Review Article
Advanced Grafting Techniques for Mitigating Biotic and Abiotic Stresses in Vegetable Crops: Breeding Strategies and Methodologies
- Prakash Singathiya 1
- Kishor Varotariya 1*
- Gandikota Brahmani 2
- Raman Choudhary 3
- Rameshwar Jangu 4
- Anuj Sohi 5
- Pragya Uikey 6
1ICAR- Indian Institute of Horticultural Research, Bengaluru, India.
2University of Horticultural Sciences, Bagalkot, Karnataka, India.
3Rani Lakshmibai Central Agricultural University, Jhansi, Uttar Pradesh, India.
4Punjab Agricultural University, Ludhiana, Punjab, India.
5Dr. Y.S. Parmar University of Horticulture and Forestry, Nauni, Solan, Himachal Pradesh, India.
6Orissa University of Agriculture and Technology, Bhubaneswar, India.
*Corresponding Author: Kishor Varotariya, ICAR- Indian Institute of Horticultural Research, Bengaluru, India.
Citation: Singathiya P, Varotariya K, Brahmani G, Choudhary R, Jangu R, et al. (2024). Advanced Grafting Techniques for Mitigating Biotic and Abiotic Stresses in Vegetable Crops: Breeding Strategies and Methodologies. Scientific Research and Reports. BioRes Scientia Publishers. 1(6):1-12. DOI: 10.59657/2996-8550.brs.24.029
Copyright: © 2024 Kishor Varotariya, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 15, 2024 | Accepted: September 05, 2024 | Published: November 20, 2024
Abstract
This review assesses the application of vegetable grafting for managing biotic and abiotic stresses in global agriculture. Vegetable grafting, a technique that unites rootstocks and scions, is effective in enhancing crop resilience to challenges such as flooding, soil-borne diseases (e.g., bacterial wilt, root-knot nematodes), and abiotic stresses like salinity and excessive temperatures. Originating in the 1920s with cucurbits, grafting has since been extended to solanaceous crops, including tomatoes and peppers. Grafting provides significant benefits, such as improved disease resistance, increased plant vigor and enhanced yield by 27-50% and fruit quality. However, the rapid evolution of plant pathogens and the complexity of abiotic stress resistance traits pose challenges. The effectiveness of rootstocks is threatened by these evolving pathogens, and the breeding of new rootstocks is hindered by the intricate genetic and physiological factors involved. The lack of specific markers for physiological traits complicates the selection process. It also included the methodology and timeline of grafting, which operates at different times with specific climates. This review highlights the need for ongoing research and technological advancements to develop new rootstocks and optimize grafting techniques. It underscores the importance of breeding efforts and the development of genetic markers to streamline rootstock selection, thereby improving the resilience and productivity of vegetable crops in diverse environmental conditions.
Keywords: grafting; rootstock; scion; climate resilience; biotic; abiotic
Introduction
Environmental factors exert varying timeframes of stress on crops. For instance, air temperature can become stressful within minutes, while soil water content issues may develop over days to weeks. Soil mineral deficiencies can take months to manifest. Abiotic stresses, such as temperature extremes (heat, cold, frost), water imbalances (drought, flooding, hypoxia), radiation (UV, ionizing radiation), chemical imbalances (mineral deficiencies or excesses, pollutants, heavy metals, pesticides), and mechanical factors (wind, soil movement, submergence), significantly impact agricultural productivity, accounting for over 50% reduction in crop yields [1]. Specifically, high temperatures, salinity, drought, and low temperatures contribute to 40%, 20%, 17%, and 15% reductions, respectively [2]. Currently, only 9% of global land is ideal for crop cultivation, with the remaining 91% experiencing various stressors. In India, ICAR estimates (2015) indicate that 36.5% of the land area, or 120.8 million hectares, is degraded due to issues such as soil erosion, salinity, alkalinity, acidity, and waterlogging. The high market demand for vegetables and limited cultivated land expose solanaceous crops (tomato, brinjal, and chili) and cucurbits (cucumber, melon, and watermelon) to various unfavorable soil and environmental conditions, including thermal stress, water stress, salinity, and organic pollutants.
Global climate change poses a significant abiotic threat to both plant and human health, with major implications for agricultural practices [3-5]. Addressing the challenge of increasing agricultural output amid climate change necessitates innovative approaches [6]. Environmental stressors are a leading cause of crop failures, typically resulting in a 50% reduction in yield [7]. Vegetables, being highly susceptible to a range of abiotic stresses such as drought, salinity, flooding, and extreme temperatures, are particularly vulnerable [6]. Key factors affecting vegetable yield include photoperiod reduction, water availability, and poor vernalization [8]. Globally, approximately 392 vegetable species from 70 plant families and 225 genera are cultivated [9]. These vegetables are highly sensitive to climate change, which can impact them biochemically, anatomically, morphologically, and physiologically [10, 11]. While some metals and biochemicals, such as silicon and phenolic acids, can help alleviate stress [12], increased temperatures and humidity may exacerbate pest and disease pressures [13]. Food security is at risk due to changing climatic conditions, which directly or indirectly affect crop production [14]. For instance, elevated CO2 concentrations can enhance photosynthesis and growth in vegetable crops, such as grafted peppers, improving their acclimatization and growth rates [15,16]. Temperature fluctuations and irregular rainfall significantly impact vegetable crop growth and yield, making climate change a critical issue for vegetable production compared to other crops [17, 18]. Effective management of abiotic stresses is essential for sustaining vegetable production, with ongoing research into breeding and genetic engineering offering potential solutions, though commercialization has been limited by the complexity of stress resistance traits [19]. Traditional breeding methods, characterized by lengthy breeding cycles, progress at a slow pace [20]. In contrast, grafting: where a scion is grafted onto a compatible rootstock offers a more rapid and effective solution to environmental stress management. This technique involves attaching susceptible or sensitive cultivars to resilient rootstocks, enhancing stress tolerance [21-23]. Grafting is a sustainable, efficient, and integrative process where both the scion and rootstock contribute to the overall resilience of the grafted plant [24]. Initially applied to watermelon in Japan, where it was used to combat Fusarium wilt by grafting onto pumpkin rootstocks, this method was traditionally associated with woody perennials but has gained prominence in managing soil-borne diseases in cucurbits [25, 26]. Today, grafting is extensively utilized by researchers to enhance environmental stress tolerance and improve yield and fruit quality in solanaceous and cucurbitaceous crops [27, 28]. It has become a prevalent practice in Asia, Europe, and the USA [29].
