Editorial
Can Gastrointestinal Parasitic Infections be Eliminated Among Tribal People in India? Yes, this Can Happen with Sustained Effective Efforts
Department of Advanced Science and Technology, National Institute of Medical Science and Research, NIMS University Rajasthan, Jaipur, Rajasthan 303121, India.
*Corresponding Author: Shanti Lal Choubisa, Department of Advanced Science and Technology, National Institute of Medical Science and Research, NIMS University Rajasthan, Jaipur, Rajasthan 303121, India.
Citation: Shanti L. Choubisa. (2024). Can Gastrointestinal Parasitic Infections Be Eliminated Among Tribal People in India? Yes, this Can Happen with Sustained Effective Efforts, Clinical and Laboratory Research, BioRes Scientia Publishers. 2(1):1-7. DOI: 10.59657/2994-6441.brs.24.010
Copyright: © 2024 Shanti Lal Choubisa, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 08, 2024 | Accepted: April 29, 2024 | Published: May 06, 2024
Abstract
According to the census, India is home to over 104 million tribal people (8.6%) belonging to diverse intermarried ethnic groups. They are socio-economically backward and live mostly isolated in forests and rural areas. The literacy rate among these people is relatively low and there is lack of health education and awareness about cleanliness and hygiene. In tribal areas of the country, the most common and endemic diseases are malaria, tuberculosis, malnutrition, sexually transmitted diseases, red cell genetic diseases (sickle cell anaemia, thalassemia, etc.). In addition to these diseases, infections by some species of gastrointestinal parasites such as protozoans (Entamoeba histolytica, E. coli, etc.) and helminths such as roundworms (Ascaris lumbricoides and Strongyloides stercoralis), hook worms (Ancylostoma duodenale), whipworms (Trichuris trichiura), pinworms (Enterobius vermicularis), tapeworms(Hymenolepis nana, H. diminuta, and Tinea solium) are also endemic and responsible for considerable morbidity and mortality in tribal individuals. However, infection with most of these parasites is often self-limiting due to their short life span and can be cured by treatment. Among tribals, >50% gastrointestinal parasitic infections have been observed and reported and most of these infected tribal subjects (>80.0%) are found to be anaemic. Eradication of these pathogenic gastrointestinal parasites is important and necessary to protect the health of tribal people. This may be possible by banning open defecation, improving economic status, sanitation, nutritional status and general awareness, imparting health education, providing clean drinking water, establishing health centres with adequate facilities for diagnosis and treatment and regular and proper monitoring. These efforts must be effective and sustainable until gastrointestinal parasitic infections among tribal people are controlled or eliminated.
Keywords: gastrointestinal parasites; health; hookworms; infection; protozoan; roundworms; soil-transmitted helminths; tribal people; whipworms; India
Introduction
The most common parasitic infections in the human population of most tropical countries of the world are some species of gastrointestinal parasites such as protozoans (Entamoeba histolytica, E. coli, etc.) and helminths such as roundworms (Ascaris lumbricoides and Strongyloides stercoralis), hookworms (Ancylostoma duodenale), whipworms (Trichuris trichiura), pinworms (Enterobius vermicularis), tapeworms (Hymenolepis nana, H. diminuta, and Tinea solium) are more prevalent and endemic. Whatsoever the case, in many countries, gastrointestinal parasitic infections pose an enormous burden and are one of the leading causes of human diseases and distress. It is estimated that one in four people has parasitic worms [1]. However, one of the most common parasitic infections worldwide is soil-transmitted helminthiases (STH) infections caused by roundworms, hookworms, and whipworms that are spread by eggs present in human faeces that contaminate the soil in areas where sanitation and hygiene are poorly practiced. Though, the World Health Organization (WHO) has classified STH infections as neglected tropical diseases (NTDs) for not being prioritized in terms of investment or research funding for greater control or treatment despite the health and economic impact of the disease to people living in under developed or developing countries. Though there are already available preventive measures or acute medical treatments for them. But these are still not universally accessible in many low-income developing countries where funding is needed to sustain treatment, making these diseases truly diseases of the poor [2]. STHs, commonly known as intestinal worm infections being aggravated by unhygienic health practices, lack of sanitation facilities, poor living conditions, and meagre access to health services, and people living in the most deprived communities and countries are hit the hardest by the burden of these parasitic diseases [3-5]. Nevertheless, the signs and symptoms depend on the type of parasitic infection. Gastrointestinal parasites cause a variety of symptoms in affected people, most of which manifest in gastrointestinal complications and general weakness [6]. Gastrointestinal conditions include inflammation of the small and/or large intestine, diarrhoea/dysentery, abdominal pain, and nausea/vomiting. These symptoms negatively impact nutritional status, including reduced micronutrient absorption, loss of appetite, weight loss, and intestinal blood loss, which can often result in anaemia. It can cause physical and mental disabilities, developmental delays in children, and irritation of the skin around the anus and vulva [7].
