in the city of Salamanca, Guanajuato.
Health Risk Analysis for Potentially Toxic Elements (PTEs) in PM2.5 Particulate Matter
The calculated risk, when assessing individual elements in the chemical composition of PM2.5 in the city of Salamanca, is presented in Table 6. In the risk levels of exposure to non-carcinogenic heavy metals through the respiratory system, for most of these elements, the risk is less than 1. This value is described in the guidelines by the Agency for Toxic Substances and Disease Registry (ATSDR) (2016), stating that some adverse health effects may occur above this value. There are only two elements in the two monitoring stations that exceed this level, which are Ni and Mn. Ni exceeds the level of 1 in the adult and children population groups at the DIF monitoring station, while in the results from the RC monitoring point, both Mn and Ni had values greater than 1 in both population groups. In a study conducted in central India in areas described with the presence of heavy industries, a characteristic like the city of Salamanca, Ni, and Mn levels are lower (2.46x10-1 for Ni and 3.32 x 10-1 for Mn) than those found in the present study. The study from India reports levels exceeding 1 for other elements such as Cd, Co, and Cr (Matawle et al., 2017).
When comparing the results found in Salamanca with risk levels from PM2.5 in Xinxiang, a city described as urbanized and located 2 km away from industrial pollution, the Mexican city's risk levels are higher. For example, the risk levels for Cr are 1.58x10-1 in children and 8.93x10-2 in adults, and the Mn risk level is 1.92x10-1 in children and 1.08x10-1 in adults (Feng et al., 2017). Considering the total risk for each group and at each station, it can be observed that the value is still greater than 1. This indicates that the exposed population is at potential risk of experiencing some effects caused by the evaluated elements, as mentioned by the Agency for Toxic Substances and Disease Registry (ATSDR, 2004), including hepatic, hematological, and neural effects, which are part of the elemental chemical composition of suspended particles with an aerodynamic diameter of 2.5 µm.
Conclusion
Regarding the chemical characterization, 21 soluble and elemental components were identified, highlighting the presence of soluble compounds such as SO4-2 and NH4+1, with elements such as Cu and Zn also standing out. Since all the analyzed elements are present in the total PM2.5 particulate matter, the risk could increase, potentially generating a combination of these elements and, through synergy, increasing the total risk compared to individual risks. The child population showed a higher risk (5.24 DIF and 4.48 RC), as the total risk values are higher compared to adults (4.40 DIF and 3.81 RC). Therefore, it is concluded that the child population is the most sensitive and vulnerable sector, and efforts should be made to reduce their exposure to PM2.5 particulate matter. The risk has been calculated only for the main inhalation route, and calculating for other routes by summing each corresponding value for the exposure route could reveal an overall increased total risk. It is expected that this study will serve as support for further environmental pollution studies in the city of Salamanca, providing a broader perspective on pollution regulation.
Declarations
Funding
The main financial support was for support of institutional projects approved by the Directorate of Support for Research and Postgraduate Studies of the University of Guanajuato, this funding was used primarily in the sampling stage.
Conflicts of interest
The authors declare not competing interests
Availability of data and material
The creation of tables and databases was carried out which are available upon request for anyone who requests it.
References
- (2004). Agency for Toxic Substances and Disease Registry (ATSDR). ATSDR Interaction Profile for Copper, Lead, Manganese, and Zinc.
Publisher | Google Scholor - Amil, N. Latif, M. T. Khan, M. F. & Mohamad, M. (2016). Seasonal variability of PM2.5 composition and sources in the Klang Valley urban-industrial environment. Atmospheric Chemistry and Physics, 16(8):5357-5381.
Publisher | Google Scholor - ATSDR. (2020). Guidance for Inhalation Exposures.
Publisher | Google Scholor - ATSDR. (2022). MINIMAL RISK LEVELS (MRLs). 1-17.
Publisher | Google Scholor - (2016). (ATSDR) Agency for Toxic Substances and Disease Registry. Exposure Dose Guidance for Determining Life Expectancy and Exposure Factor.