History
Scientific vegetable grafting began in the late 1920s in Japan and Korea, where watermelon was grafted onto gourd rootstocks to manage soilborne diseases [30]. By the early 1930s, Japan had commercialized grafting watermelon onto bottle gourd (Lagenaria siceraria) and summer squash (Cucurbita moschata) to enhance resistance to Fusarium wilt [31]. In the 1950s, aubergine (Solanum melongena) was first grafted onto scarlet aubergine (Solanum integrifolium) [32]. The practice of grafting tomatoes (Solanum lycopersicum) began in the 1960s, driven by the high demand for grafted vegetables in East Asia, which remains the largest market for this technique [33].
In India, grafting research has been initiated by Dr. Bhatt and his team at the IIHR in Bangalore, with studies on brinjal grafting using Solanum nigrum as the rootstock at TNAU, Coimbatore. Additionally, cucurbit grafting at the NBPGR regional station in Thrissur, Kerala, achieved a 98% success rate using Momordica cochinchinensis as rootstock. CSKHPKV, Palampur, has also conducted grafting research on cucurbits and solanaceous crops, identifying over 22 rootstocks that provide resistance to bacterial wilt and nematodes. Private companies such as "VNR Seed Private Limited" and "Takii Seed India Private Limited" are now specializing in vegetable grafting and seedling supply [34].
Why graft? Top of Form
Vegetable grafting, was initially employed to produce large gourds for rice storage [35], has evolved into a versatile technique used to manage soil-borne and foliar diseases, enhance plant vigor, extend harvesting periods, improve yield and fruit quality, and extend postharvest life across various countries. This method has become a distinctive cultural practice that reduces pesticide dependency, boosts yield and production efficiency, and enhances economic viability in both open-field and protected cultivation systems [36].
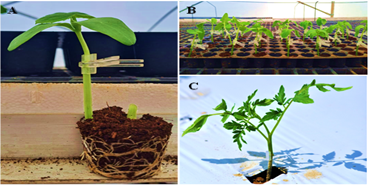
Figure 1: Newly grafted cucumber (A&B) and tomato (C) at ICAR-IIHR developed Centre of Excellence (COE) on protected cultivation of horticultural crops, ICAR-IIHR, Bangalore
In vegetable production, grafting merges the advantageous traits of two plants vigorously growing rootstocks and high-yielding scions to bolster crop resilience and productivity. Scientifically, grafting resists soil-borne pathogens, pests, and nematodes while improving tolerance to abiotic stresses such as drought, salinity, and extreme temperatures. It also enhances nutrient and water uptake, resulting in healthier plants and increased yields. Moreover, grafting reduces the reliance on chemical inputs, thereby supporting sustainable agricultural practices. By leveraging genetic synergy to optimize plant performance, vegetable grafting addresses essential challenges in contemporary horticulture.
Purpose of Vegetable Grafting
Tolerance to Soil-Borne Diseases
Grafting is an effective strategy for managing soil-borne diseases, such as Fusarium wilt in cucurbitaceous crops (e.g., cucumber, melon) and bacterial wilt in solanaceous crops (e.g., tomato, pepper). It is also useful against phytophthora blight in various crops [32, 37]. This technique provides a rapid and efficient means of controlling Fusarium oxysporum f.sp. melonis races 1 and 2 in melon [38].
Increased Plant Vigor
Grafted plants generally exhibit greater vigor compared to non-grafted plants. This is due to the more robust root systems of selected rootstocks, which enhance the absorption of water and nutrients. As a result, irrigation and pesticide applications can be reduced, as observed in grafted cucumber plants [39].
Enhanced Yield Performance
Grafting has been employed to improve crop yields in soils with suboptimal productivity. Research from Korea and Japan demonstrated that grafted tomato, melon, pepper, eggplant, and watermelon plants produced 25 to 50 percent higher yields compared to non-grafted counterparts. Similar yield increases have been reported in watermelon, cucumber, melon, pepper, and eggplant [33, 40, 41].
Improvement of Qualitative and Quantitative Traits
The root system plays a significant role in determining the quality of fruit produced. Grafting eggplants onto Solanum torvum has been shown to increase fruit size without compromising quality. The choice of rootstock can affect various quality attributes, including sugar content, flavor, colour, carotene levels, and texture [42, 43]. Quality characteristics such as fruit shape, skin colour, smoothness, and soluble solids concentration are influenced by the rootstock, which translocates essential solutes through the scion via the xylem [44].
Table 1: Standardized grafting techniques at Indian Institute of Horticulture
| Scion plant | Rootstock | Method of grafting |
| Eggplant | Solanum torvum | Tongue and cleft method |
| Solanum sissymbrifolium | Cleft method | |
| Solanum khasianum | Both tongue and cleft methods | |
| Tomato | Solanum pimpinellifolium | Cleft method |
| Solanum nigrum | Tongue and cleft methods | |
| Sweet pepper | Capsicum annum | Splice grafting |
| Cucumber | Cucurbita moschata | Hole insertion and tongue method |
| Cucurbita maxima | Tongue method | |
| Cucurbita ficifolia | Hole insertion and tongue method | |
| Watermelon | Benincasa hispida | Hole insertion and tongue method |
| C. moschata | Hole insertion and tongue method | |
| C. melo | Cleft method | |
| C. moschata × C. maxima | Hole insertion method | |
| Lagenaria siceraria | Splice grafting | |
| Sicyos angulatus | Hole insertion and cleft method | |
| Bitter gourd | C. moschata | Hole insertion and tongue method |
| Lagenaria siceraria | Hole insertion method | |
| Bottle gourd | C. moschata, Luffa sp. | Hole insertion and tongue method |
| Pak-Choi | R. sativus var. longipinnatus | Splice grafting |
Prerequisites for Grafting
- Choice of Rootstock and Scion: Ensure the rootstock and scion are compatible for successful grafting.