However, children are at greatest risk of morbidity due to helminth infections, as the disease has debilitating effects on their physical and cognitive health, such as anaemia, malnutrition, stunted growth, and delayed learning development [8]. Children aged 5–14 years are at greatest risk of morbidity, accounting for approximately half of the global disease burden of STH infection [9]. Furthermore, it is estimated that one third of the world's population is infected with STH [8], making it the most widespread and disabling chronic infection as well as the most common among NTDs [10]. Statistical findings revealed that STH infects approximately 2 billion of the world's population, with children being the most affected [11]. According to World Health Organization (WHO) estimates, 870 million children live in high-prevalence areas. Africa, South Asia and South America are the most affected regions of the world [12]. India alone contributes about 25% of the total global cases, with 220.6 million children requiring preventive chemotherapy [13, 14]. Is it possible to eliminate these parasitic infections among tribals? If so, how could this happen is the focus of the present editorial.
Tribals in India
In India, according to the census, there are more than 104 million tribal people (8.6%) belonging to diverse intermarried ethnic groups residing in several states. These people are socio-economically backward and live mostly in isolated in forests and rural and remote areas. The literacy rate among these people is relatively low and there is lack of health education and awareness about cleanliness and hygiene. These people do not get enough nutritious food and clean drinking water is also not available to them in sufficient quantity. Most of these areas still lack proper toilet facilities for defecation. Therefore, due to poverty, people are often forced to defecate in the open without wearing shoes and slippers.
Gastrointestinal parasitic infections in tribals
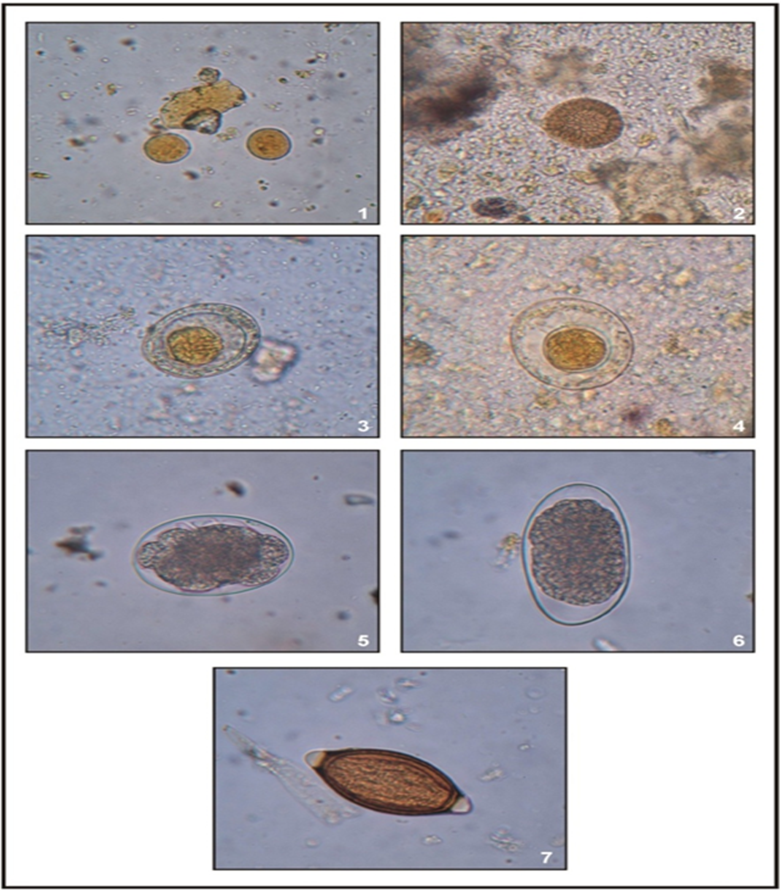
Though, many infectious and non-communicable diseases are endemic in tribal areas. But the most common diseases in tribal provinces are malaria, tuberculosis, malnutrition, sexually transmitted diseases, red cell genetic diseases (sickle-cell anaemia, thalasseamia and glucose-6-phosphate dehydrogenase deficiency), dracunculiasis, etc. [15-49]. Dracunculiasis, one of the nematode worm parasitic diseases endemic in tribal areas, has now been eliminated, but in its place, another disease, fluorosis, has developed among the tribal people [50–59] as well as in their domestic animals [60–74]. In fact, fluorosis disease in India, especially in tribal areas, is the result of the National Dracunculus Eradication Programme [75–77]. Apart from these diseases, among tribal people the most common diseases due to infection of gastrointestinal parasites, protozoans (E. histolytica, E. coli, etc.) and helminths (A. lumbricoides, S. stercoralis, A. duodenale, T. trichiura, E. vermicularis, H. nana, H. diminuta, and T. solium) (Figures 1-7) are also hyperendemic. However, among these intestinal parasites, soil-transmitted helminth parasites (STHs) such as roundworms (A. lumbricoides and S. stercoralis), hookworms (A. duodenale), and whipworms (T. trichiura) are the commonest parasites are found in tribal