Publisher | Google Scholor - Azarmi, F., & Kumar, P. (2016). Ambient exposure to coarse and fine particle emissions from building demolition. Atmospheric Environment, 137:62-79.
Publisher | Google Scholor - Bayraktar, H. Turalioǧlu, F. S. & Tuncel, G. (2010). Average mass concentrations of TSP, PM 10, and PM 2.5 in Erzurum urban atmosphere, Turkey. Stochastic Environmental Research and Risk Assessment, 24(1):57-65.
Publisher | Google Scholor - Buxton, M. A. Perng, W. Tellez-Rojo, M. M. Rodríguez-Carmona, Y. Cantoral. et. al. (2020). Particulate matter exposure, dietary inflammatory index and preterm birth in Mexico City, Mexico. Environmental Research, 189.
Publisher | Google Scholor - Cortina–Januchs, M. G. Quintanilla–Dominguez, J. Vega–Corona, A. & Andina, D. (2015). Development of a model for forecasting of PM10 concentrations in Salamanca, Mexico. Atmospheric Pollution Research, 6(4):626-634.
Publisher | Google Scholor - Das, R. Khezri, B. Srivastava, B. Datta, S. Sikdar et.al. (2015). Trace element composition of PM2.5 and PM10 from Kolkata–a heavily polluted Indian metropolis. Atmospheric Pollution Research, 6(5):742-750.
Publisher | Google Scholor - Duarte, A. L. Schneider, I. L. Artaxo, P. & Oliveira, M. L. S. (2022). Spatiotemporal assessment of particulate matter (PM10 and PM2.5) and ozone in a Caribbean urban coastal city. Geoscience Frontiers, 13(1).
Publisher | Google Scholor - Fan, J. & Wang, Y. (2016). Atmospheric emissions of as, sb, and se from coal combustion in Shandong Province, 2005-2014. Polish Journal of Environmental Studies, 25(6):2339-2348.
Publisher | Google Scholor - Feng, J. Yu, H. Liu, S. Su, X. Li, Y. Pan, Y. & Sun, J. (2017). PM2.5 levels, chemical composition, and health risk assessment in Xinxiang, a seriously air-polluted city in North China. Environmental Geochemistry and Health, 39(5):1071-1083.
Publisher | Google Scholor - Feng, J. Yu, H. Liu, S. Su, X. Li, et.al. (2017). PM2.5 levels, chemical composition, and health risk assessment in Xinxiang, a seriously air-polluted city in North China. Environmental Geochemistry and Health, 39(5):1071-1083.
Publisher | Google Scholor - Gao, Y. Guo, X. Li, C. Ding, H. Tang. et.al. (2015). Characteristics of PM2.5 in Miyun, the northeastern suburb of Beijing: chemical composition and evaluation of health risk. Environmental Science and Pollution Research, 22(21):16688-16699.
Publisher | Google Scholor - Goudarzi, G. Alavi, N. Geravandi, S. Idani, E. Behrooz.et.al. (2018). Health risk assessment on humans exposed to heavy metals in the ambient air PM10 in Ahvaz, southwest Iran. International Journal of Biometeorology, 62(6):1075-1083.
Publisher | Google Scholor - Hannam, K. McNamee, R. Baker, P. Sibley, C. & Agius R. (2014). Air pollution exposure and adverse pregnancy outcomes in a large UK birth cohort: Use of a novel spatio-temporal modeling technique. Scandinavian Journal of Work, Environment and Health, 40(5):518-530.
Publisher | Google Scholor - Hansen, C. Neller, A. Williams, G. & Simpson, R. (2006). Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. BJOG: An International Journal of Obstetrics and Gynecology, 113(8):935-941.
Publisher | Google Scholor - Harrison, R. M. Bousiotis, D. Mohorjy, A. M. Alkhalaf, A. K. Shamy. et.al. (2017). Health risk associated with airborne particulate matter and its components in Jeddah, Saudi Arabia. Science of the Total Environment, 590-591:531-539.
Publisher | Google Scholor - Hassanvand, M. S. Naddafi, K. Faridi, S. Nabizadeh, R. Sowlat. et.al. (2015). Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Science of the Total Environment, 527-528:100-110.