- Grafting Tools: Utilize various tools such as grafting clips, tubes, pins, and blades to aid in the grafting process.
- Screening House: Use a controlled environment to cultivate seedlings before they undergo grafting.
- Healing Chamber: Maintain a temperature of 28-29°C, relative humidity of 90-95%, and complete darkness for the initial 1-2 days to support callus development and acclimatization of the grafted seedlings over a period of 5-7 days.
Steps in Grafting
- Selection of Rootstock and Scion Species: Choosing appropriate rootstock and scion species is crucial for successful grafting. The compatibility between the two is essential for establishing a healthy graft union.
- Creation of a Graft Union: This involves physically aligning and joining the rootstock and scion. The graft union is created by making precise cuts on both the rootstock and scion to ensure a successful union.
- Healing of the Union: After creating the graft union, it must heal properly. This step involves maintaining appropriate conditions to promote the formation of vascular connections between the rootstock and scion.
- Acclimatization of the Grafted Plant: Once the graft has healed, the grafted plant is gradually acclimated to its environment to ensure it can thrive.
Grafting Methods
Grafting methods vary based on factors such as the number of grafts, the purpose of grafting, labour availability, and access to machinery and infrastructure [36].
Tongue/Approach Grafting
This method involves using rootstock and scion of similar size. Scion seeds are planted 5-7 days prior to rootstock seeds to achieve synchronized growth. The grafting cut is made at a 30–40° angle relative to the perpendicular axis. Often, one cotyledonary leaf is removed to avoid crowding. The scion is inserted into the rootstock, and specially designed grafting clips secure the union [41].
Cleft/Apical/Wedge Grafting
In cleft grafting, the lower stem of the scion is cut at a slant to form a wedge. This wedge is inserted into a split made in the rootstock, and a clip is used to hold the scion and rootstock together [32]. This method is particularly common for solanaceous crops.
Hole/Top Insertion Grafting
This method is often used for grafting watermelon due to the smaller size of watermelon seedlings compared to the larger rootstocks like bottle gourd or squash. Optimal temperatures of 21-36°C are required during transplanting to ensure successful grafting. This technique is widely practiced in China [45].
Splice Grafting/Tube Grafting/One Cotyledon Splice Grafting
Splice grafting involves making a slanted cut (35–45°) on the rootstock to remove the growth point and one cotyledonary leaf. A prepared scion is then matched to this cut. This method is extensively used by commercial producers for cucurbit and solanaceous crops [41].
Pin Grafting
Pin grafting employs specially designed pins to hold the grafted position instead of traditional grafting clips. The Takii Seed Company in Japan has developed ceramic pins with a hexagonal cross-section, measuring 15 mm in length and 0.5 mm in width. This method reduces time and effort as the pins do not need to be removed like clips [41].
Mechanical Grafting
Grafting machines and robots are increasingly used in modern grafting procedures. The first robotic 'one-cotyledon grafting method' for cucurbit vegetables was developed by Iam Brain in Japan during the 1980s, with prototypes created in 1987 and modified in 1989. This automated method allows for a transplant to be completed in 4.5 seconds with a 95% success rate [46].
Post-Graft Healing Environment
The success of grafting is significantly influenced by the maintenance of the grafted plants in their initial stages. During the first two days post-grafting, scions are highly susceptible to water loss, which can lead to wilting and potentially cause graft failure. To mitigate this risk, it is essential to maintain high humidity levels to prevent excessive water loss, ideally ensuring that it does not exceed 5% [47]. To enhance humidity, grafted plants should be covered with a black plastic sheet for 5-7 days after grafting. This practice helps to maintain moisture, reduce light intensity, and facilitate the healing process. Additionally, during the healing phase, it is crucial to protect the grafted plantlets from direct sunlight to prevent stress and promote successful graft union formation.
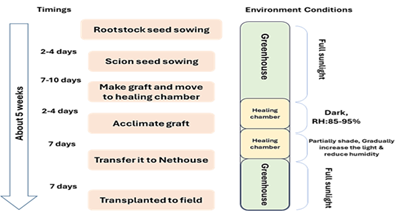
Figure 2: Grafting timeline for vegetable crops
Cucurbitaceae
The practice of grafting cucurbitaceous crops began in the 1920s, with Cucurbita moschata used as a rootstock for watermelon. In Greece, grafting has emerged as a viable alternative to methyl bromide for managing soil-borne diseases in cucumbers. Among the various soil-borne diseases affecting cucurbit crops, Fusarium wilt, caused by Fusarium oxysporum, is one of the most widespread and damaging [26]. At the genetic level, microRNAs (miRNAs) play a crucial role in regulating plant growth and development, and they exhibit specific responses to various environmental stresses. According to Li et al. [48], when watermelon (Citrullus lanatus) was grafted onto bottle gourd (Lagenaria siceraria) or squash (Cucurbita maxima × Cucurbita moschata) rootstocks, the expression of over 40 miRNAs was altered. Further investigations into the molecular mechanisms of 17 selected miRNAs in grafted cucumber plants under drought stress were conducted using pumpkin (Cucurbita moschata) rootstock. The study, which involved mini-watermelon cv. Ingrid grafted with rootstock ‘PS 1313’ (Cucurbita maxima × Cucurbita moschata), found that the grafted plants had improved yield, nutritional content, and fruit quality compared to non-grafted plants. However, there was no significant difference in gas exchange and leaf water relations between grafted and non-grafted plants. Despite similar water stress sensitivity, grafted plants achieved a higher marketable yield. This research highlighted the benefits of rootstock ‘PS 1313’ and supported the broader recommendation of using grafted rootstocks, particularly under drought conditions, to manage drought stress [49]. Additionally, another study comparing drought-tolerant rootstocks for watermelon found that wax gourd was superior to bottle gourd in drought-prone environments [50].
Cucurbita spp
The genus Cucurbita encompasses several widely used rootstocks, including those for watermelon, bottle gourd, and cucumber. Interspecific crosses within this genus are an effective method for developing novel genotypes that combine desirable traits from different parent species. Cucurbita maxima serve as a valuable bridge for cross-species grafting due to its compatibility with various Cucurbita species. Cucurbita moschata is noted for its resilience to both biotic and abiotic stresses [51]. Grafting melons and watermelons onto hybrid Cucurbita rootstocks has been shown to reduce the incidence of Fusarium wilt and improve yields [52]. Additionally, grafted watermelons demonstrate enhanced resistance to Verticillium wilt [53].