individuals. The prevalence of these parasitic infections has been found to be more than 50% among tribals in different geographical regions of India [76–80]. Interestingly, most tribal people infected with any of these parasites have been found to suffer from anaemia (Hb < 7g/dl). However, tribal children and pregnant women are more vulnerable to STHs infection. Therefore, the prevalence of these parasitic infections among them is relatively high. Whatsoever, infection with these intestinal parasites is responsible for considerable morbidity and mortality in tribal people. However, infection with most of these parasites is often self-limiting due to their short life span and can be cured by treatment.
Figures 1-7: Cyst and ova or eggs (x400) of diverse species of gastrointestinal parasites recovered in tribals of Rajasthan, India. 1. E. coli, 2. T. solium, 3. H. nana, 4. H. diminuta, 5. A. duodenale, 6. S. stercoralis, and 7. T. trichiura. Source: [81]
Can gastrointestinal parasitic infections be eliminated?
Yes definitely, this may be possible by breaking the transmission chain of gastrointestinal parasite infection. This can be achieved by banning open defecation and improving sanitation and hygiene practices with preventive strategies. Improvement in economic status, nutritional status, and general awareness, dissemination of general health education, providing clean drinking water, establishment of health centres with adequate facilities for diagnosis and treatment and regular and proper surveillance in tribal areas are also effective preventive efforts. These efforts should be sustainable until gastrointestinal parasitic infections are completely controlled or eliminated in tribal populations or in tribal areas. WHO also recommends drugs for the treatment of helminth worm infections-Albendazole (400 mg) and Mebendazole (500 mg) – as effective, inexpensive and easy to administer by non-medical personnel (such as teachers). They have been extensively safety tested and used in millions of people, but there have been few side effects. Towards the control or elimination of STH from India, the Department of Drinking Water and Sanitation, Ministry of Jal Shakti, Government of India is running a large-scale project or programme named "Swachh Bharat Mission-Gramin". This is the world's largest cleanliness initiative which is running successfully in diverse rural areas in the country. The main goal under this mission is to make villages open defecation free (ODF). For this, individual household toilets are being constructed in villages at the Government level. There is no doubt that this programme is successful and is moving towards its achievement. The Medical and Health Department of each state also celebrates “National Deworming Day” in all states and union territories (UTs) every year on 10 February, as well as Mop-up Day on 15 February. Some states/UTs also conduct a bi-annual round on 10th August depending on the status of the prevalence of STHs infections. On this day, deworming with 400 mg Albendazole is given to all high-risk groups including pregnant women [6]. However, the development of resistance to Albendazole may pose a challenge to control efforts of STH infection. Therefore, it is necessary to monitor the effectiveness of anthelmintic drugs in controlling parasitic infections.