Publisher | Google Scholor - Hernandez, H. & Rodriguez, R. (2012). Geochemical evidence for the origin of vanadium in an urban environment. Environmental Monitoring and Assessment, 184(9):5327-5342.
Publisher | Google Scholor - Herrera Murillo, J. Campos Ramos, A. Ángeles García, F. Blanco Jiménez, S. Cárdenas. et. al. (2012). Chemical composition of PM 2.5 particles in Salamanca, Guanajuato Mexico: Source apportionment with receptor models. Atmospheric Research, 107:31-41.
Publisher | Google Scholor - Hulskotte, J. H. J. van der Gon, H. A. C. D. Visschedijk, A. J. H. & Schaap, M. (2007). Brake wear from vehicles as an important source of diffuse copper pollution. Water Science and Technology, 56(1):223-231.
Publisher | Google Scholor - Instituto Nacional de Estadística y Geografía. (2021). Panorama sociodemográfico de Guanajuato Censo de Población y Vivienda 2020.
Publisher | Google Scholor - IQAir. (2018). 2018 WORLD AIR QUALITY REPORT Region & City PM2.5 Ranking.
Publisher | Google Scholor - IQAir. (2020). 2020 World Air Quality Report Region & City PM2.5 Ranking.
Publisher | Google Scholor - Izydorczyk, G. Mikula, K. Skrzypczak, D. Moustakas, K. Witek-Krowiak. (2021). Potential environmental pollution from copper metallurgy and methods of management. Environmental Research, 197.
Publisher | Google Scholor - Kermani, M. Farzadkia, M. Kalantari, R. R. & Bahmani, Z. (2018). Fine particulate matter (PM2.5) in a compost facility: heavy metal contaminations and health risk assessment, Tehran, Iran. Environmental Science and Pollution Research, 25(16):15715-15725.
Publisher | Google Scholor - Kretzschmar, J. G. (1994). PARTICULATE MATTER LEVELS AND TRENDS IN MEXICO CITY, SAO PAULO, BUENOS AIRES AND RIO DE JANEIRO. Atmospheric Environment, 28(19):3181-3191.
Publisher | Google Scholor - Kumar, P. Morawska, L. Birmili, W. Paasonen, P. Hu. et.al. (2014). Ultrafine particles in cities. Environment International, 66 :1-10.
Publisher | Google Scholor - Liao Z, Sun J, Liu J, Guo S, & Fan S. (2018). Long-term trends in ambient particulate matter, chemical composition, and associated health risk and mortality burden in Hong Kong (1995–2016). Air Quality, Atmosphere and Health, 11(7):773-783.
Publisher | Google Scholor - Linares B, Guizar J. M, Amador N, Garcia A, Miranda. et.al. (2010). Impact of air pollution on pulmonary function and respiratory symptoms in children. Longitudinal repeated-measures study. BMC Pulmonary Medicine, 10.
Publisher | Google Scholor - Lippmann, M. (2012). Particulate matter (PM) air pollution and health: Regulatory and policy implications. Air Quality, Atmosphere and Health, 5(2):237-241.
Publisher | Google Scholor - Marabini L, Ozgen S, Turacchi S, Aminti S, Arnaboldi F. et.al. (2017). Ultrafine particles (UFPs) from domestic wood stoves: genotoxicity in human lung carcinoma A549 cells. Mutation Research - Genetic Toxicology and Environmental Mutagenesis, 820:39-46.
Publisher | Google Scholor - Matawle J. L, Pervez S, Shrivastava A, Tiwari S, Pant P. et.al. (2017). PM2.5 pollution from household solid fuel burning practices in central India: 1. Impact on indoor air quality and associated health risks. Environmental Geochemistry and Health, 39(5):1045-1058.
Publisher | Google Scholor - Minguillón M. C, Campos A. A, Cárdenas B, Blanco S, Molina L T.et. al. (2014). Mass concentration, composition, and sources of fine and coarse particulate matter in Tijuana, Mexico, during Cal-Mex campaign. Atmospheric Environment, 88:320-329.