Table: 2
| Rootstock | Scion | Biotic stress resistance |
| C. maxima × C. moschata | Cucumber, Melon, Watermelon | Fusarium wilt |
| Fig leaf gourd | Cucumber, Melon | Fusarium wilt, Phytophthora and RKN |
| Luffa cylindrica | Cucumber | RKN |
| Burr cucumber | Cucumber | Fusarium wilt and nematode resistance, low-temperature tolerance |
| Cucurbita ficifolia | Cucumber, Watermelon | Fusarium wilt |
| Cucumus pustules | Cucumber | Fusarium wilt and RKN |
Bottle Gourd (Lagenaria siceraria)
Since the 1920s, Lagenaria siceraria (bottle gourd) has been employed as a rootstock in grafting. Screening of bottle gourd germplasm collections has identified several accessions with notable tolerance to various stresses, including flooding, powdery mildew, whitefly, zucchini yellow mosaic virus, Fusarium wilt, Verticillium wilt, and other soil-borne diseases [54, 41]. Due to quality concerns associated with Cucurbita spp. and their interspecific hybrids, L. siceraria has become a preferred rootstock, especially for watermelon. L. siceraria is effective in managing soil-borne diseases and tolerating low soil temperatures [55]. Evaluations of rootstock potential have assessed graft compatibility, plant growth, and resistance to Fusarium wilt, leading to the selection of several promising accessions [54]. Furthermore, bottle gourd rootstocks have been found to effectively control Verticillium wilt in watermelon [53].
Cucumis spp
Lagenaria siceraria has been suggested as a rootstock for cucumber, melon, and watermelon to manage root-knot nematodes and Fusarium wilt [56]. Additionally, Cucumis melo rootstocks offer an alternative to Cucurbita genotypes for providing resistance to Fusarium wilt, nematodes, Monosporascus root rot, and vine decline, while enhancing marketable yield without compromising fruit quality [57]. Among Cucumis species, Cucumis metuliferus stands out as a particularly promising rootstock. Melons grafted onto C. metuliferus accessions show resistance to nematodes [58]. Cucumis sativus var. hardwickii has also been evaluated as a rootstock for cucumbers [59]. Furthermore, Cucumis ficifolius, C. zeyheri, C. africanus, C. anguria, and C. myriocarpus are among the Cucumis species that provide resistance to nematodes and/or Fusarium wilt [60].
Table 3
| Rootstock | Scion | Biotic stress Resistance |
| Cucumis pustulatus | Melon, Cucumber, Watermelon | Fusarium wilt, RKN |
| CNPH 01-962 and CNPH 01-963 C. melo subsp. melo | Melon | RKN |
| C. melo | Cucurbita | Monosporascus root rot |
| C. metuliferus | Melon, Cucumber | RKN |
| C. ficifolius, C. zeyheri, C. africanus, C. anguria and and C. myriocarpus | Melon, Cucumber | Fusarium and RKN |
| Pumpkin, squash, fig leaf gourd, cucumber, bottle gourd | Melon | Fusarium wilt, Gummy Stem blight |
| Cucurbita martinezi | Melon | Powdery mildew, verticilium wilt |
| Momordica charantia | Melon | RKN |
| C. sativus var hardwickii | Cucumber, Melon | Fusarium wilt, RKN |
Watermelon (Citrullus lanatus)
The citron (Citrullus lanatus var. citroides) has emerged as a promising rootstock for watermelon due to its lower susceptibility to galling compared to Cucurbita hybrids and bottle gourd rootstocks [61]. It is particularly effective in managing root-knot nematodes and enhancing resistance to Fusarium wilt in watermelon scions [52]. Recent screenings of foreign Citrullus collections and their horticultural evaluations have identified novel and potentially valuable rootstock sources for watermelon [62].
Table 4
| Rootstock | Scion | Biotic stress resistant |
| C. lanatus var. citroides | Watermelon | RKN |
| Citron | Watermelon | Fusarium wilt |
| Pumpkin (Cucubita moschata) | Watermelon | RKN, Fusarium wilt |
| Bottle gourd | Watermelon | Fusarium spp. |
| Squash (Cucurbita moschata) | Watermelon | Fusarium wilt |
| Cucumis metuliferus | Watermelon | RKN |
| Pumpkin | Watermelon | Successful grafting |
| B. hispida | Watermelon | Fusarium spp., Verticillium spp. and nematodes |
Solanaceae
The practice of grafting within the Solanaceae family began in the 1950s with the grafting of eggplant (Solanum incanum) onto scarlet eggplant (Solanum integrifolium) [32]. Today, tomato, eggplant, and pepper are among the most frequently grafted solanaceous crops. Similar to cucurbits, the primary motivation for grafting these crops is to enhance resistance to soil-borne diseases and nematodes [63].
Solanum lycopersicum
Among the genus Solanum, rootstocks derived from Solanum lycopersicum (tomato) are extensively utilized, with Solanum species also commonly used for eggplant rootstocks [64]. Interspecific hybrids used as rootstocks can enhance fruit quality by increasing the accumulation of macro and microelements, phenolic compounds, vitamin C, lycopene, and flavonoids in the fruit of grafted plants [65]. In a 2015 study conducted by Bahadur et al., tomato hybrids such as Arka Rakshak and Arka Samrat were grafted onto various eggplant rootstocks (IC-354557, IC-111056, IC-374873, and CHBR-2) and subjected to waterlogged conditions. The results showed that these grafted plants did not exhibit symptoms of leaf chlorosis or wilting, and there was a minimal decrease in chlorophyll levels throughout all stages of growth.