Conclusion
Tribal people in India are very backward socio-economically and live mostly in isolated in diverse forests and rural and remote areas. The literacy rate among these people is relatively low and there is lack of health education and awareness about cleanliness and hygiene. A large number of parasitic and non-parasitic diseases are prevalent among these people. However, among these, gastrointestinal parasitic infections are most common among tribals. >50% of the tribals are found to suffer from single or multiple protozoan and helminth parasitic infections. Due to their infection, most of tribal people suffer from anaemia and general weakness and are affected by diarrhoea/dysentery, stomach ache, nausea/vomiting and many other gastrointestinal health problems. However, tribal children and pregnant women are found to be more vulnerable to soil-transmitted helminth parasites, such as roundworms, hookworms, and whipworms. In tribal areas of India by banning open defecation, improving economic status, sanitation, nutritional status, and general awareness, giving health education, providing clean drinking water, establishing health centres with adequate facilities for diagnosis and treatment, and with regular monitoring, these parasitic infections can be eliminated. These efforts must be effective and sustainable until gastrointestinal parasitic infections among tribal people are controlled or eliminated.
Declarations
Funding sources
No any funding sources for this work.
Acknowledgements
The author thanks to Vishvjeet Jaroli, Head, Department of Zoology, Shri Ratanlal Kanwarlal Patni Girls’ College, Kishangarh, Rajasthan 305801, India and Prof. Darshana Choubisa, Department Prosthodontics and Crown & Bridge, Geetanjali Dental and Research Institute, Udaipur, Rajasthan 313002, India for their cooperation.
References
- WHO. (2012). Parasitic Diseases. World Health Organization, Geneva.
Publisher | Google Scholor - Remme JH, Feenstra P, Lever P, Médici A, Morel C, Noma M, et al. (2006). Tropical Diseases Targeted for Elimination. In: Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC), 433.
Publisher | Google Scholor - Crompton D, Savioli L. (1993). Intestinal parasitic infections and urbanization. Bull WHO, 71(1):1-7.
Publisher | Google Scholor - De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. (2003). Soil-transmitted helminth infections: updating the global picture. Trends Parasit, 19(12):547-551.
Publisher | Google Scholor - Montresor A, Crompton D, Hall A, Bundy D, Savioli L. (1998). Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization, 1-49.
Publisher | Google Scholor - WHO. (2023). Soil-transmitted helminth infections. World Health Organization, Geneva.
Publisher | Google Scholor - Ashtiani MTH, Monajemzadeh M, Saghi B, Shams S, Mortazavi SH, Khaki S, et al. (2011). Prevalence of intestinal parasites among children referred to children's medical centre during 18 years (1991–2008), Tehran, Iran. Annals Trop Med Parasit, 105 (7):507-513.
Publisher | Google Scholor - Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. (2006). Soil transmitted helminth infections: ascariasis, trichuriasis, and hookworm. The Lancet, 367(9521):1521-1532.
Publisher | Google Scholor - Hotez PJ, Fenwick A, Savioli L, Molyneux DH. (2009). Rescuing the bottom billion through control of neglected tropical diseases. The Lancet, 373(9674):1570-1575.
Publisher | Google Scholor - Bieri FA, Li Y-S, Yuan L-P, He Y-K, Gray DJ, Williams GM, et al. (2014). School-based health education targeting intestinal worm-further support for integrated control. PLoS Negl Trop Dis, 8(3): e2621.
Publisher | Google Scholor - Savioli L, Albonico M. (2004). Soil-transmitted helminthiasis. Nat Rev Microbiol, 2:618-619.