Publisher | Google Scholor - Moreno-Ríos A. L, Tejeda-Benítez L. P, & Bustillo-Lecompte, C. F. (2022). Sources, characteristics, toxicity, and control of ultrafine particles: An overview. Geoscience Frontiers, 13(1).
Publisher | Google Scholor - Motesaddi Zarandi S, Shahsavani A, Khodagholi F, & Fakhri Y. (2019). Concentration, sources and human health risk of heavy metals and polycyclic aromatic hydrocarbons bound PM2.5 ambient air, Tehran, Iran. Environmental Geochemistry and Health, 41(3):1473-1487.
Publisher | Google Scholor - Mukta T. A, Hoque M. M. M, Sarker M. E, Hossain M. N, & Biswas G. K. (2020). Seasonal variations of gaseous air pollutants (SO2, NO2, O3, CO) and particulates (PM2.5, PM10) in Gazipur: An industrial city in Bangladesh. Advances in Environmental Technology, 6(4):195-209.
Publisher | Google Scholor - Pan Y, Tian S, Li X, Sun Y, Li Y. et.al. (2015). Trace elements in particulate matter from metropolitan regions of Northern China: Sources, concentrations and size distributions. Science of the Total Environment, 537:9-22.
Publisher | Google Scholor - Peña C. E, Carter D. E, Ayala-Fierro F, Superfund A, Research B. et. al. (2001). TOXICOLOGIA AMBIENTAL: Evaluación de Riesgos y Restauración Ambiental.
Publisher | Google Scholor - Perrino C. (2010). ATMOSPHERIC PARTICULATE MATTER. In Proceedings of a C.I.S.B. Minisymposium.
Publisher | Google Scholor - Peter A. E, Shiva Nagendra S. M, & Nambi I. M. (2018). Comprehensive analysis of inhalable toxic particulate emissions from an old municipal solid waste dumpsite and neighborhood health risks. Atmospheric Pollution Research, 9(6):1021-1031.
Publisher | Google Scholor - Pham D. T, Eldukhri E. E, Soroka A. J, Cortina M. G, Mendoza U. S. et.al. (2008). Forecasting SO2 air pollution in Salamanca, Mexico using an ADALINE. Innovative Production Machines and Systems, 232-237.
Publisher | Google Scholor - Ramírez O, Sánchez de la Campa A. M, Amato F, Moreno T, Silva L. F. et. al. (2019). Physicochemical characterization and sources of the thoracic fraction of road dust in a Latin American megacity. Science of the Total Environment, 652:434-446.
Publisher | Google Scholor - Santibáñez-Andrade M, Quezada-Maldonado E. M, Osornio-Vargas Á, Sánchez-Pérez Y, & García-Cuellar C. M. (2017). Air pollution and genomic instability: The role of particulate matter in lung carcinogenesis. Environmental Pollution, 229:412-422.
Publisher | Google Scholor - Schauer, J. J. (2006). Characterization of Metals Emitted from Motor Vehicles.
Publisher | Google Scholor - Schneider I. L, Teixeira E. C, Silva L. F. O, & Wiegand, F. (2015). Atmospheric particle number concentration and size distribution in a traffic–impacted area. Atmospheric Pollution Research, 6(5):877-885.
Publisher | Google Scholor - Diario oficial. (1993). NOM-035-SEMARNAT-1993,
Publisher | Google Scholor - Sierra-Vargas M. P, Guzman-Grenfell A. M, Blanco-Jimenez S, Sepulveda-Sanchez J. D, Bernabe-Cabanillas R. M. et. al. (2009). Airborne particulate matter PM 2.5 from Mexico City affects the generation of reactive oxygen species by blood neutrophils from asthmatics: an in vitro approach. Journal of Occupational Medicine and Toxicology, 4(1).
Publisher | Google Scholor - Sistema Meteorológico Nacional. (2010). Normales Climatológica por Estado.
Publisher | Google Scholor - Smith, J. T. (2007). Are passive smoking, air pollution, and obesity a greater mortality risk than major radiation incidents? BMC Public Health, 7.