Different tomato cultivars and rootstocks have shown diverse physiological responses. Rivero et al. [66] reported that tomato cv. RX-335 exhibited increased PAL activity and elevated levels of total phenols and o-diphenols, but also decreased PPO and GPX activity, and reduced dry weight. Bloom et al. [67] found that grafted tomato cv. LA1778 maintained root hydraulic and stomatal conductance. Zhou et al. [68] noted that grafting cucumber cv. Jinyan No. 4 onto Figleaf Guard (Cucurbita ficifolia) resulted in less reduction in carboxylation and RuBisCO activity, enhancing CO2 assimilation. Venema et al. [69] observed that grafting tomato cv. Moneymaker with Solanum habrochaites LA1777 increased root mass ratio and total leaf carbon concentration. The grafted tomato cv. UC 82-B with eggplant cv. Black Beauty had larger leaf area, higher leaf fresh and dry weight, increased chlorophyll fluorescence, and more pollen grains per flower [70].
Table 5
| Rootstock | Scion | Biotic stress resistance |
| Jimson weed (Datura stramonium L.) | Tomato | RKN |
| Maxifort’ (S. lycopersicum × S. habrochaites) | Tomato | Fol races 1 and 2 and crown rot |
| CRA 66 or Hawaii 7996 | Heirloom tomatoes | Fusarium wilt |
| Solanum pimpinellifolium, S. pennellii, S. Chilense | Tomato | Tomato yellow leaf curl virus |
| Capsicum annuum (Chilli) | Tomato | Phytophthora blight, bacterial wilt |
| S. melongena (Brinjal) | Tomato | Bacterial wilt |
| S. lycopersicum | Tomato | Vertcilium wilt |
| Capsicum chacoence (Chilli) | Tomato | RKN |
| EG195 and EG203 | Tomato | Fusarium wilt |
| German Johanson | GRA 66 | TSWV |
| Solanum torvum | Kashi Aman, Kashi Vishesh and Hissar Lalit | RKN |
| BN10-2Hawai-7996 | Arkasamrat | Bacterial wilt, Rkn |
| Beufort, Maxifort, Big power, Robusta, RST-04-10 | Tomato | TMV, Corky Root, Fusarium Wilt, Verticillium Wilt, RKNp |
Solanum melongena
Aubergine (Solanum melongena) and its wild relatives are commonly used as rootstocks for both tomato and aubergine cultivation. In certain conditions, S. melongena can serve as an alternative rootstock for tomatoes, providing resistance to bacterial wilt (Ralstonia solanacearum) [71]. Solanum torvum, a wild species native to India and closely related to cultivated aubergine, is also utilized as a rootstock due to its resistance to various soil-borne diseases, including root-knot nematodes [72] and bacterial wilt [73]. Recently, the AVRDC has suggested the rootstock VI006378 for enhancing flood tolerance in tomatoes for East Asia. For eggplants, the recommended rootstocks are VI045276, VI046103, VI034845, VI046104, and VI046101 [74]. Rootstocks from Solanum aethiopicum have been shown to improve fruit set, quantity, and mass in tomato scions, as well as enhance disease resistance and extend fruit shelf life. However, they do not affect Brix or acidity levels [71]. Additionally, accessions of S. aethiopicum have demonstrated resistance to three tobacco viruses and pepper mild mottle virus [75].
Table 6
| Rootstock | Scion | Biotic stress resistance |
| S. Melongena | Brinjal, Tomato | Bacterial Wilt, Corky root rot |
| S. torvum | Brinjal, Tomato | Soil born and RKN |
| S. aethiopicum | Brinjal | Tobacco viruses, Fusarium wilt |
| S. aethiopicum | Chilli | Pepper mild mottle virus |
| EG195 and EG203 | Brinjal | Bacterial wilt, RKN |
| Jimson weed (Datura stramonium L.) | Brinjal | RKN |
| Wild brinjal | M-9 Brinjal | Bacterial wilt |
Capsicum annuum
Cultivars and intraspecific hybrids of Capsicum annuum are commonly used as rootstocks for pepper scions. However, accessions from other Capsicum species, such as C. baccatum, C. chinense, and C. frutescens, as well as their hybrids with C. annuum, have also been evaluated for their suitability as rootstocks [36]. Penella et al. [76] investigated the water stress responses of different pepper cultivars, specifically Capsicum annum L. cv. Verset and Capsicum annum L. cv. Atlante, PI-15225, and ECU-973. Their study found that these cultivars demonstrated efficient osmotic adjustment under water stress conditions. This adaptation enabled the peppers to maintain optimal functionality of their photosynthetic machinery despite the lack of water. The ability to regulate osmotic pressure effectively helps these cultivars sustain photosynthesis and overall plant health, highlighting their potential resilience and adaptability to challenging environmental conditions. Genotypes of C. annuum and C. frutescens exhibit moderate to high resistance to Meloidogyne incognita and Meloidogyne javanica, but they are highly sensitive to Meloidogyne enterolobii [77]. Conversely, C. baccatum has been tested for graft compatibility and resistance to Meloidogyne nematodes, showing potential for effective use as a rootstock [78]. Additionally, C. frutescens genotypes have been employed as rootstocks for sweet peppers and demonstrated good resistance to M. incognita [77]. C. chinense genotypes are also currently being explored for their rootstock potential.
Table 7
| Rootstock | Scion | Biotic stress resistance |
| C. annum | Chilli | F. oxysporum, M. incognita and M. javanica |
| SCM334 | Chilli | Root rot, Wilt |
| C. baccatum | Chilli | M. javanica |
| C. frutescent | Chilli | M. incognita |
| PR 920, PR 921, PR 922 | Nokkwang | Phytophthora blight, Bacterial wilt |
| Arka Harita | Chilli | Phytophthora root rot, RKN |
| Arka Mohini | Chilli | Bacterial Wilt |
Challenges in Grafting
- Lack of Knowledge: Farmers in remote areas may lack sufficient information about rootstock-scion compatibility, impacting their ability to effectively utilize grafting techniques.
- Limited Adoption: While greenhouse hydroponic tomato producers are currently the primary users of grafted seedlings, open-field vegetable growers often remain unfamiliar with grafting procedures.
- Synchronization Requirements: Successful grafting requires precise synchronization between rootstocks and scions, along with high germination rates and successful establishment post-transplantation. Achieving these conditions consistently can be challenging.
- Environmental Control: Optimal graft establishment demands controlled temperature, humidity, and light conditions during the post-grafting phase, which can be difficult to maintain in open-field environments.