Publisher | Google Scholor - Lobo DA, Velayudhan R, Chatterjee P, Kohli H, Hotez PJ. (2011). The neglected tropical diseases of India and South Asia: review of their prevalence, distribution, and control or elimination. PLoS Negl Trop Dis, 5:e1222.
Publisher | Google Scholor - N Salam, S Azam. (2017). Prevalence and distribution of soil-transmitted helminth infections in India. BMC Pub Health, 17:201.
Publisher | Google Scholor - Ulaganeethi R., Saya, GK, Rajkumari N, Kumar, SS, Ganapathy K, Dorairajan G. (2023). Soil-transmitted helminth infections among antenatal women in primary care settings in Southern India: prevalence, associated factors and effect of anti-helminthic treatment. Trop Med Infect Dis, 8:48.
Publisher | Google Scholor - Choubisa SL, Choubisa L. (1991). Prevalence of vaginal Trichomoniasis in Dungarpur district, Rajasthan. Indian J Parasit, 15(2):97-99.
Publisher | Google Scholor - Choubisa SL, Choubisa L. (1992). Prevalence of intestinal and malaria parasitic infections in tribal students of Dungarpur (Rajasthan). Indian J Parasit, 16(2):55-57.
Publisher | Google Scholor - Choubisa SL, Sharma PN, Parmar L. (1984). Haemoglobin-A2 and glucose-6-phosphate dehydrogenase deficiency in relation to malaria. Indian J Parasit, 8(2):247-249.
Publisher | Google Scholor - Choubisa SL. (1985). Sickle cell haemoglobin, glucose-6-phosphate dehydrogenase deficiency and thalassaemic genes in relation to malaria endemicity. Indian J Parasit, 9(2):131-133.
Publisher | Google Scholor - Choubisa SL, Choubisa DK, Choubisa L. (2003). Focus on red cell genetic defects and malaria. J Parasit Dis, 27(2):99-103.
Publisher | Google Scholor - Choubisa SL, Choubisa DK, Choubisa L. (2005). The ABO blood groups and malaria. J Parasit Dis, 29(2):109-111.
Publisher | Google Scholor - Choubisa SL. (2002). Guinea worm (Dracunculus medinensis) in Rajasthan, India: A case report. J Parasit Dis, 26(2):105-106.
Publisher | Google Scholor - Choubisa SL, Choubisa L. (2005). Trichomonas veginalis parasitic infection (Trichomoniasis) in subjects inhabiting rural environment of tribal region of southern Rajasthan. J Parasit Dis, 29(1):77-80.
Publisher | Google Scholor - Choubisa SL. (2009). Amoebiasis among tribals of Rajasthan (India). J Commun Dis, 41(1):53-55.
Publisher | Google Scholor - Choubisa SL, Verma R, Choubisa L. (2010). Dracunculiasis in tribal region of Rajasthan (India): A case report. J Parasit Dis, 34(2):94-96.
Publisher | Google Scholor - Choubisa SL. (2011). Parasitic infection in tribals and their domestic animals of southern Rajasthan. A technical report. Indian Council of Medical Research, New Delhi. 1-14.
Publisher | Google Scholor - Choubisa SL, Jaroli VJ. (2013). Gastrointestinal parasitic infections in diverse species of domestic ruminants inhabiting tribal rural areas of southern Rajasthan, India. J Parasit Dis, 37(2): 271-275.
Publisher | Google Scholor - Choubisa SL. (2022). A historical dreaded human nematode parasite, Dracunculus worm (Dracunculus medinensis) whose awe is still alive in elderly of India! Can't it reappear in India? Austin Pub Health, 6(1): 1-4.
Publisher | Google Scholor - Choubisa SL, Choubisa P. (2024). Are freshwater sources safe for the health of humans and domestic animals in terms of deadly trematodiases? Med Discoveries, 3(1): 1-7.
Publisher | Google Scholor - Jain RC, Andrew AMR, Choubisa SL, Acharya A, Joshi KC. (1983). Sickle cell gene in the Mina tribal population of Kherwara tehsil of Udaipur district in Rajasthan. Indian J Med Res, 78:522-555.