Publisher | Google Scholor - Sosa Iglesias G, & Ortega López F. (2004). Caracterización intensiva de la calidad del aire local y regional en la ciudad de Salamanca, Gto.: Campaña de monitoreo atmosférico 2003. Revista Del Instituto Mexicano de Ingenieros Químicos, 45(11-12):33-43.
Publisher | Google Scholor - Straffelini G, Ciudin R, Ciotti A, & Gialanella S. (2015). Present knowledge and perspectives on the role of copper in brake materials and related environmental issues: A critical assessment. Elsevier Ltd. In Environmental Pollution, 207:211-219.
Publisher | Google Scholor - Sun J, & Zhou T. (2017). Health risk assessment of China’s main air pollutants. BMC Public Health, 17(1).
Publisher | Google Scholor - Sun Y, Wang Y, & Zhang C. (2010). Vertical observations and analysis of PM2.5, O3, and NOx at Beijing and Tianjin from towers during summer and Autumn 2006. Advances in Atmospheric Sciences, 27(1):123-136.
Publisher | Google Scholor - Takaoka M, Shiota K, Imai G, & Oshita, K. (2016). Emission of particulate matter 2.5 (PM2.5) and elements from municipal solid waste incinerators. Journal of Material Cycles and Waste Management, 18(1):72-80.
Publisher | Google Scholor - Tan J, Zhang L, Zhou X, Duan J, Li Y. et. al. (2017). Chemical characteristics and source apportionment of PM2.5 in Lanzhou, China. Science of the Total Environment, 601-602, 1743-1752.
Publisher | Google Scholor - Teixeira E. C, Meira L, de Santana E. R. R, & Wiegand F. (2009). Chemical composition of PM10 and PM2.5 and seasonal variation in South Brazil. Water, Air, and Soil Pollution, 199(1-4):261-275.
Publisher | Google Scholor - Tian H. Z, Zhu C. Y, Gao J. J, Cheng K, Hao J. M. et.al. (2015). Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmospheric Chemistry and Physics, 15(17):10127-10147.
Publisher | Google Scholor - Tian M, Gao J, Zhang L, Zhang H, Feng C. (2021). Effects of dust emissions from wind erosion of soil on ambient air quality. Atmospheric Pollution Research, 12(7).
Publisher | Google Scholor - (2022). USEPA. (n.d.). Integrated Risk Information System. Retrieved 19
Publisher | Google Scholor - (1996). USEPA. Air Quality Criteria for Particulate Matter.
Publisher | Google Scholor - (2009). USEPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment).
Publisher | Google Scholor - Viana, M. Kuhlbusch, T. A. J. Querol, X. Alastuey, A. Harrison. et.al. (2008). Source apportionment of particulate matter in Europe: A review of methods and results. Journal of Aerosol Science, 39(10):827-849.
Publisher | Google Scholor - Viana M, Querol X, Götschi T, Alastuey A, Sunyer J. et.al. (2007). Source apportionment of ambient PM2.5 at five Spanish centers of the European Community Respiratory Health Survey (ECRHS II). Atmospheric Environment, 41(7):1395-1406.
Publisher | Google Scholor - Watson J. G, Chow J. C, & Houck J. E. (2001). PM 2.5 chemical source profiles for vehicle exhaust, vegetative burning, geological material, and coal burning in Northwestern Colorado during 1995. Chemosphere, 43:1141-1151.
Publisher | Google Scholor - Wen J, Wang X, Zhang Y, Zhu H, Chen et.al. (2018). PM2.5 source profiles and relative heavy metal risk of ship emissions: Source samples from diverse ships, engines, and navigation processes. Atmospheric Environment, 191:55-63.
Publisher | Google Scholor - Zhang J, Zhou X, Wang Z, Yang L, Wang J. et.al. (2018). Trace elements in PM2.5 in Shandong Province: Source identification and health risk assessment. Science of the Total Environment, 621:558-577.
Publisher | Google Scholor - Zhang N, Han B, He F, Xu J, Niu C. et.al. (2015). Characterization, health risk of heavy metals, and source apportionment of atmospheric PM2.5 to children in summer and winter: an exposure panel study in Tianjin, China. Air Quality, Atmosphere and Health, 8(4):347-357.
Publisher | Google Scholor