- Handling and Vulnerability: Newly grafted seedlings are delicate and susceptible to diseases and pests, making their handling and care a significant challenge.
- Need for Skilled Personnel: The grafting process requires trained professionals to ensure success. The scarcity of skilled staff, particularly in rural areas, poses a substantial obstacle.
- Time Consumption: Grafting is a labor-intensive propagation method, which can be time-consuming and resource-demanding.
- Consistency in Production: Large-scale growers often face difficulties in achieving uniform production of high-quality rootstocks, which can hinder overall grafting success.
Conclusion
The grafting genetically diverse vegetable crops presents a promising approach for mitigating environmental stressors in the face of climate change. To fully realize the benefits of grafting, future research should focus on evaluating diverse germplasm for viable rootstocks and developing automated grafting systems that enhance scalability and accessibility. Key factors such as scion-rootstock compatibility, geographical conditions, and the interplay between shoot and root systems must be carefully considered. Advancements in grafting technology can make this technique more economically feasible and integral to modern vegetable production, helping farmers adapt to climate change and promote sustainable agricultural practices worldwide.
References
- Wang C. (2007). Variability of the Caribbean low-level jet and its relations to climate. Clim Dyn., 29: 411-422.
Publisher | Google Scholor - Ashraf M, Athar HR, Harris PJC, Kwon, TR. (2008). Some prospective strategies for improving crop salt tolerance. Adv Agron., 97:45-110.
Publisher | Google Scholor - Costello A, Abbas M, Allen A, Ball S, Bell S, Bellamy R et al. (2009). Managing the health effects of climate change: lancet and University College London Institute for Global Health Commission. The Lancet., 373(9676): 1693-1733.
Publisher | Google Scholor - Olesen JE, Bindi M. (2002). Consequences of climate change for European agricultural productivity, land use and policy. Eur J Agron., 16(4): 239-262.
Publisher | Google Scholor - Baer P, Risbey JS. (2009). Uncertainty and assessment of the issues posed by urgent climate change. An editorial comment. Clim Change., 92(1): 31-36.
Publisher | Google Scholor - McMichael P. (2007). Feeding the world: agriculture, development and ecology. Social Regist., 43: 1-24.
Publisher | Google Scholor - Reimers, F. 1st ed. (2000). Unequal schools, unequal chances: The challenges to equal opportunity in the Americas (Vol. 5). Cambridge, MA: Harvard University Press.
Publisher | Google Scholor - Sharma A, Kiran R. (2013). Corporate social responsibility: Driving forces and challenges. Int J Bus Res Dev., 2(1): 18-27.
Publisher | Google Scholor - Kays SJ, Dias JCS. (1995). Common names of commercially cultivated vegetables of the world in 15 languages. Econ Bot., 1:115-152.
Publisher | Google Scholor - Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, et al. (2017). Crop production under drought and heat stress: plant responses and management options. Front Plant Sci., 8: 1147.
Publisher | Google Scholor - Singh A, Sharma S, Singh B. (2017). Effect of germination time and temperature on the functionality and protein solubility of sorghum flour. J Cereal Sci., 76: 131-139.
Publisher | Google Scholor - Kaushik P, Saini DK. (2019). Silicon as a vegetable crops modulator - A review. Plants., 8(6): 148.
Publisher | Google Scholor - Chauhan BS, Mahajan G, Randhawa RK, Singh H, Kang MS. (2014). Global warming and its possible impact on agriculture in India. Adv Agron., 123: 65-121.
Publisher | Google Scholor - Schmidhuber J, Tubiello FN. (2007). Global food security under climate change. Proc Natl Acad Sci., 104(50): 19703-19708.
Publisher | Google Scholor - Xu Z, Jiang Y, Jia B, Zhou G. (2016). Elevated-CO2 response of stomata and Its dependence on environmental factors. Front Plant Sci., 7: 1-15.
Publisher | Google Scholor - Jang Y, Mun B, Do K, Um Y, Chun C. (2014). Effects of photosynthetic photon flux and carbon dioxide concentration on the photosynthesis and growth of grafted pepper transplants during healing and acclimatization. Hortic Environ Biotechnol., 55: 387-396.
Publisher | Google Scholor - Wurr DCE, Fellows J, Fuller MP. (2004). Simulated effects of climate change on the production pattern of winter cauliflower in the UK. Sci Hortic., 101(4): 359-372.
Publisher | Google Scholor - Rao CS, Gopinath KA, Prasad JVNS, Singh AK. (2016). Climate resilient villages for sustainable food security in tropical India: concept, process, technologies, institutions, and impacts. In: Advances in Agronomy (D. Sparks, ed.). Elsevier; 101-214.
Publisher | Google Scholor - Colla G, Rouphael Y, Leonardi C, Bie Z. (2010). Role of grafting in vegetable crops grown under saline conditions. Sci Hortic., 127(2): 147–155.
Publisher | Google Scholor - Atlin G, Cairns J, Das B. (2017). Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob Food Secur., 12:31-37.
Publisher | Google Scholor - Edelstein M. (2017). Grafting vegetable-crop: pros and cons. Acta Hortic., 235-238.
Publisher | Google Scholor - Altunlu H, Gul A. (2012). Increasing drought tolerance of tomato plants by grafting. Acta Hortic., 960: 183-190.
Publisher | Google Scholor - Nilsen ET, Freeman J, Grene R, Tokuhisa J. (2014). A rootstock provides water conservation for a grafted commercial tomato (Solanum lycopersicum L.) line in response to mild-drought conditions: a focus on vegetative growth and photosynthetic parameters. PLoS One., 9(12): e115380.
Publisher | Google Scholor - Sakata Y, Ohara T, Sugiyama M. (2007). The history and present state of the grafting of cucurbitaceous vegetables in Japan. Acta Hortic., 731: 159-170.
Publisher | Google Scholor - He Y, Zhu Z, Yang J, Ni X, Zhu B. (2009). Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ Exp Bot., 66(2): 270-278.
Publisher | Google Scholor - Davis A, Perkins-Veazie P, Sakata Y, Lopez-Galarza S, Maroto J, Lee SG, et al. (2008). Cucurbit grafting. Crit Rev Plant Sci., 27(1): 50-74.