Publisher | Google Scholor - Jain RC, Andrew AMR, Choubisa SL. (1983). Sickle cell and thalassaemic genes in tribal population of Rajasthan. Indian J Med Res, 78:836-840.
Publisher | Google Scholor - Jain RC, Choubisa SL, Acharya A, Andrew AMR, Chhaparwal JK, Joshi KC. (1983). Incidence of G-6-PD deficiency in the tribal population of southern Rajasthan. J Assoc Physicians India, 32(3):266-267.
Publisher | Google Scholor - Choubisa SL, Parmar L, Purohit VK. (1984). Abnormal haemoglobins in subjects belonging to scheduled castes of Udaipur district (Rajasthan). Indian J Med Res, 80:463-468.
Publisher | Google Scholor - Choubisa SL, Choubisa L, Pande S, Srivastava YK. (1987). Incidence of abnormal haemoglobins and G-6-PD deficiency in school children of Udaipur (Rajasthan), India. J Trop Med Hyg, 90:215-216.
Publisher | Google Scholor - Choubisa SL. (1988). Abnormal haemoglobins, thalassaemia and G-6-PD deficiency in school children belonging to scheduled castes and tribes of Rajasthan, India. Indian J Physi Anthrop Hum Genet,14(3):31-40.
Publisher | Google Scholor - Choubisa SL. (1990). Sickle cell traits in Damor tribe of Dungarpur district, southern Rajasthan. Man in India, 70(4):454-458.
Publisher | Google Scholor - Choubisa SL. (1990). Distribution of Hb-Bart’s (α-thalassaemia) in various population of Dungarpur district of Rajasthan (India). Indian J Physi Anthrop Hum Genet, 6(1&2):43-48.
Publisher | Google Scholor - Choubisa SL. (1991). Abnormal haemoglobins, thalassaemia and G-6-PD enzyme deficiency in Rajasthan (Western-India). Haematologia, 24(3):153-165.
Publisher | Google Scholor - Choubisa SL, Choubisa DK. (1994). Erythrocyte glucose-6-phosphate dehydrogenase deficiency in senile cataracts. Man in India, 74(3):267-270.
Publisher | Google Scholor - Choubisa SL, Choubisa DK. Mutant Hbs. (1996). ß-thalassaemia and G-6PD enzyme deficiency in tribal population of southern Rajasthan (India). Proceedings of 9th International Congress of Human Genetics, Rio de Jaeiro, Brazil, 18-23.
Publisher | Google Scholor - Choubisa SL. (1997). Study of certain haematological genetic polymorphic systems in relation to malaria endemicity in tribes residing in arid and humid ecosystems of Rajasthan. A technical report, Indian Council of Medical Research, New Delhi, India. 1-24.
Publisher | Google Scholor - Choubisa SL, Choubisa DK, Khare S. (2000). α-thelassaemia (Hb-Bart’s) in Rajasthan (India). Haematologia, 30(3): 209-213.
Publisher | Google Scholor - Choubisa SL, Choubisa DK, Choubisa L. (2004). Erythrocyte genetic disorders in inhabitants of Aravali hilly environment. Indian J Physi Anthrop Hum Genet, 23(2):145-159.
Publisher | Google Scholor - Choubisa SL, Choubisa L. (2006). Erythrocyte mutant genes in inhabitants of arid environment of Western-Rajasthan (India). Indian J Physi Anthrop Hum Genet, 25(1):45-50.
Publisher | Google Scholor - Choubisa SL. (2009). Sickle cell haemoglobin, thelassaemia and G-6-PD enzyme deficiency genes in Garasiya tribe inhabited malaria endemic areas of Sirohi district, Rajasthan (India). J Commun Dis, 41(1):13-18.
Publisher | Google Scholor - Choubisa SL. (2010). Haemoglobin-C gene in India? Curr Sci, 99(7):860.
Publisher | Google Scholor - Choubisa SL, Choubisa A. (2021). Status of erythrocyte genetic disorders in people of desert and humid environments, Rajasthan, India: focus on natural selection in tribals against malaria. Proc Indian Natl Sci Acad, 87(3):433-445.