Publisher | Google Scholor - Mohamed F, El-Hamed K, Elwan M, Hussien MA. (2012). Impact of grafting on watermelon growth, fruit yield and quality. Veg Crops Res Bulletin., 76: 99-118.
Publisher | Google Scholor - Johnson S, Inglis D, Miles C. (2014). Grafting effects on eggplant growth, yield, and verticillium wilt incidence. Int J Veg Sci., 20(1): 3-20.
Publisher | Google Scholor - Witzel K, Neugart S, Ruppel S, Schreiner M, Wiesner M, Baldermann S. (2015). Recent progress in the use of ‘omics’ technologies in brassicaceous vegetables. Front Plant Sci., 6: 244.
Publisher | Google Scholor - Ashita E. (1927). Grafting of Watermelons Korea (Chosun). Agricl Newsltr., 1: 1-9.
Publisher | Google Scholor - Oda M. (2002). Grafting of vegetable crops. Scientific report of the graduate school of agriculture and biological sciences, Osaka Prefecture University., 54: 49-72.
Publisher | Google Scholor - Oda M. (1999). Grafting of vegetables to improve greenhouse production. Food and Fertilizer Technology Center Extension Bulletin, 1-11.
Publisher | Google Scholor - Lee JM, Oda M. (2003). Grafting of herbaceous vegetables and ornamental crops. Hort W., 28: 201-209.
Publisher | Google Scholor - Kumar R, Rajasree V, Sagar L, Ahuja A, Savithiri N, Karthick K, Mehta A, Saini R. (2018). Vegetable grafting: A recent advance in olericulture: A review. Int J Curr Microbiol App Sci., 7: 1877-1882.
Publisher | Google Scholor - Hong MS, Lee. (1710) Forest Economics 1: 38-39.
Publisher | Google Scholor - Lee JM, Kubota C, Tsao SJ, Bie Z, Echevarria PH, Morra L, Oda M. (2010). Current status of vegetable grafting: diffusion, grafting techniques, automation. Sci Hort., 127: 93-105.
Publisher | Google Scholor - Jang Y, Cho M, Um Y, Ko K, Chun C. (2012). Effect of grafting on growth and incidence of Phytophtora blight and bacteria wilt pepper (Capsicum annuum L.). Hort Env Biot., 53: 9-19.
Publisher | Google Scholor - Nisini PT, Colla G, Granati E, Temperini O, Crino P, Saccardo F. (2002). Rootstock resistance to fusarium wilt and effect on fruit yield and quality of two muskmelon cultivars. Sci Hortic., 93(3-4): 281-288.
Publisher | Google Scholor - Mohammadi H, Salehi R, Esmaeili M. (2015). Yield and fruit quality of grafted and non-grafted muskmelon (Cucumis melo L.) affected by planting density. Acta Hort., 247-254.
Publisher | Google Scholor - Kacjan-marsic N, Osvald J. (2004). The influence of grafting on yield of two tomato cultivars (Lycopersicon esculentum Mill.) grown in a plastic house. Acta Agric Slov., 83(2), 243-249.
Publisher | Google Scholor - Colla G, Perez-Alfocea F, Schwarz D. (2017). Vegetable Grafting: Principles and Practices. CABI., 30-51.
Publisher | Google Scholor - Flores FB, Sanchez-Bel P, Estan MT, Martinez-Rodriguez MM, Moyano E, Morales B. (2010). The effectiveness of grafting to improve tomato fruit quality. Scia Hort., 125: 211–217.
Publisher | Google Scholor - Davis AR, Perkins-Veazie P, Sakata Y, Lopez-Galarza S, Maroto JV, Lee SG, et al. (2008). Cucurbit grafting critical reviews in plant sciences. Tyl Frs Onl; 27: 50-74.
Publisher | Google Scholor - Nicoletto C, Tosini F, Sambo P. (2013). Effect of grafting and ripening conditions on some qualitative traits of ‘Cuore di bue’ tomato fruits. J Sci Food Agri., 93: 1397-1403.
Publisher | Google Scholor - Oda M. (1994). Effects of uniconazole and gibberellic acid application on elongation of hypocotyls and internodes in fig leaf gourd for rootstock. Japan Agri Res Qua., 28: 195-199.
Publisher | Google Scholor - Ito T. (1992). Present state of transplant production practices in Japanese horticultural industry. In: Kurata K and Kozai T transplant production system, Yokohama. Klr Acd Pub., 65-82.
Publisher | Google Scholor - Dong W, Zhou ZC, Bu YL, Zhuo JQ, Chen LZ, Li YZ. (2015). Research and application of grafted seedlings healing room. Acta Hort., 1: 51-57.
Publisher | Google Scholor - Li, C., Li, Y., Bai, L., He, C. & Yu, X. (2016). Dynamic expression of miRNAs and their targets in the response to drought stress of grafted cucumber seedlings. Hort Plant J., 2(1): 41-49.
Publisher | Google Scholor - Rouphael Y, Cardarelli M, Colla G, Rea E. Yield, (2008). mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. Hort Sci., 43(3): 730-736.
Publisher | Google Scholor - Muneer S, Ko CH, Wei H, Chen Y, Jeong BR. (2016). Physiological and proteomic investigations to study the response of tomato graft unions under temperature stress. PLoS One., 11(6): e0157439.
Publisher | Google Scholor - Paris HS, Kabelka E. (2009). Gene list for Cucurbita species. Cucurbit Genetics Cooperative Report., 32: 44-69.
Publisher | Google Scholor - Keinath AP, Hassell RL. (2014). Control of Fusarium wilt of watermelon by grafting onto bottle gourd or interspecific hybrid squash despite colonization of rootstocks by Fusarium. Plt Dis., 98:255-266.
Publisher | Google Scholor - Buller S, Inglis D, Miles C. (2013). Plant growth, fruit yield and quality and tolerance to verticillium wilt of grafted watermelon and tomato in field production in the Pacific Northwest. Hortic Sci Hort., 48: 1003-1009.
Publisher | Google Scholor - Yetisir H, Caliskan ME, Soylu S, Sakar M. (2006). Some physiological and growth responses of watermelon [Citrullus lanatus (Thunb.) Matsum. And Nakai] grafted onto Lagenaria siceraria to flooding. Envir Exper Bot., 58:1-8.