Publisher | Google Scholor - Choubisa SL, Choubisa A. (2021). A brief review of sickle-cell haemoglobin, β-thalassaemia and G-6-PD deficiency genes among tribals of scheduled area of Rajasthan, India: focus on tribal health. J Biomed Res Environ Sci, 2(12):1187-1196.
Publisher | Google Scholor - Choubisa SL. (2022). How to prevent and control sickle-cell anaemia and β-thalassemia major in the tribal people of the scheduled area of Rajasthan (India)? Annal Hemat Onco, 9(6):1-5.
Publisher | Google Scholor - Choubisa SL. (2023). How do sickle cell genes protect tribal people from deadly malaria? Is this a type of natural selection? Annal Hemat Onco, 10(5):1-6.
Publisher | Google Scholor - Choubisa SL, Sompura K, Bhatt SK, Choubisa DK, Pandya H, Joshi SC, Choubisa L. Prevalence of fluorosis in some villages of Dungarpur district of Rajasthan. Indian J Environ Health, 38(2):119-126.
Publisher | Google Scholor - Choubisa SL, Sompura K, Choubisa DK, Sharma OP. (1996). Fluoride in drinking water sources of Udaipur district of Rajasthan. Indian J Environ Health, 38(4):286-291.
Publisher | Google Scholor - Choubisa SL, Sompura K. (1996). Dental fluorosis in tribal villages of Dungarpur district (Rajasthan). Poll Res, 15(1):45-47.
Publisher | Google Scholor - Choubisa SL, Sompura K. (1996). Dental fluorosis in tribal villages of Dungarpur district (Rajasthan). Poll Res, 15(1):45-47.
Publisher | Google Scholor - Choubisa SL. (1999). Chronic fluoride intoxication (fluorosis) in tribes and their domestic animals. Intl J Environ Stud, 56(5):703-716.
Publisher | Google Scholor - Choubisa SL. (2001). Endemic fluorosis in southern Rajasthan (India). Fluoride, 34(1): 61-70.
Publisher | Google Scholor - Choubisa SL, Choubisa L, Choubisa DK. (2001). Endemic fluorosis in Rajasthan. Indian J Environ Health, 43(4):177-189.
Publisher | Google Scholor - Choubisa SL. (2012). Fluoride in drinking water and its toxicosis in tribals, Rajasthan, India. Proc Natl Acad Sci, India Sect B: Biol Sci, 82(2):325-330.
Publisher | Google Scholor - Choubisa SL, Choubisa D. (2019). Genu-valgum (knock-knee) syndrome in fluorosis- endemic Rajasthan and its current status in India. Fluoride, 52(2):161-168.
Publisher | Google Scholor - Choubisa SL, Choubisa D, Choubisa A. (2023). Fluoride contamination of groundwater and its threat to health of villagers and their domestic animals and agriculture crops in rural Rajasthan, India. Environ Geochem Health, 45:607-628.
Publisher | Google Scholor - Choubisa SL, Pandya H, Choubisa DK, Sharma OP, Bhatt SK, Khan IA. (1996). Osteo-dental fluorosis in bovines of tribal region in Dungarpur (Rajasthan). J Environ Biol, 17(2):85-92.
Publisher | Google Scholor - Choubisa SL. (1999). Some observations on endemic fluorosis in domestic animals of southern Rajasthan (India). Vet Res Commun, 23(7):457-465.
Publisher | Google Scholor - Choubisa SL. (2007). Fluoridated ground water and its toxic effects on domesticated animals residing in rural tribal areas of Rajasthan (India). Intl J Environ Stud, 64(2):151-159.
Publisher | Google Scholor - Choubisa SL. (2010). Osteo-dental fluorosis in horses and donkeys of Rajasthan, India. Fluoride, 43(1):5-10.
Publisher | Google Scholor - Choubisa SL. (2010). Fluorosis in dromedary camels of Rajasthan, India. Fluoride, 43(3):194-199.
Publisher | Google Scholor - Choubisa SL, Modasiya V, Bahura CK, Sheikh Z. (2012). Toxicity of fluoride in cattle of the Indian Thar Desert, Rajasthan, India. Fluoride, 45(4):371-376.
Publisher | Google Scholor - Choubisa SL. (2013). Fluorotoxicosis in diverse species of domestic animals inhabiting areas with high fluoride in drinking waters of Rajasthan, India. Proc Natl Acad Sci, India Sect B: Biol Sci, 83(3):317-321.
Publisher | Google Scholor - Choubisa SL. (2013). Fluoride toxicosis in immature herbivorous domestic animals living in low fluoride water endemic areas of Rajasthan, India: an observational survey. Fluoride, 46(1):19-24.
Publisher | Google Scholor - Choubisa SL. (2021). Chronic fluoride exposure and its diverse adverse health effects in bovine calves in India: an epitomised review. Glob J Biol Agric Health Sci, 10(3): 1-6.
Publisher | Google Scholor - Choubisa SL. (2022). A brief and critical review of chronic fluoride poisoning (fluorosis) in domesticated water buffaloes (Bubalus bubalis) in India: focus on its impact on rural economy. J Biomed Res Environ Sci, 3(1):96-104.
Publisher | Google Scholor - Choubisa SL. (2022). A brief review of chronic fluoride toxicosis in the small ruminants, sheep and goats in India: focus on its adverse economic consequences. Fluoride, 55(4):296-310.
Publisher | Google Scholor - Choubisa SL. (2023). Endemic hydrofluorosis in cattle (Bos taurus) in India: an epitomised review. Int J Vet Sci Technol, 8(1):1-7.
Publisher | Google Scholor - Choubisa SL. (2023). Chronic fluoride poisoning in domestic equines, horses (Equus caballus) and donkeys (Equus asinus). J Biomed Res, 4(1):29-32.
Publisher | Google Scholor - Choubisa SL. (2023). A brief review of endemic fluorosis in dromedary camels (Camelus dromedarius) and focus on their fluoride susceptibility. Austin J Vet Sci & Anim Husb, 10(1):1-6,
Publisher | Google Scholor - Choubisa SL. (2023). A brief and critical review of endemic fluorosis in domestic animals of scheduled area of Rajasthan, India: focus on its impact on tribal economy. Clin Res Anim Sci, 3(1):1-11.
Publisher | Google Scholor - Choubisa SL. (2018). Fluoride distribution in drinking groundwater in Rajasthan, India. Curr Sci, 114(9):1851-1857.
Publisher | Google Scholor - Choubisa SL. (2018). A brief and critical review of endemic hydrofluorosis in Rajasthan, India. Fluoride, 51(1):13-33.
Publisher | Google Scholor - Choubisa SL. (2018). A brief and critical review on hydrofluorosis in diverse species of domestic animals in India. Environ Geochem Health, 40(1): 99-114.
Publisher | Google Scholor - Chakma T, Rao PV, Tiwary RS. (2000). Prevalence of anaemia and infection in tribal areas of Madhya Pradesh. J Med Assoc,98(9):567-570.
Publisher | Google Scholor - Rao VG, Yadav R, Bhondeley MK, Das S, Agrawal MC, Tiwary RS. (2002). Worm infestation and anaemia: a public health problem among tribal pre-school children of Madhya Pradesh. J Commun Dis, 34(2):100-105.
Publisher | Google Scholor - Choubisa SL, Choubisa L. (2006). Intestinal helminthic infections in tribal population of southern Rajasthan, India. J Parasit Dis, 30(2):163-167.
Publisher | Google Scholor - Choubisa SL, Jaroli VJ, Choubisa P, Mogra N. (2012). Intestinal parasitic infection in Bhil Tribe of Rajasthan, India. J Parasit Dis, 36(2):143-148.
Publisher | Google Scholor - Kaliappan SP, George S, Francis MR, Kattula D, Sarkar R, Minz S, et al. (2013). Prevalence and clustering of soil-transmitted helminth infections in a tribal area in southern India. Trop Med Int Health, 18(12):1452-1462.
Publisher | Google Scholor