Publisher | Google Scholor - Bekhradi F, Kashi A, Delshad M. (2011). Effect of three cucurbits rootstocks on vegetative and yield of ‘Charleston Gray’ watermelon. Intl J of Plant Prod., 5:105–110.
Publisher | Google Scholor - Liu B, Ren J, Zhang Y, An J, Chen M, Chen H, et al. (2015). A new grafted rootstock against root-knot nematode for cucumber, melon, and watermelon. Agro Sust Dev; 35: 251-259.
Publisher | Google Scholor - Jang Y, Huh YC, Dong-Kum P, Mun B, Lee S, Um Y. (2014). Greenhouse evaluation of melon rootstock resistance to Monosporascus root rot and vine decline as well as of yield and fruit quality in grafted ‘Inodorus’ melons. Korean Soc for Hort Sci., 32: 614-622.
Publisher | Google Scholor - Siguenza C, Schochow M, Turini T, Ploeg A. (2005). Use of Cucumis metuliferus as a rootstock for melon to manage Meloidogyne incognita. J Nematol., 37(3): 276.
Publisher | Google Scholor - Nienhuis J, Lower RL. (1980). Influence of reciprocal donor scions on fruit setting characteristics of recipient scions of Cucumis sativus and C. hardwickii R. Cuc Gene Coop Rep., 3: 17-19.
Publisher | Google Scholor - Trionfetti N P T, Colla G, Granati E, Temperini E, Crino P, Saccardo F. (2002). Rootstock resistance to fusarium wilt and effect on fruit yield and quality of two muskmelon cultivars. Sci Hort., 93: 281-288.
Publisher | Google Scholor - Thies JA, Levi A. (2007). Characterization of watermelon (Citrullus lanatus cv. citroides) germplasm for resistance to root knot nematodes. Hortsci., 42: 1530-1533.
Publisher | Google Scholor - Edelstein M, Tyutyunik J, Fallik E, Meir A, Tadmor Y, Cohen R. (2014). Horticultural evaluation of exotic watermelon germplasm as potential rootstocks. Scia Hort., 165: 196-202.
Publisher | Google Scholor - King SR, Davis AR, Liu Wand Levi A. (2008). Grafting for disease resistance. Hort Sci., 43: 1673-1676.
Publisher | Google Scholor - Ioannou N. (2001). Integrating soil solarization with grafting on resistant rootstocks for management of soil-borne pathogens of eggplant. J Hortic Sci Biotechnol., 76(4), 396-401.
Publisher | Google Scholor - Riga P, Benedicto L, Garcia-Flores L, Villano D, Medina S, Gil-Izquierdo A. (2016). Rootstock effect on serotonin and nutritional quality of tomatoes produced under low temperature and light conditions. J Food Comp Anal., 46: 50-59.
Publisher | Google Scholor - Rivero RM, Ruiz JM, Sanchez E, Romero L. (2003). Does grafting provide tomato plants an advantage against H2O2 production under conditions of thermal shock? Physiol Plant., 117(1):44-50.
Publisher | Google Scholor - Bloom AJ, Zwieniecki MA, Passioura JB, Randall LB, Holbrook NM, St. Clair DA. (2004). Water relations under root chilling in a sensitive and tolerant tomato species. Plant Cell Env., 27(8): 971-979.
Publisher | Google Scholor - Zhou Y, Zhou J, Huang L, Ding X, Shi K, Yu J. (2009). Grafting of Cucumis sativus onto Cucurbita ficifolia leads to improved plant growth, increased light utilization and reduced accumulation of reactive oxygen species in chilled plants. J Plant Res., 122(5):529-540.
Publisher | Google Scholor - Venema JH, Dijk BE, Bax JM, Van Hasselt PR, Elzenga JTM. (2008). Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ Exp Bot., 63(1–3): 359-367.
Publisher | Google Scholor - Abdelmageed AHA, Gruda N. (2009). Influence of grafting on growth, development, and some physiological parameters of tomatoes under controlled heat stress conditions. Eur J Hortic Sci. 74(1):16-20.
Publisher | Google Scholor - Black L, Wu DL, Wang JF, Kalb T, Abbass D, Chen JH. (2003). Grafting tomatoes for production in the hot-wet season Shanhua, Taiwan. AVRDC Publi., 03-551.
Publisher | Google Scholor - Bagnaresi P, Sala T, Irdani T, Scotto C, Lamontanara A, Beretta M, Rotino GL, Sestili S, Cattivelli L, Sabatini E. (2013). Solanum torvum responses to the root-knot nematode (Meloidogyne incognita). BMC Genom., 14:540.
Publisher | Google Scholor - Petran A, Hoover E. (2014). Solanum torvum as a compatible rootstock in interspecific tomato grafting. J Hort., 1:103.
Publisher | Google Scholor - Peng Y, Dong Y, Tu B, Zhou Z, Zheng B, Luo L, et al. (2013). Roots play a vital role in flood-tolerance of poplar demonstrated by reciprocal grafting. Flora: Morphol Distrib Funct Ecol Plants., 208(8-9): 479-487.
Publisher | Google Scholor - Tzortzakakis EA, Bletsos FA, Avgelis AD. (2006). Evaluation of solanum rootstock accessions for control of root-knot nematodes and tobamo viruses. J Plant Dis Prot., 113: 188-189.
Publisher | Google Scholor - Penella C, Nebauer SG, San Bautista A, Lopez-Galarza S, Calatayud A. (2014). Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: physiological responses. J Plant Physiol., 171(10): 842-885.
Publisher | Google Scholor - Pinheiro JB, Boiteux LS, Almeida MRA, Pereira RB, Galhardo LCS, Carneiro RMDG. (2015). First report of Meloidogyne enterolobii in capsicum rootstocks carrying the Me1 and Me3/Me7 genes in Central Brazil. Nematropica., 45: 184-188.
Publisher | Google Scholor - Oka Y, Offenbach R, Pivonia S. (2004). Pepper rootstock graft compatibility and response to Meloidogyne javanica and M. incognita. J Nemt., 36: 137-141.
Publisher | Google Scholor